Polyvinyl Acetate: Keeping It Together in School
It’s a brand new back to school year with new opportunities, new tools, and more paper glue?? That’s right! Almost every back to school supplies list has paper glue or some form of adhesive. Do you know the ingredient that is often times used to make glue?
Polyvinyl acetate (PVA) is one of those low-profile polymers, unlike polyethylene or polystyrene. PVA molecule likes to hide and can usually be found between 2 pieces of wood or paper glued together. The reason for this is because PVA is the glue/adhesive between wood and paper.
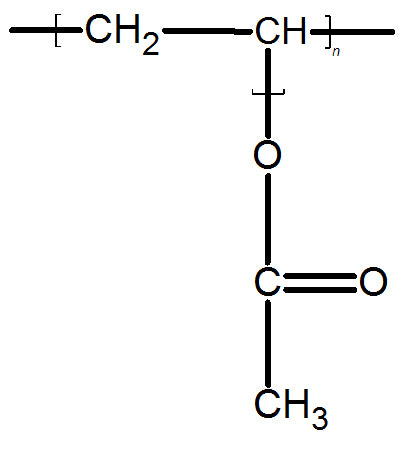
PVA has a chemical formula of (C4H6O2)n. It’s an aliphatic rubbery synthetic polymer which belongs to the polyvinyl ester family. Polyvinyl acetate can often time be confused with polyvinyl alcohol since base hydrolysis can convert polyvinyl acetate to polyvinyl alcohol and acetic acid.
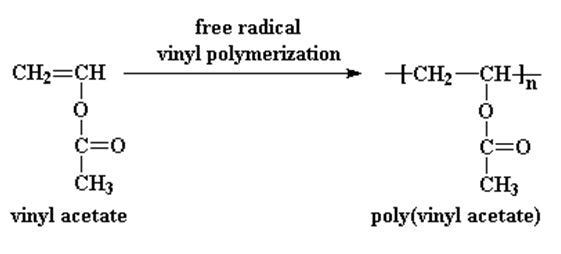
Polyvinyl acetate is prepared by the polymerization of the vinyl acetate monomer, through free radical vinyl polymerization of the vinyl acetate monomer.
The next time you take up your paper/wood glue to build some art and crafts, think of PVA!
What does PVA look like in Chemistry?
Let’s Get Building!
Using your Student Molecular Model Set from Duluth Labs let’s create Polyvinyl acetate! You’ll need:
-
8 Carbon Atoms
-
4 Oxygen Atoms
-
6 Hydrogen Atoms
-
12 Small connectors (compact small bonds for hydrogen)
-
12 Medium Connectors
-
4 Long connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building with Our First Monomer PVA unit.
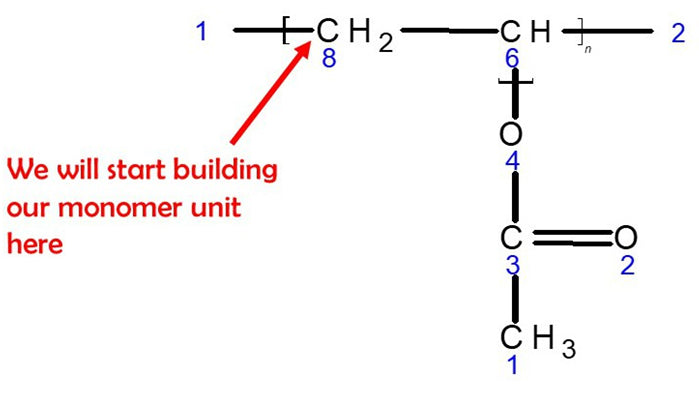
Let’s Start!
Steps:
-
1

1.Get a carbon atom(Carbon 8)and using a medium connector attach this to the left of Carbon 8. Then use 2 small connectors to attach 2 hydrogen atoms to Carbon 8.
-
2

2. Then, using a medium connector, attach Carbon 6 to Carbon 8. Use a small connector to attach a hydrogen atom to Carbon 6 and a medium connector to the right of Carbon 6.
-
3

3. Using a medium connector, attach an Oxygen atom to Carbon 6.
-
4
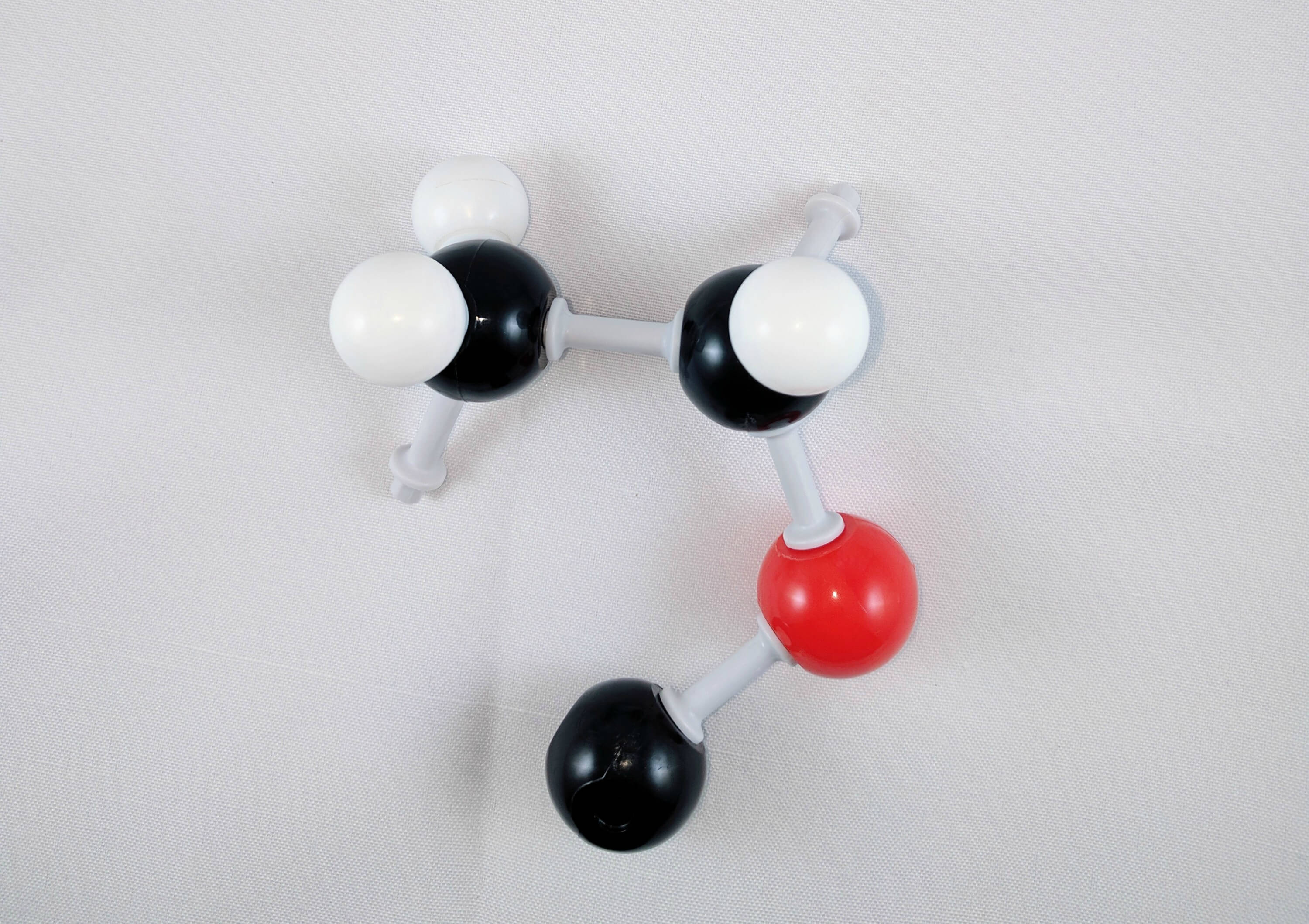
4. Then, use a medium connector to attach a carbon atom (Carbon 3) to the oxygen atom.
-
5

5. Using 2 long connectors, attach another oxygen atom to Carbon 3
-
6
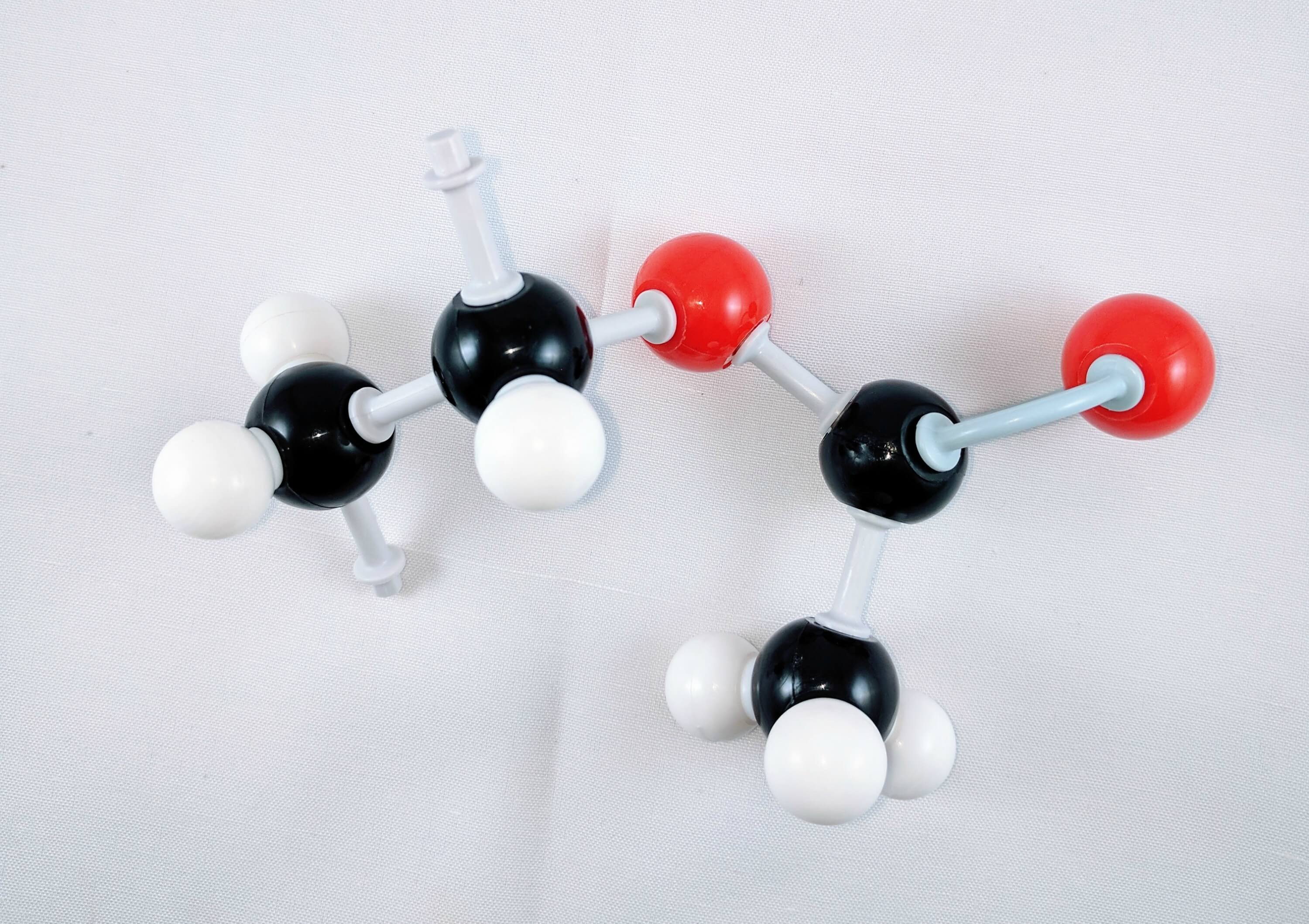
6. Finally, use a medium connector to attach a methyl group. This is a carbon atom (Carbon 1) with 3 hydrogen atoms attached using 3 small connectors.
-

Yay! We've just built our first Monomer Unit!
Note: Re-do the steps above by creating another PVA monomer unit.
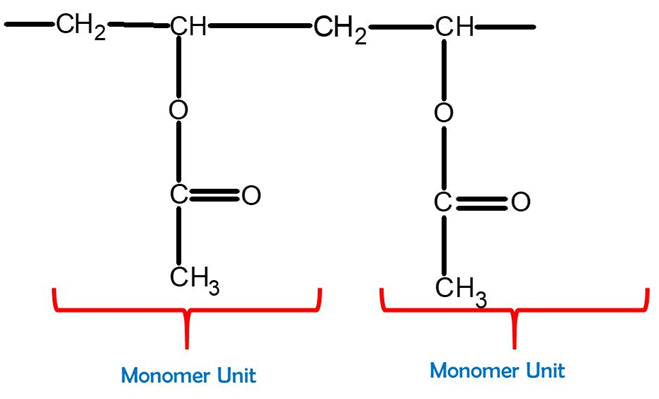
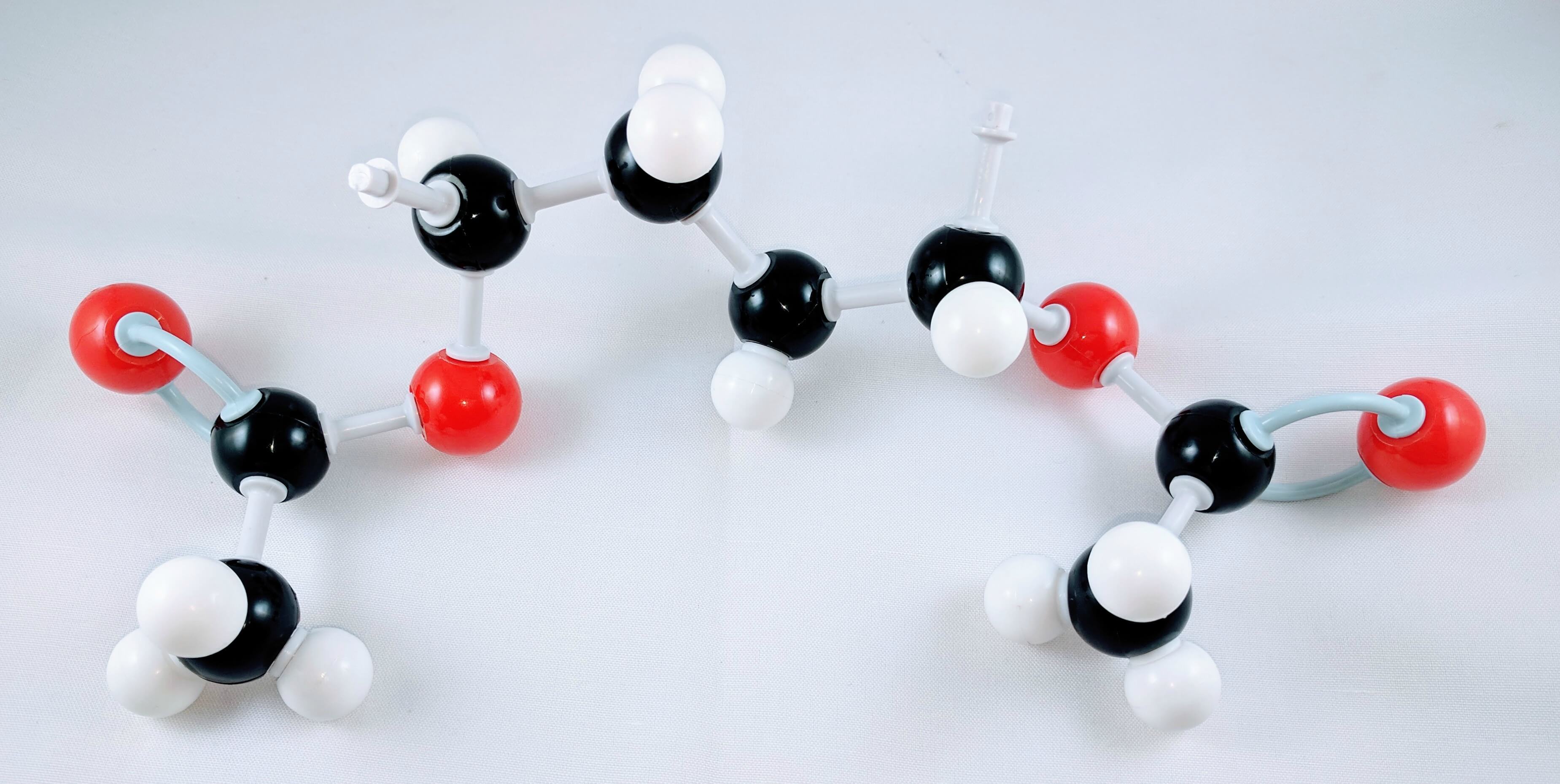
Polyvinyl Acetate
Feel free to build a bigger PVA molecule by joining more monomer units together. This can be better represented with one of our larger Molecular Model Kits (MM-004 or MM-006).
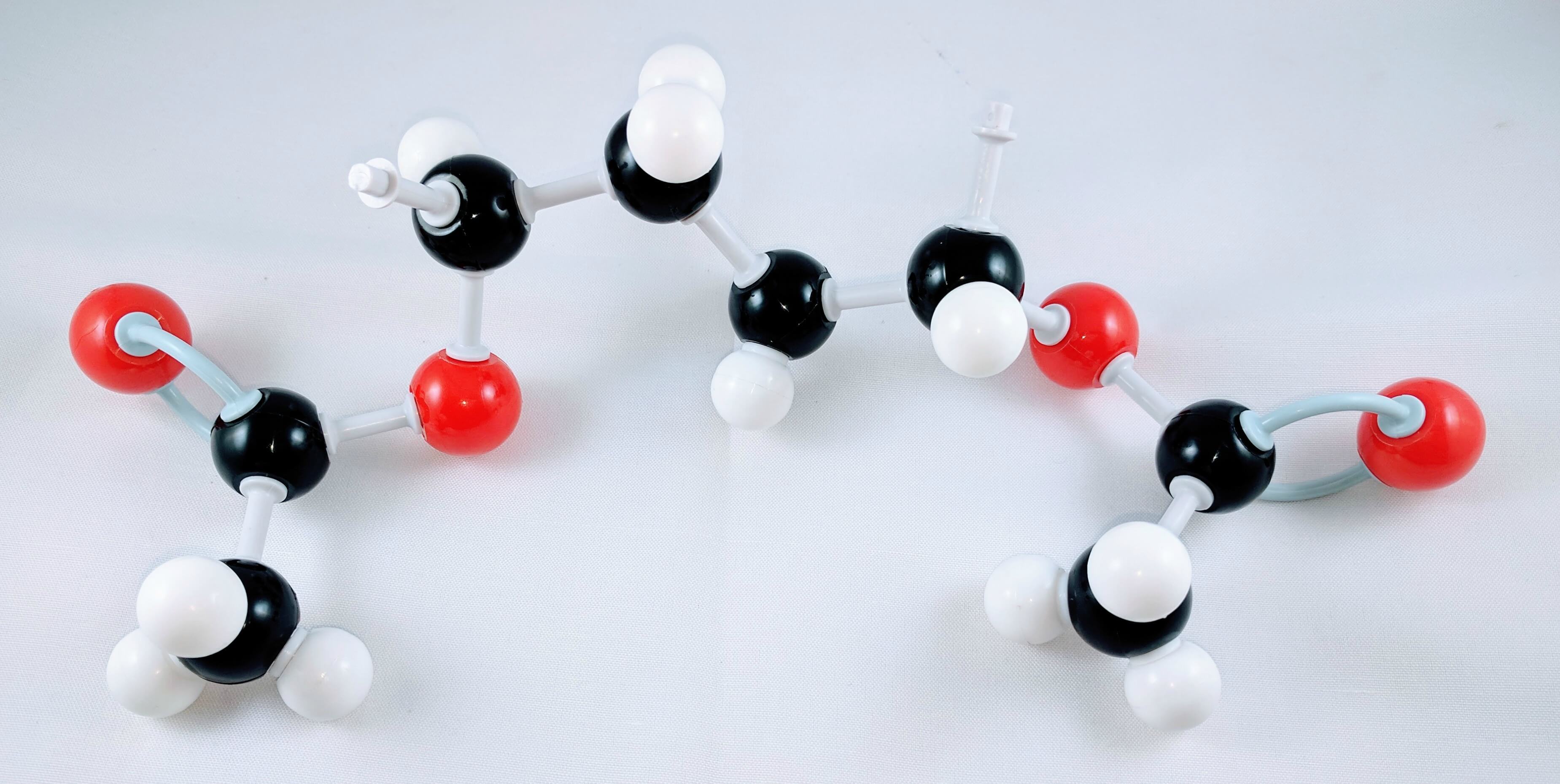
Great work! Now we have our newly-built Polyvinyl Acetate molecule
Place your molecule beside your Elmers Glue then share it with your science/chemistry teacher! Let them know you learned something new.













discount viagra https://viagaracon.com/# – ed pills online
viagra pills price
viagra for sale [url=https://viagaracon.com/#]low price viagra[/url] canadian viagra online pharmacy
Commenting is a key aspect of the photo experience, whatever message they’re sending. But for those of us too lazy to come up with witticisms, there’s always the like button.
It’s only funny when it’s someone else. Untag and pretend it doesn’t exist. The more you comment the more it shows up on news feeds.
‘Why is Exeter Academy the best choice ? It is a small school where every student immediately becomes an individual person. It’s lesson system allowed me to be at the right level to suit me according to my personal progress.’
카지노사이트
best canadian pharmacy https://goviagarato.com/# – buy viagra pills
canadian pharmacies
red viagra pills [url=https://goviagarato.com/]viagra from canada[/url] generic viagra from us pharmacy
cialis canadian pharmacy https://gecialiscan.com/ – order cialis online
cialis soft tabs
cost of cialis buy cialis cialis 5mg
generic viagra without a doctor prescription https://reviagaraget.com/# – red viagra pills
best price for viagra
order viagra online viagra 100mg india pharmacy viagra