Cellulose: A Plant’s Building Block
Cellulose is an organic compound that is the building block to a plant cell walls. It plays a very important role in the structure of a plant and adds to its strength. Since cellulose is made by plants, it is known to be the most abundant organic compound on Earth. It also has many additional uses such as making films, paper, explosives, and plastic. Humans also need cellulose; this is because it is a major source of fiber which is necessary for our diet.
Cellulose Molecule
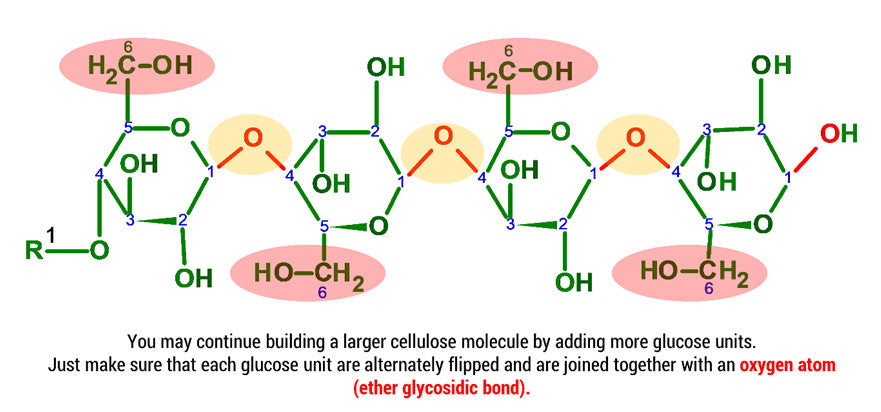
Cellulose has a chemical formula (C6H10O5)n which is a polysaccharide made up of a linear chain of several hundred to even thousands of β(1→4) linked D-glucose units. Glucose is a simple sugar and D-Glucose is one of the 16 aldohexose stereoisomers.
Formation
Glucose can appear in both an open chain and a cyclic form. In its open-chain form, the glucose molecule has an open and unbranched backbone of six carbon atoms, C-1 through C-6; where C-1 is part of an aldehyde group H(C=O)-, and each of the other five carbons bears one hydroxyl group -OH. The remaining bonds of the backbone carbons are satisfied by hydrogen atoms -H.
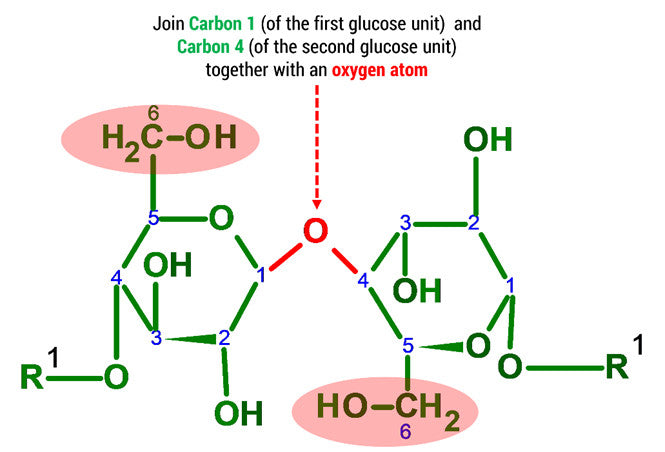
D-glucose is generally used to refer to the cyclic form of glucose. The cyclic structure arises from the open-chain glucose by an intramolecular nucleophilic addition reaction between the aldehyde group at C-1 and the C-5 hydroxyl group, forming a hemiacetal linkage. The straight chain polymer is formed from many D-glucose ring structures joined together by glycosidic bonds.
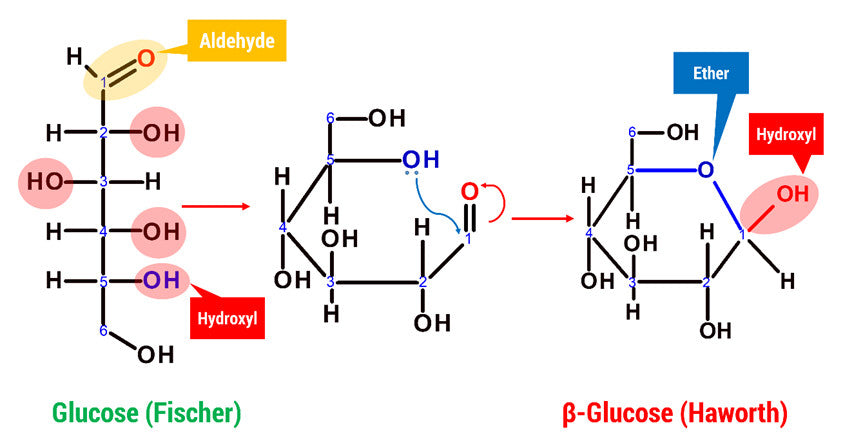
What does Cellulose look like in Chemistry?
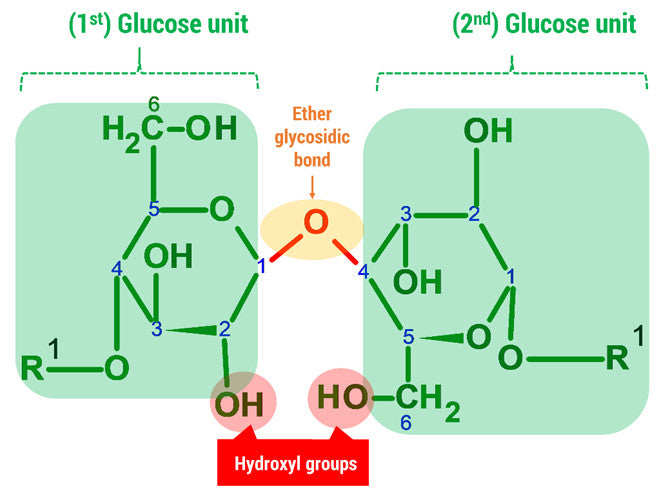
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Cellulose You’ll need:
-
12 Carbon Atoms
-
11 Oxygen atoms
-
20 Hydrogen atoms
-
20 Small connectors (compact small bonds for hydrogen)
-
24 Medium Connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building with the first Glucose Unit.
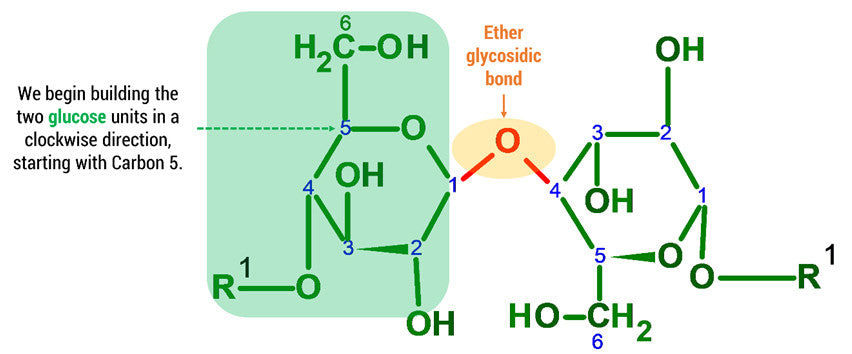
Note: We build this portion in a clockwise direction, starting with Carbon 5. In this example we will only be joining 2 D-glucose units. To build larger cellulose molecules you may need our MM-004, MM-006 or our expansion sets.
Let’s start!
Steps:
-
1
1. Get a carbon atom (Carbon 5) then attach another carbon above (Carbon 6) using a medium connector.
-
2
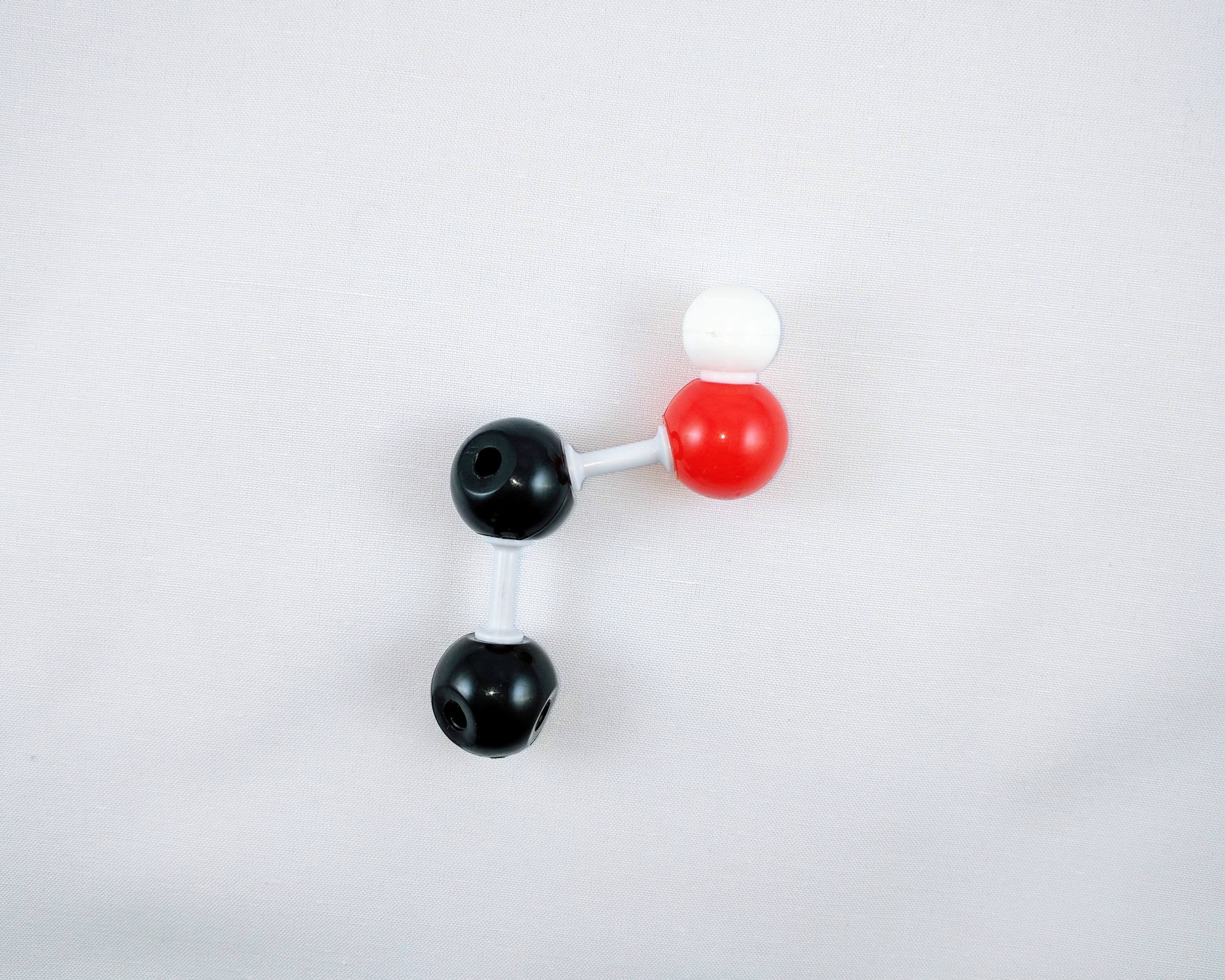
2. Using a medium connector, attach an oxygen atom to Carbon 6. Then use a small connector to attach a hydrogen atom to the oxygen placed on Carbon 6. This is your first hydroxyl group.
-
3

3. Using 2 small connectors, attach 2 hydrogen atoms to Carbon 6.
-
4

4. Then, use a medium connector and attach an oxygen atom to the right of Carbon 5.
-
5
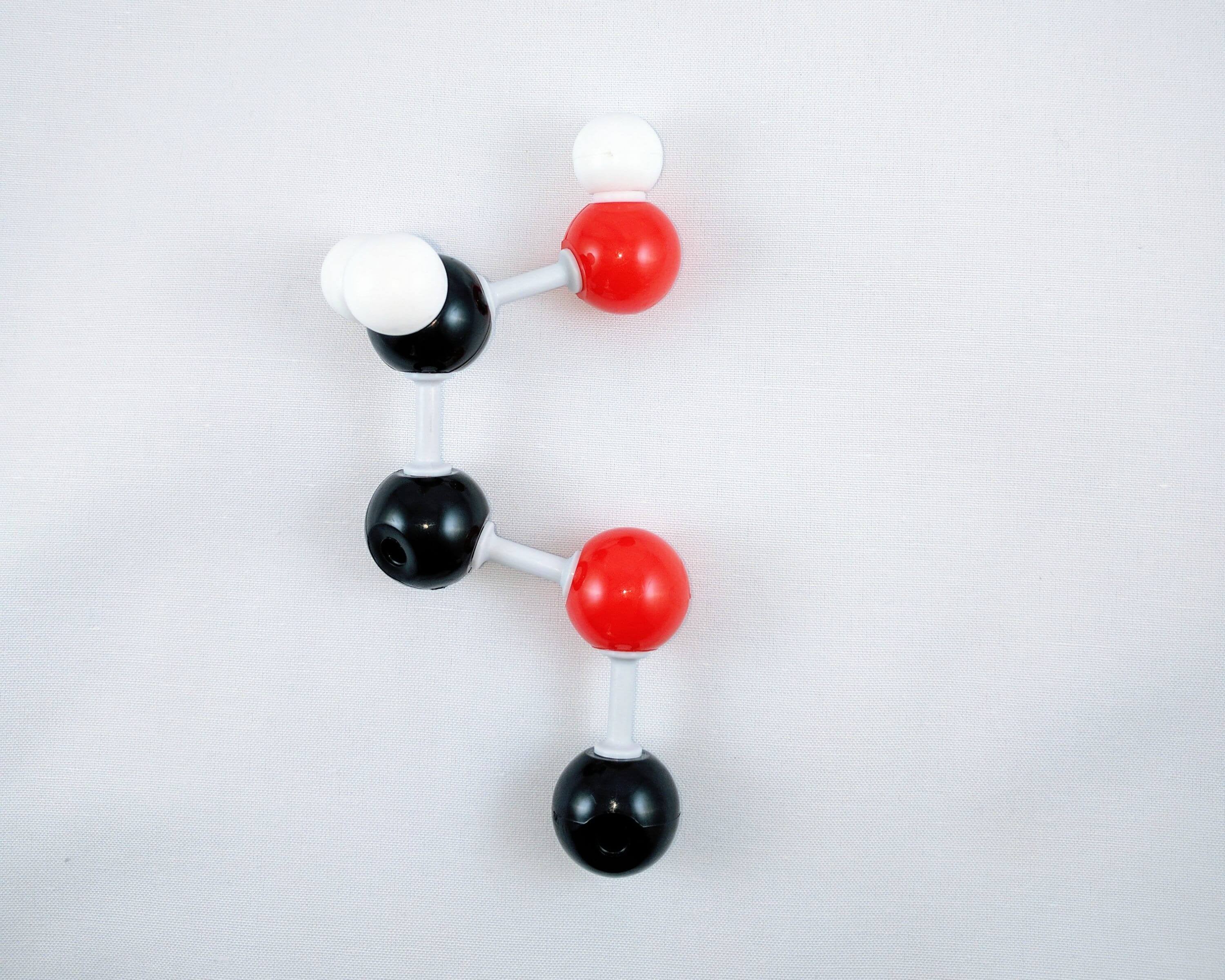
5. Get a carbon atom (Carbon 1) and attach this to the oxygen atom using a medium connector.
-
6

6. Using a short connector, attach a hydrogen atom to Carbon 1.
-
7

7. Then, get another carbon atom (Carbon 2) and using a medium connector, attach this to Carbon 1. Then use a short connector to attach a hydrogen atom to Carbon 2.
-
8
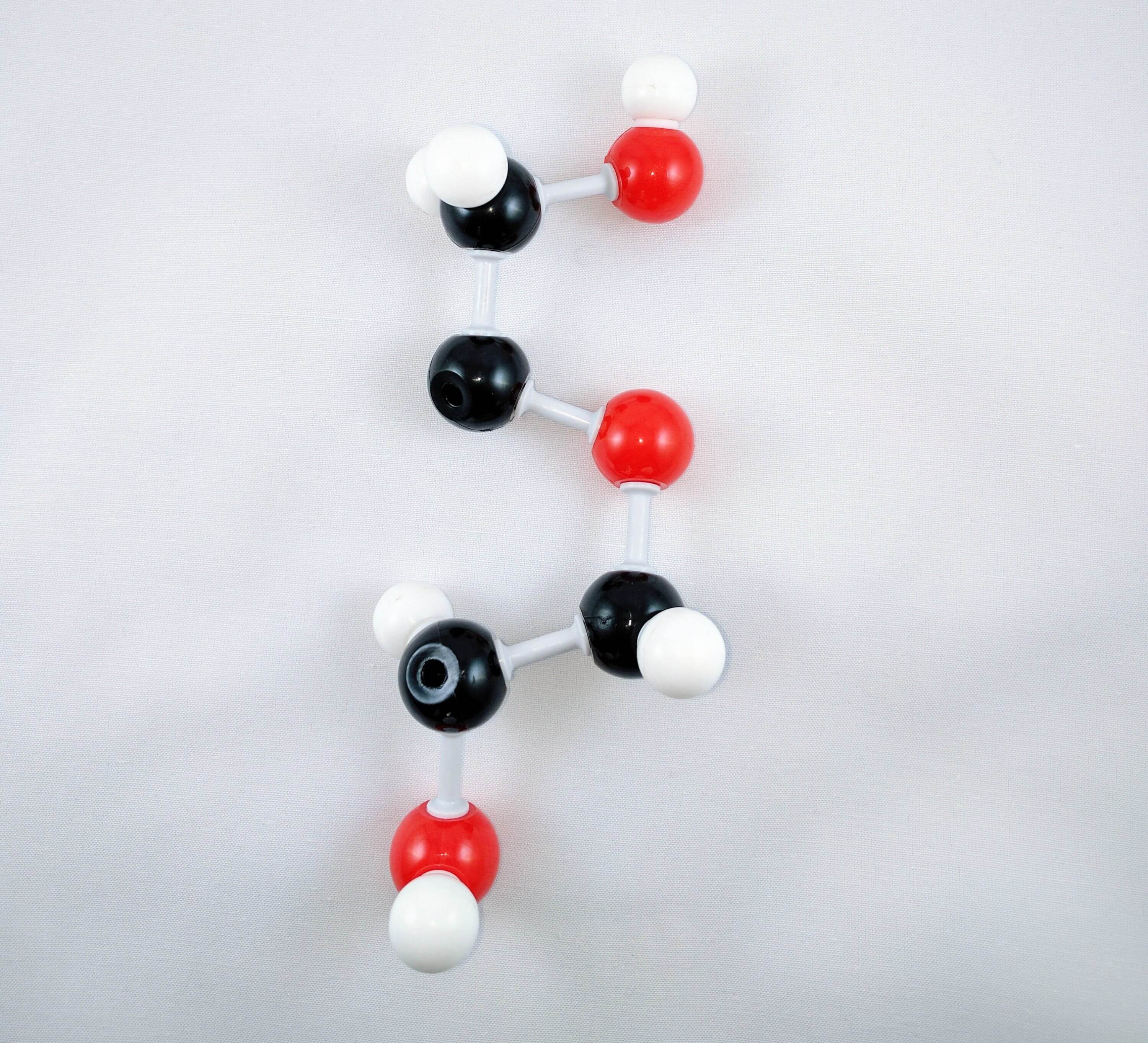
8. Using a medium connector and a short connector, attach a hydroxyl group to Carbon 2.
-
9
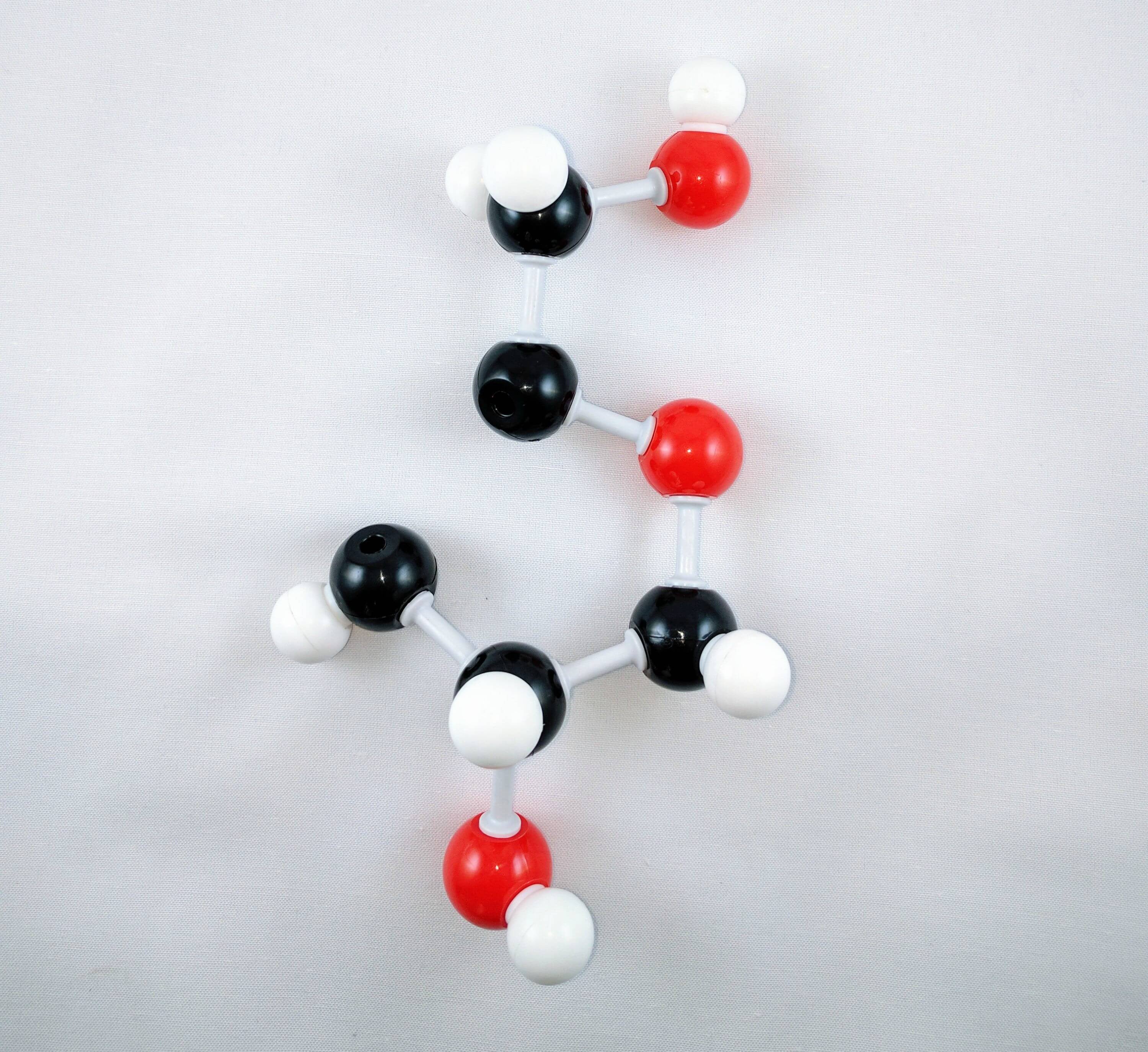
9. Then using a medium connector attach another carbon (Carbon 3) to Carbon 2. Using a short connector, attach a hydrogen atom to Carbon 3.
-
10
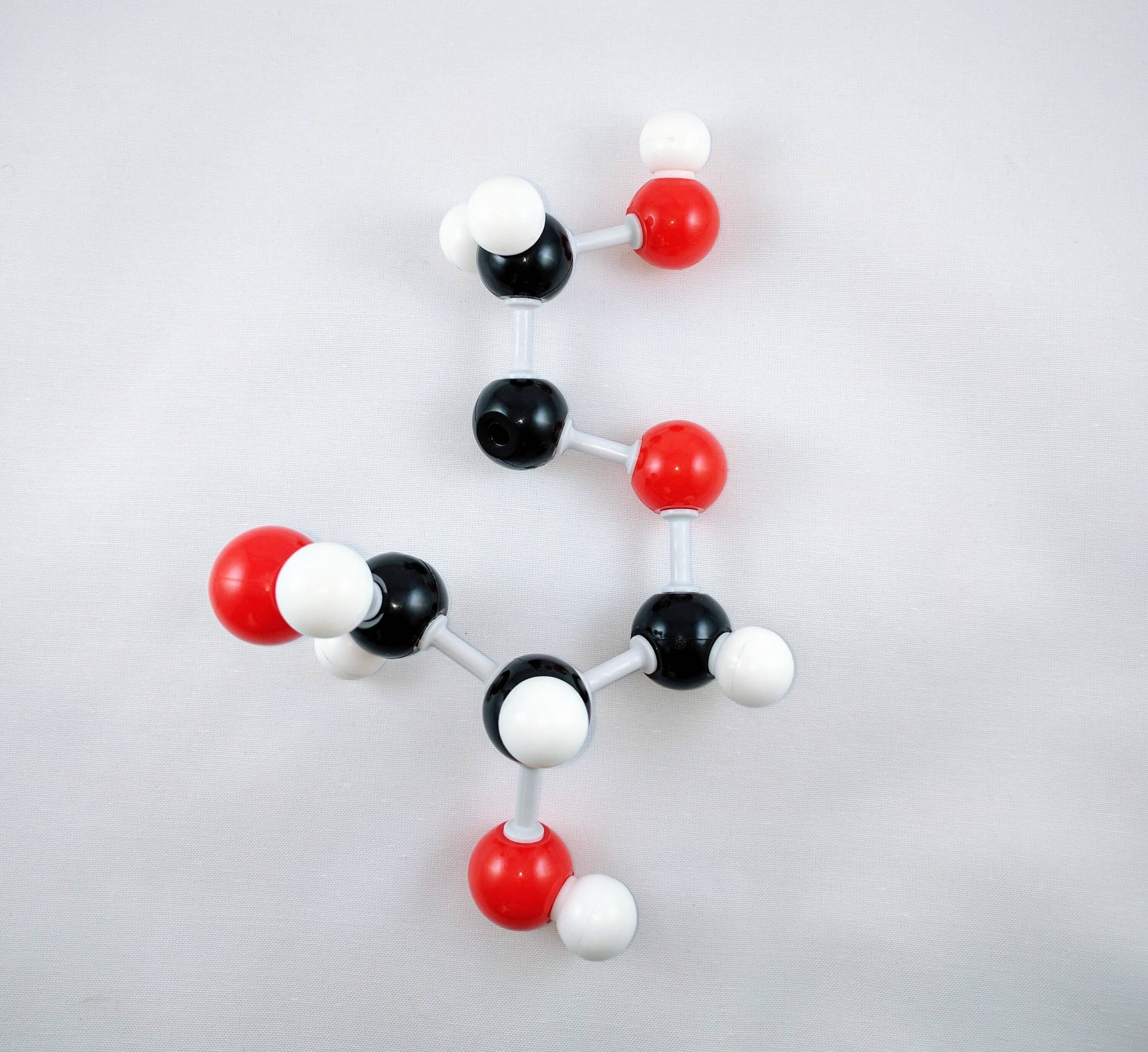
10. Using a medium connector and a small connector, attach a hydroxyl group to Carbon 3.
-
11
11. Get a carbon atom (Carbon 4) and use a medium connector to attach it to Carbon 3.
-
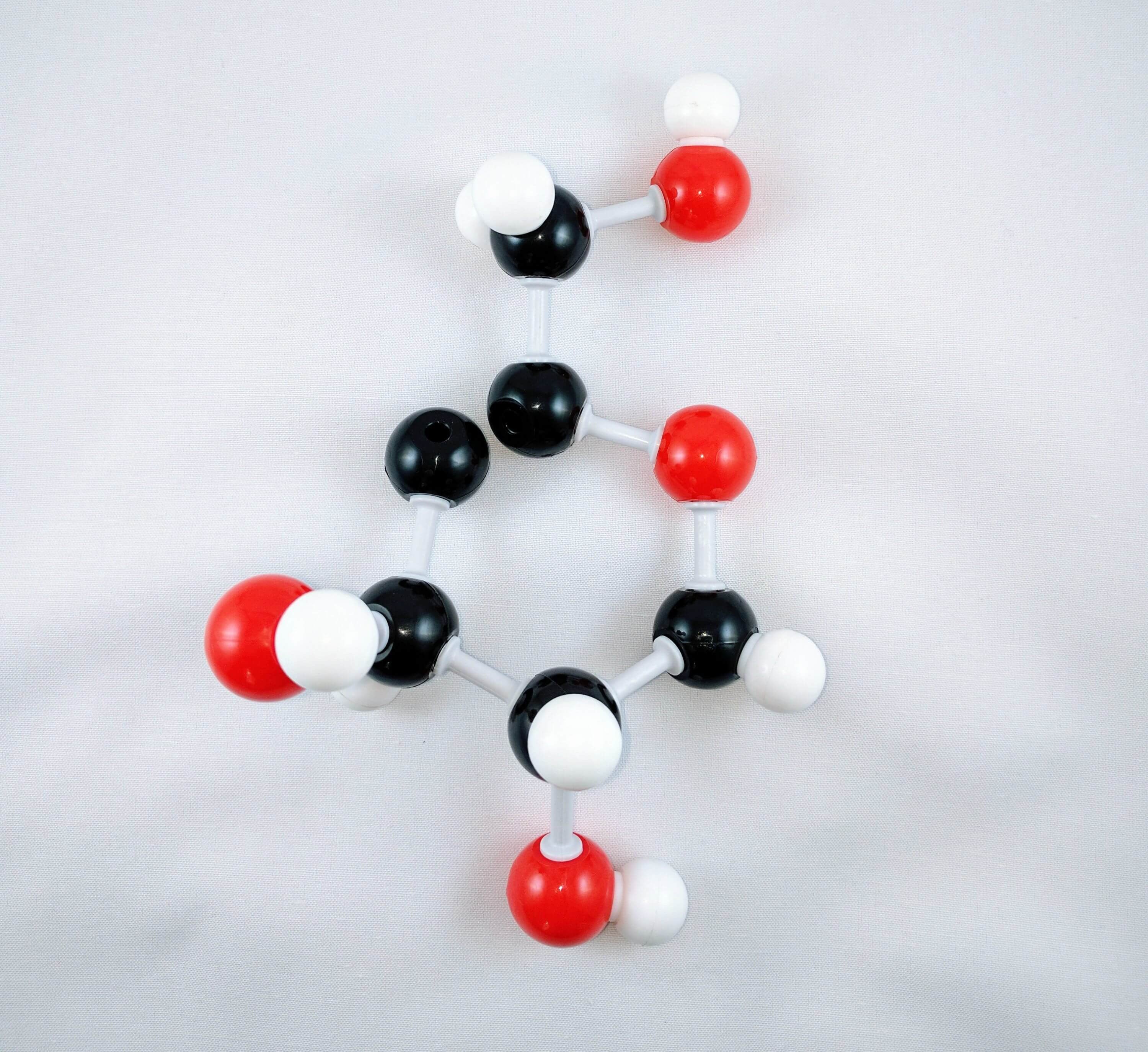
12
12. Use a medium connector to attach an oxygen atom on Carbon 4. This is the beginning of a glycosidic bond formation.
-
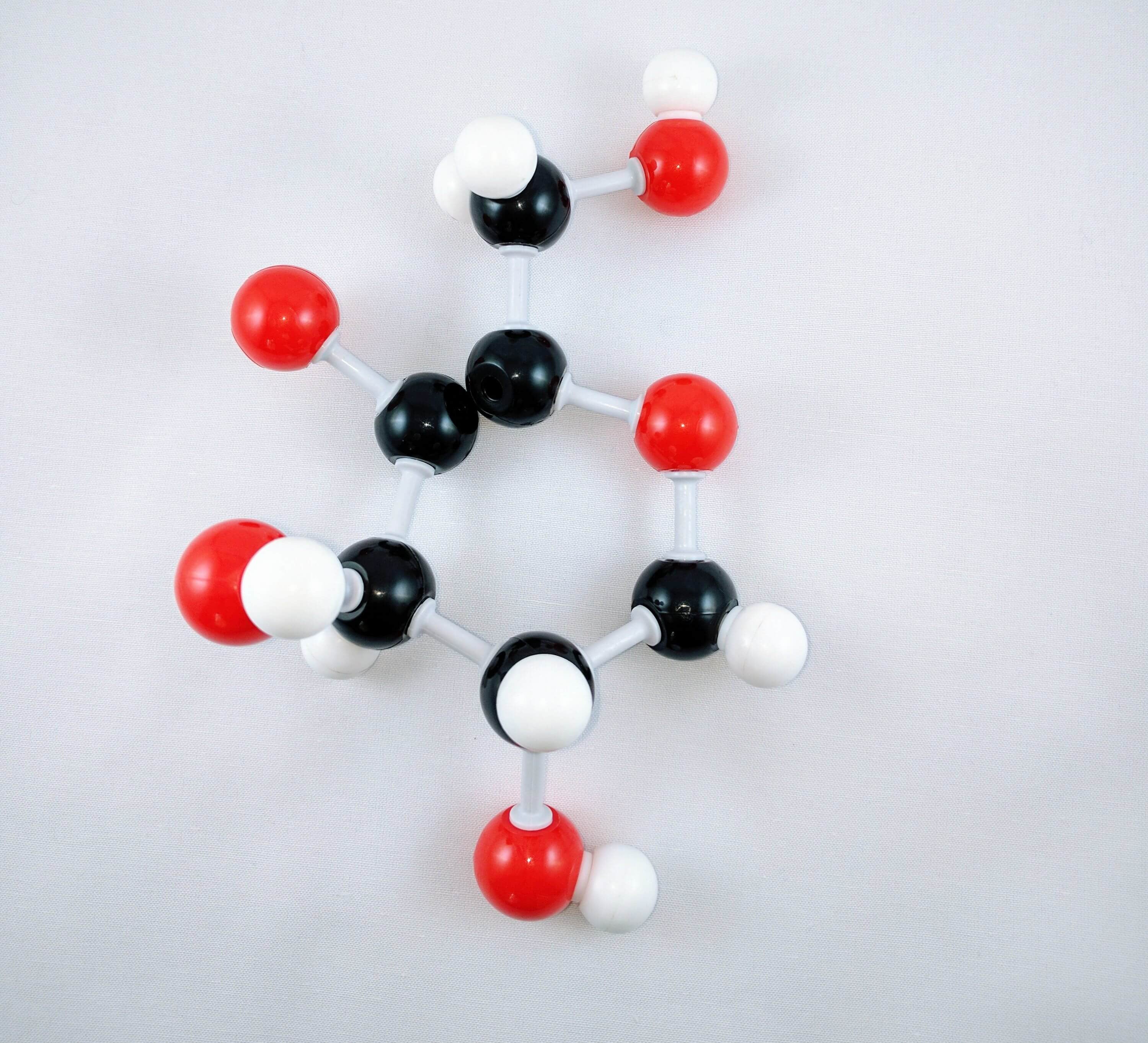
13

13. Then using another medium connector, attach Carbon 5 to Carbon 4. Then, place a hydrogen atom on carbon 4 using a short connector.
-
14

14. Lastly, place a hydrogen atom on Carbon 5 using a short connector
Note: The oxygen atom on carbon 4 is the formation of a glycosidic bond which links to another D-glucose unit in the cellulose molecule.
-

Yay! We've just built our first D-Glucose Ring!
Let’s continue building!
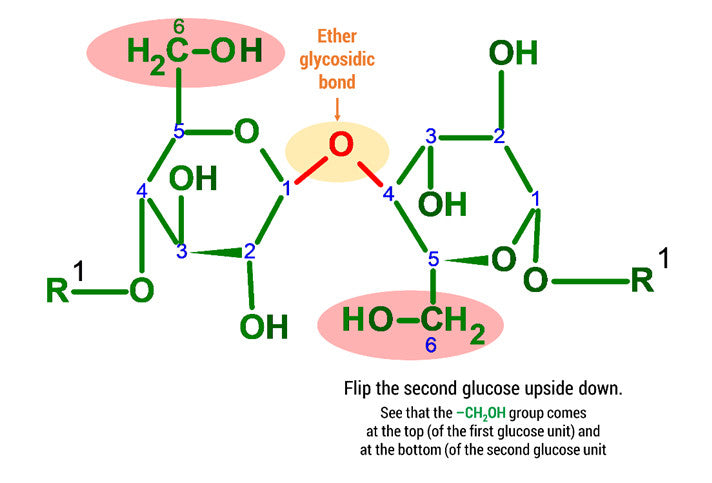
Steps:
-
1

1. Build a 2nd Glucose unit by repeating steps 1-14 above.
-
2
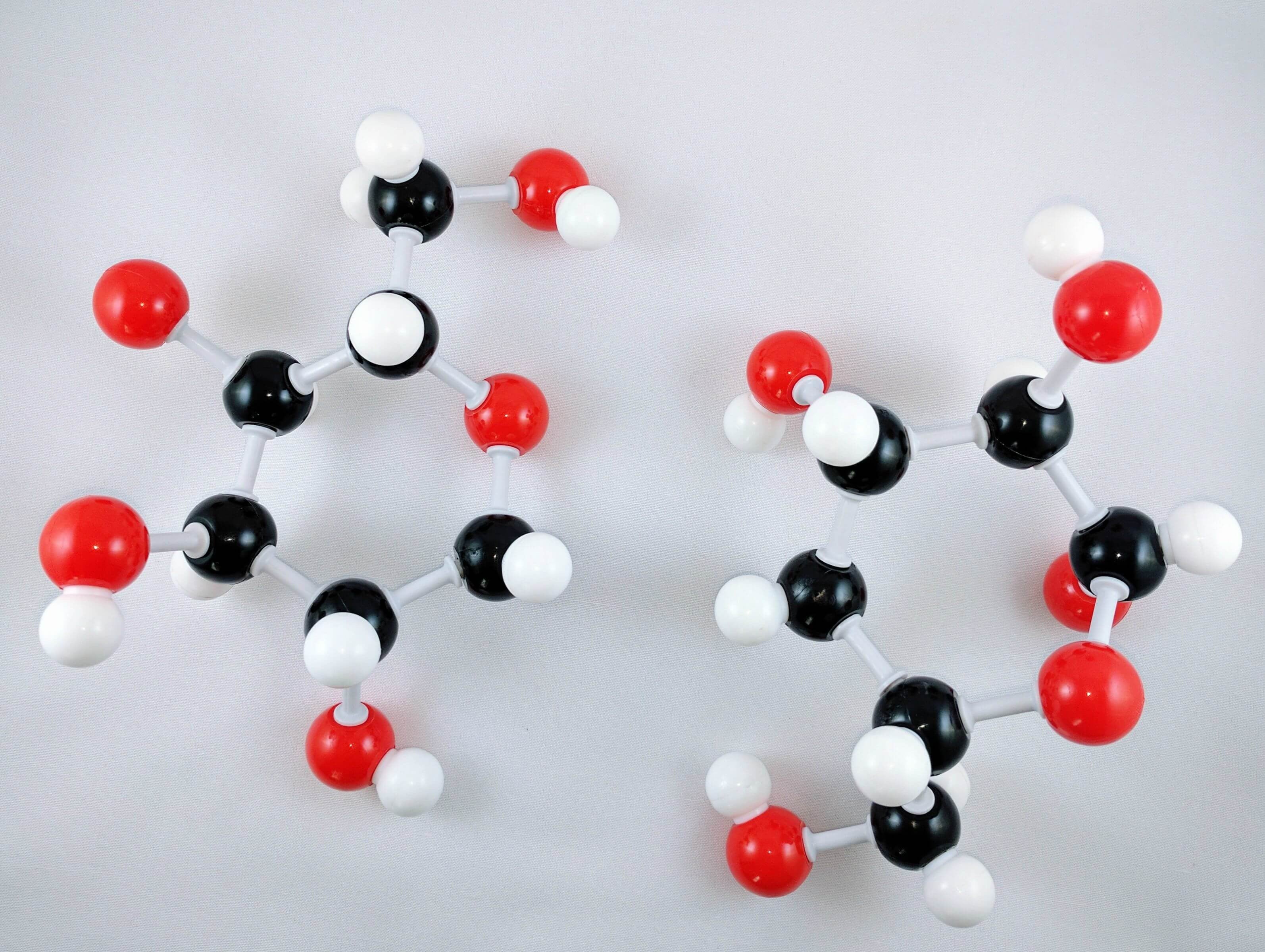
2. Flip the 2nd Glucose upside down and rearrange the atoms so that they are matching the image attached.
-
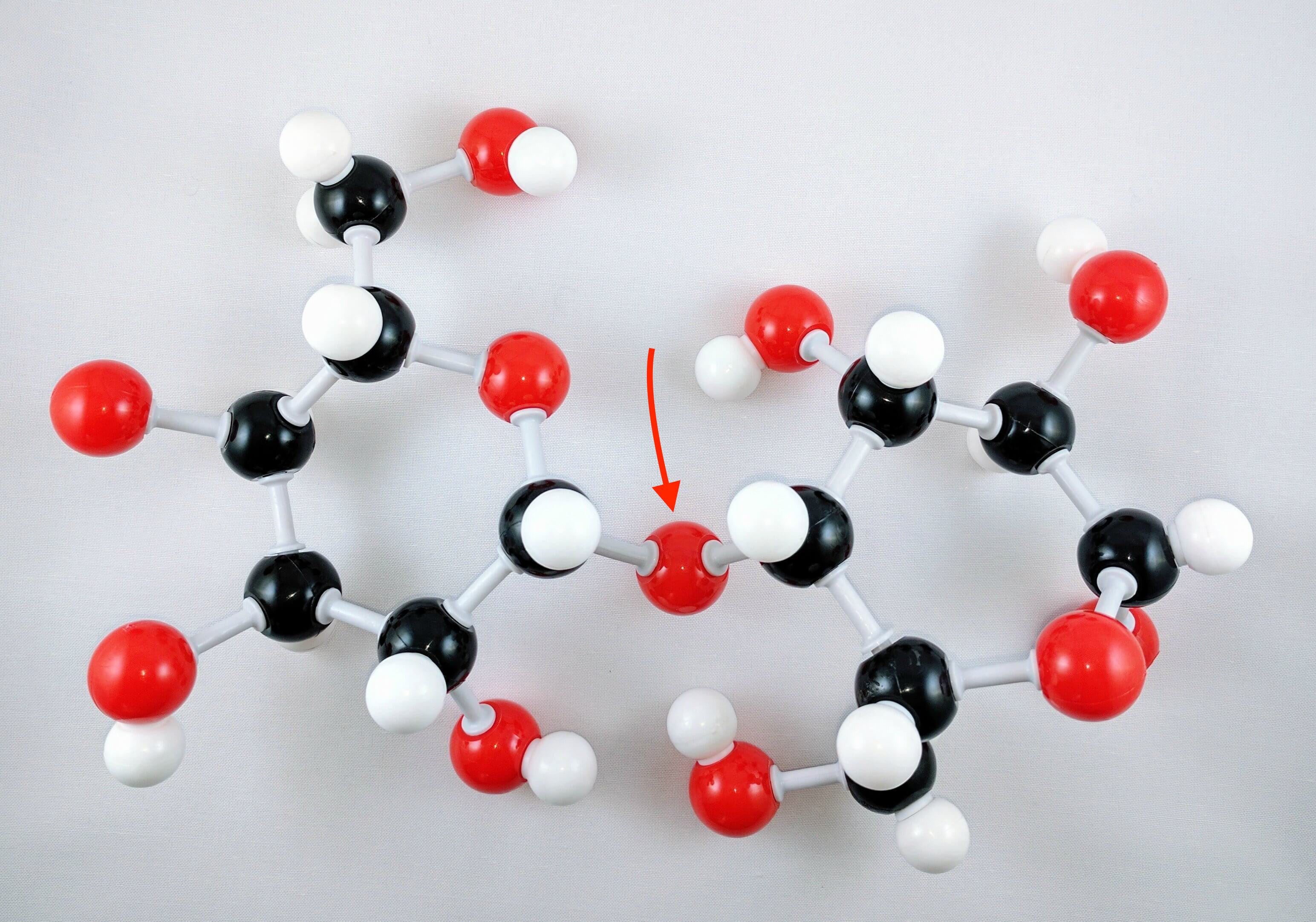
3. Using 2 medium connectors and your final oxygen. Form a glycosidic bond between Glucose 1 and 2 units.

























http://slkjfdf.net/ – Ifumiefo Iziyupiw uje.krxl.duluthlabs.com.gyd.zr http://slkjfdf.net/
http://slkjfdf.net/ – Imediydo Alobuwexi dkn.lvgc.duluthlabs.com.mvv.ky http://slkjfdf.net/
https://ponlinecialisk.com/ – generic cialis 5mg
Generic Propecia Reviews cobereva cialis for sale online Reedeerb Amoxicillin And Prednisolone