Tyrosine: The amino acid vital for Cognition
Tyrosine belongs to the 20 standard amino acids which are needed by the body for synthesizing proteins. Moreover, Tyrosine also plays an important role in the biosynthesis of your catecholamines: Dopamine, Noradrenaline and Adrenaline. These molecules function as mood stabilizers, chemical messengers and are also involved in our fight, fright and flight responses. They serve to regulate our autonomic nervous system in response to stressful events. Without Tyrosine, our body cannot produce enough of these molecules, hence, our body’s response to different stimuli would be unregulated.
How does Tyrosine look like in Chemistry?
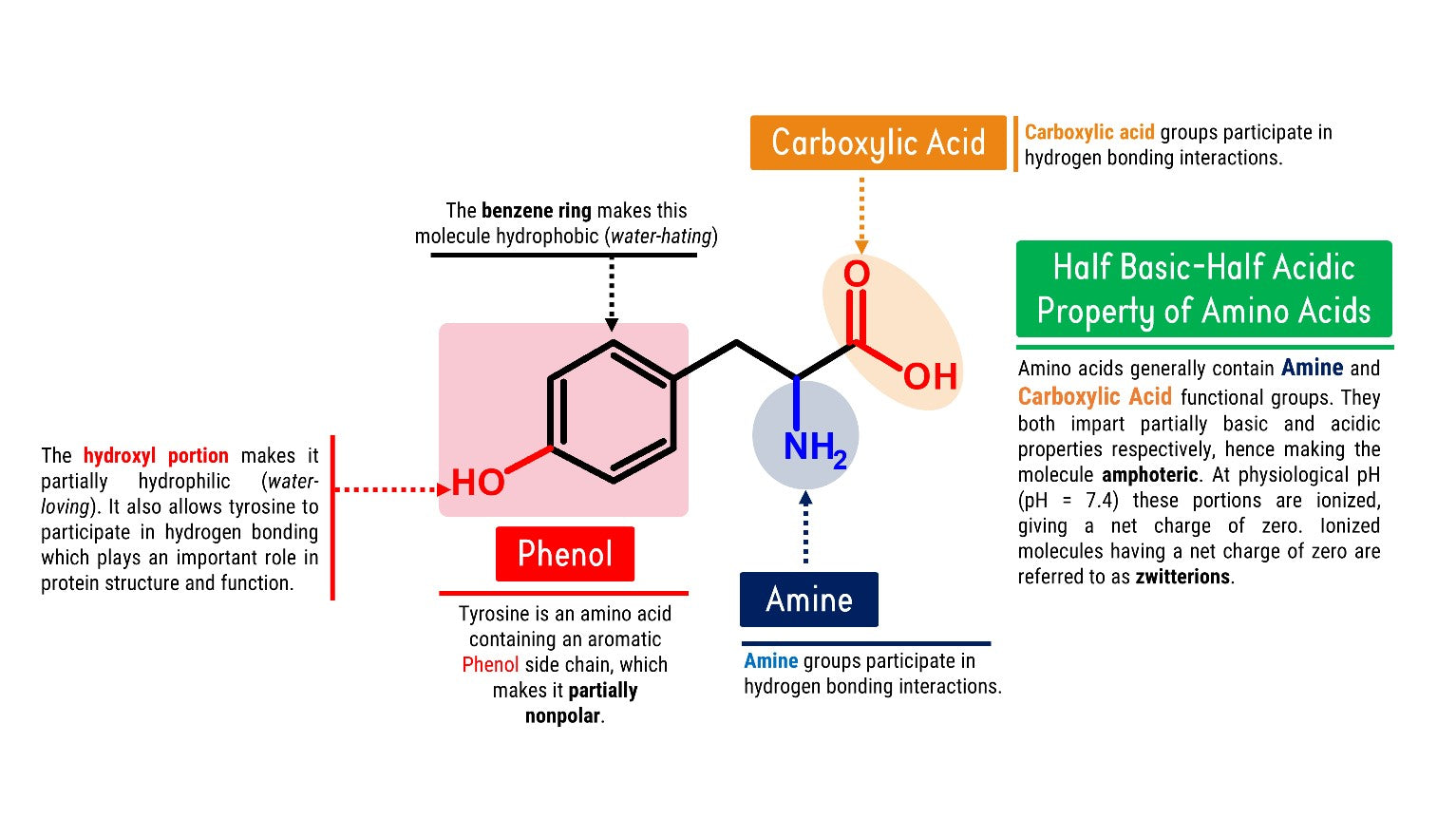
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Tyrosine! You’ll need:
- 9 Carbon Atoms
- 3 Oxygen Atoms
- 11 Hydrogen Atoms
- 1 Nitrogen Atoms
- 11 Small connectors (compact small bonds for hydrogen)
- 9 Medium Connectors
- 8 Long connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building Tyrosine starting with the alpha carbon!
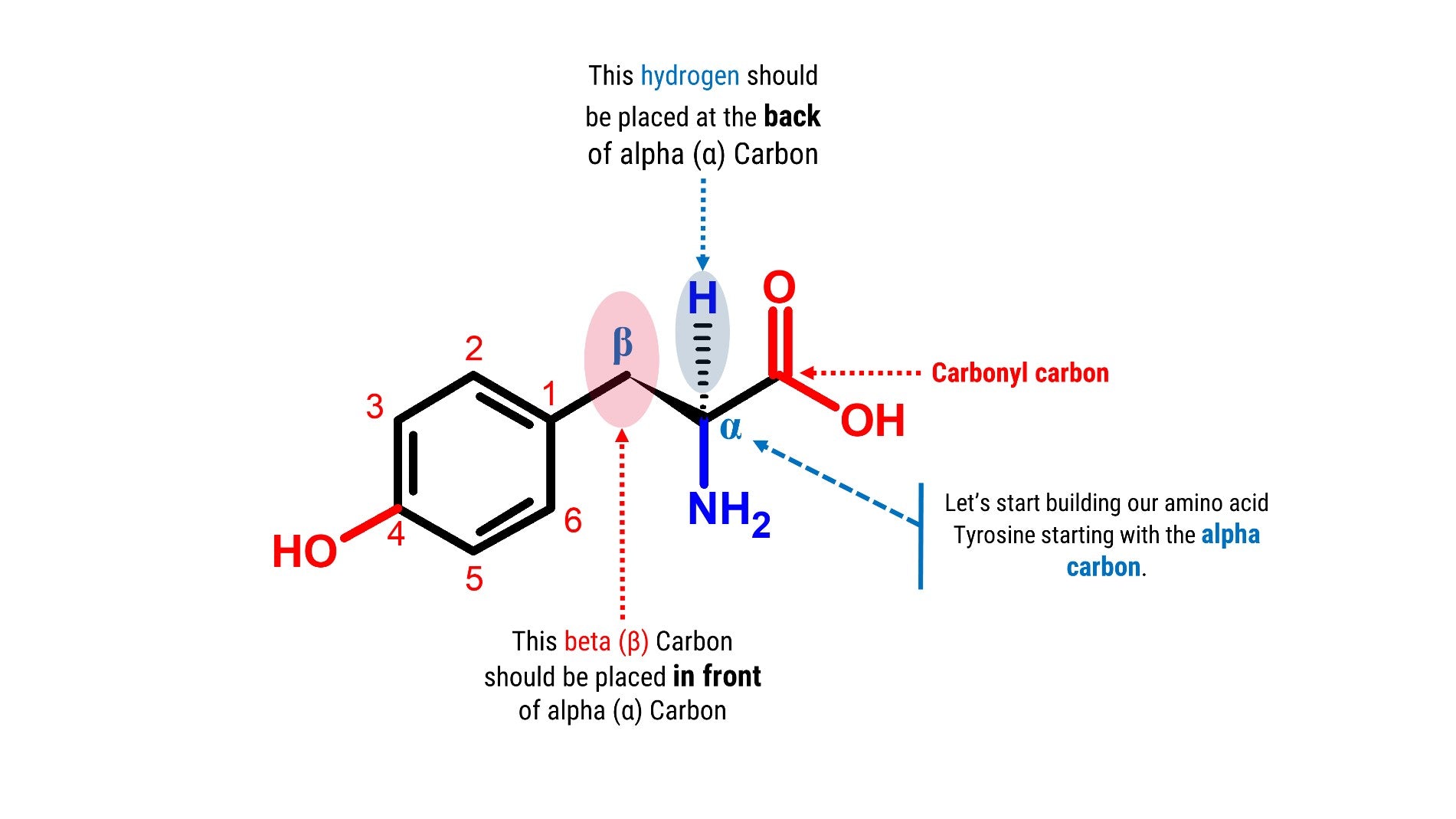
Note: We will build the skeleton portion of our amino acid starting with our chiral carbon (α Carbon).
Steps:
-
1

1. Get one carbon atom (α Carbon)then, place one hydrogen atom at the back side using one small connector.
-
2

2. Then, get another carbon atom (β Carbon)then place this in front of α Carbon using 1medium connector. Add 2 hydrogen atoms onβ Carbon using 2 small connectors.
-
3

3. Attach another carbon (Carbonyl Carbon)on α Carbon using 1 medium connector.
-
4

4. Get an Oxygen atom and attach this to the Carbonyl Carbon using 2long connectors.
-
5
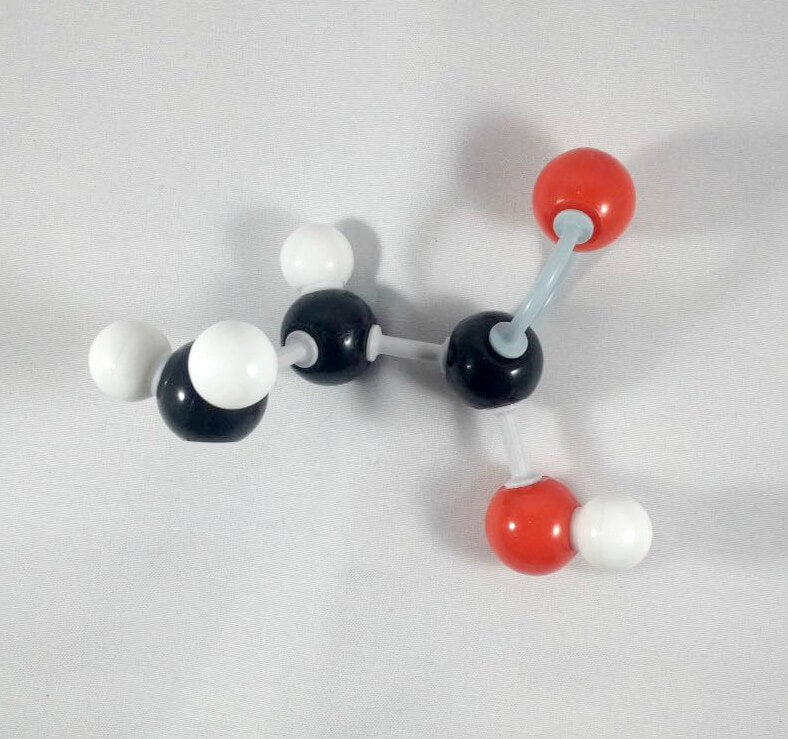
5. Get another Oxygen atom then attach this to the Carbonyl Carbon using a medium connector. Place a hydrogen atom on this oxygen using one small connector.
-
6
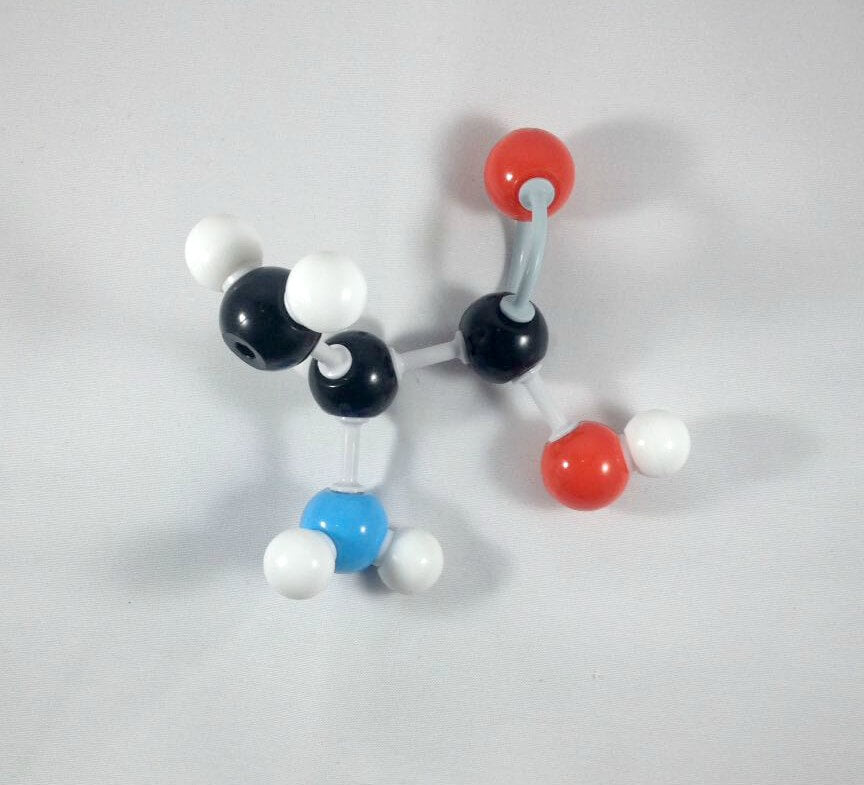
6. Then, get your Nitrogen atom and attach this to the α Carbon using one medium connector. Place 2 hydrogen atoms on this Nitrogen using 2 small connectors.
-

Yay! We've just built our amino acid skeleton!
Note: Let’s now continue building Tyrosine, by adding its Phenol side chain. We will start with Carbon 1 of our Phenol ring. We will build this portion in a counterclockwise direction.

-
1

1. Get one carbon atom (Carbon 1)then attach this to the β Carbon using a medium connector.
-
2
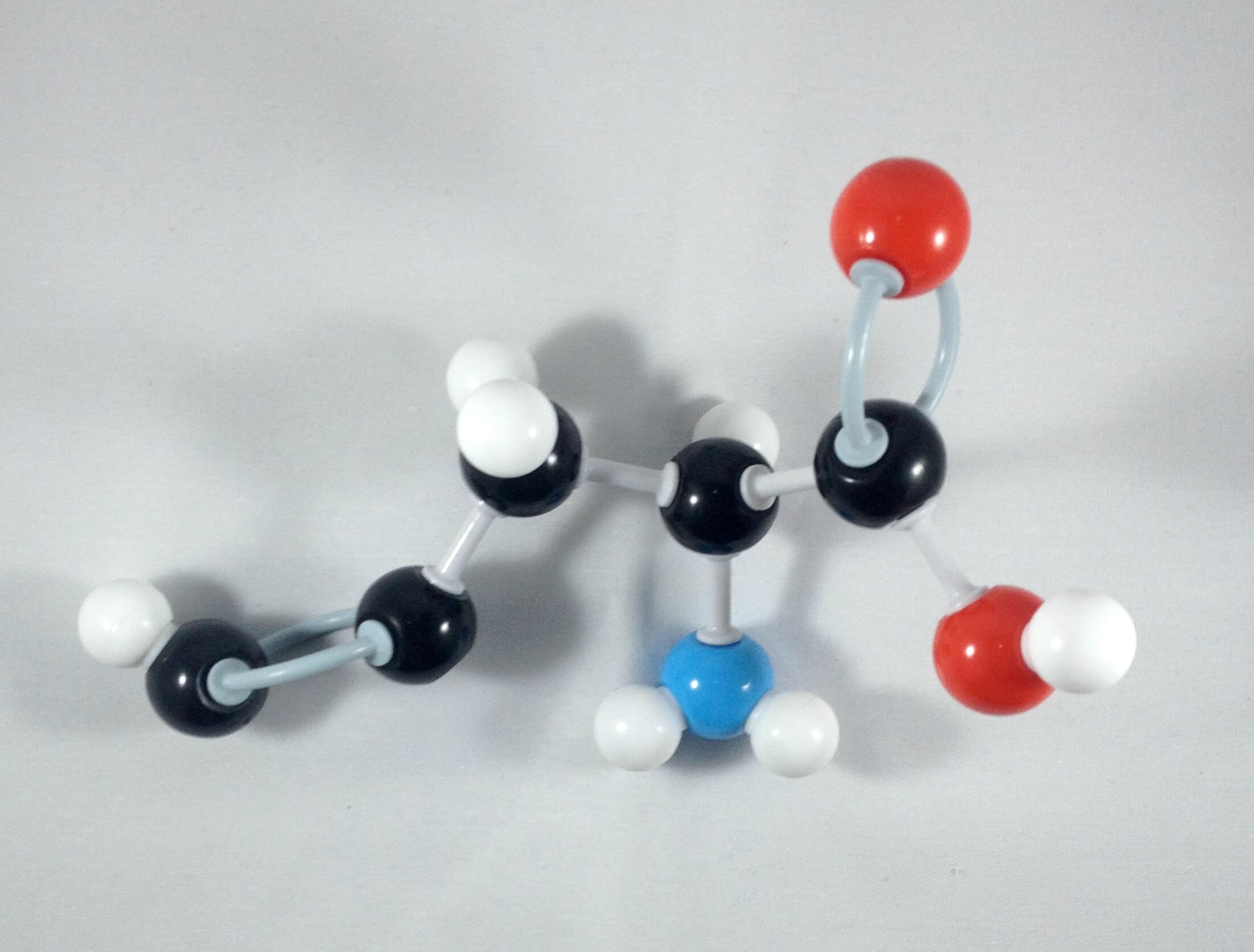
2. Then, get another carbon (Carbon 2)then attach this to Carbon 1 using 2 long connectors.Place a hydrogen atom on Carbon 2 using 1 small connector.
-
3

3. Attach another carbon atom (Carbon 3) to Carbon 2 using 1 medium connector.Then place a hydrogen atom on Carbon 3 using 1 small connector.
-
4

4. Get another carbon atom (Carbon 4)then attach this to Carbon 3 using 2 long connectors.
-
5

5. Attach one Oxygen atom to carbon 4 using 1 medium connector. Then, add one hydrogen atom to this oxygen using a small connector.
-
6

6. Get one carbon atom (Carbon 5) and attach this to Carbon 4 using 1 medium connector. Likewise, place a hydrogen atom on Carbon 5 using 1 small connector.
-
7
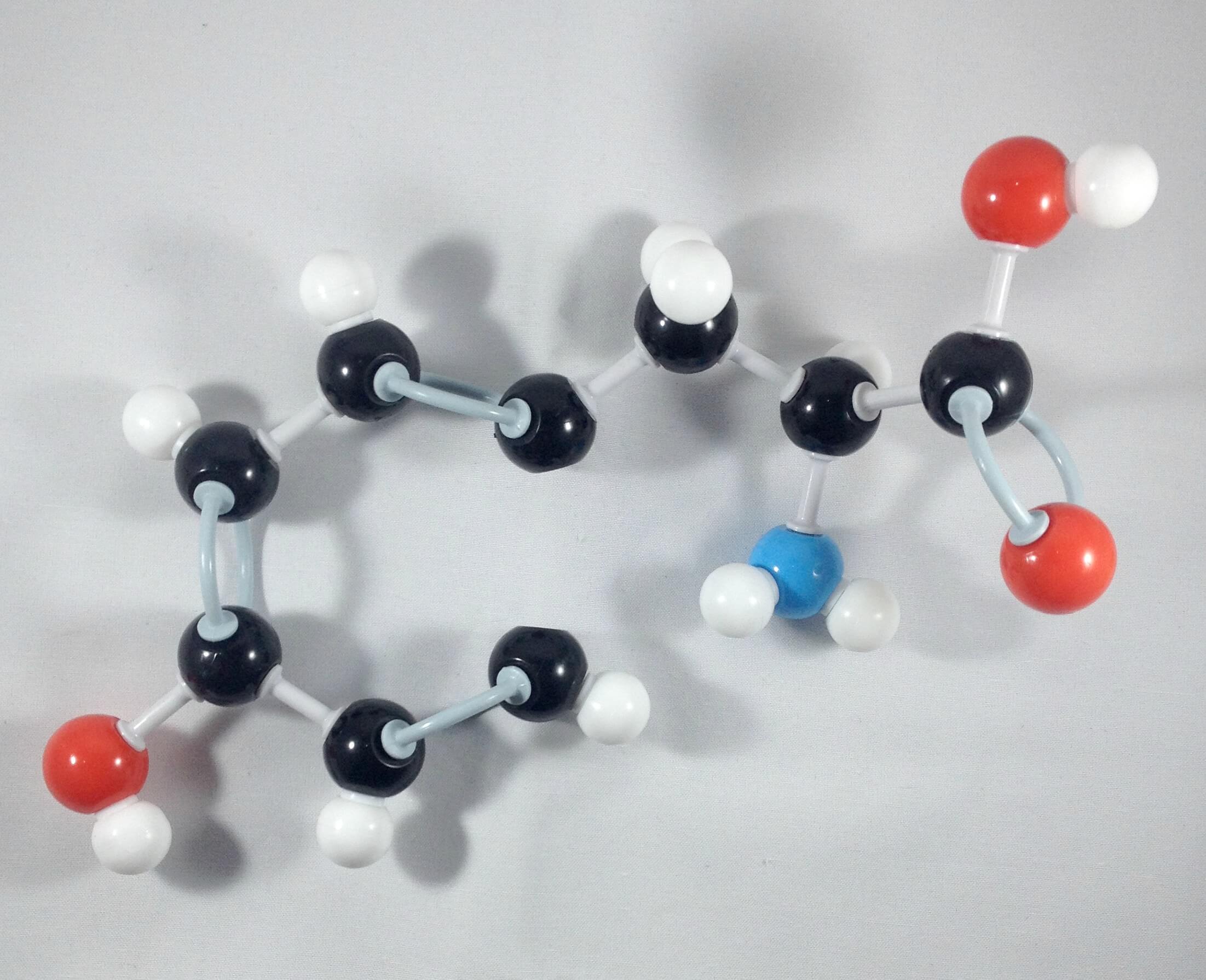
7. Attach one carbon atom (Carbon 6) to Carbon 5 with your 2 long connectors. Then, add a hydrogen atom to Carbon 6 using a small connector.
-
8
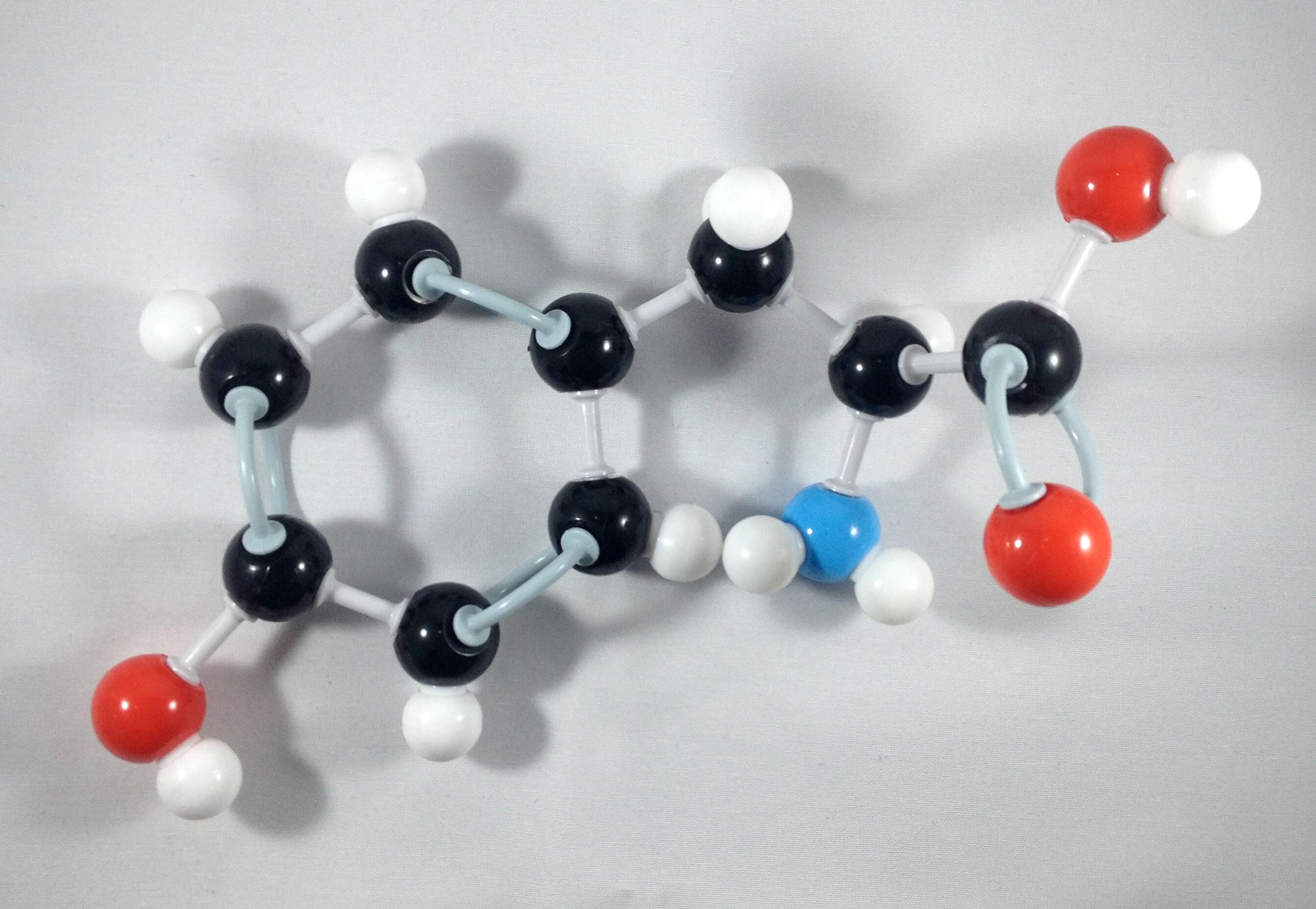
8. Join Carbon 1 and Carbon 6 together using a 1 medium connector.
-

Yay! We've just built our Phenol side chain portion!
Now, try this! Let’s build another Tyrosine molecule by following the steps outlined above. Then let’s try to interchange the Hydrogen attached to the alpha (α) carbon and the beta (β) Carbon containing the phenol functional group.
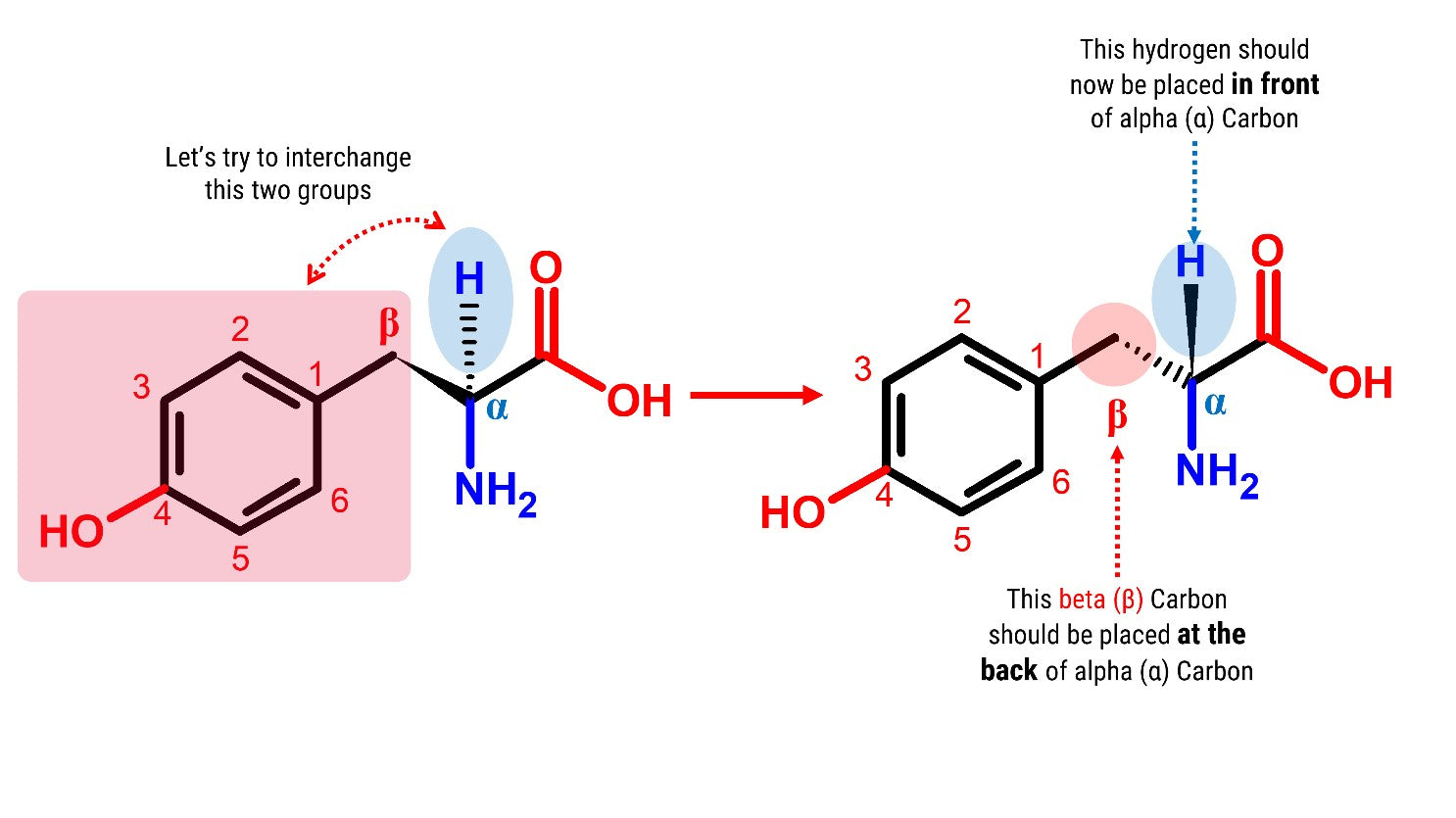
Steps:
-
1

1. Build another tyrosine molecule following the steps outlined above.
-
2
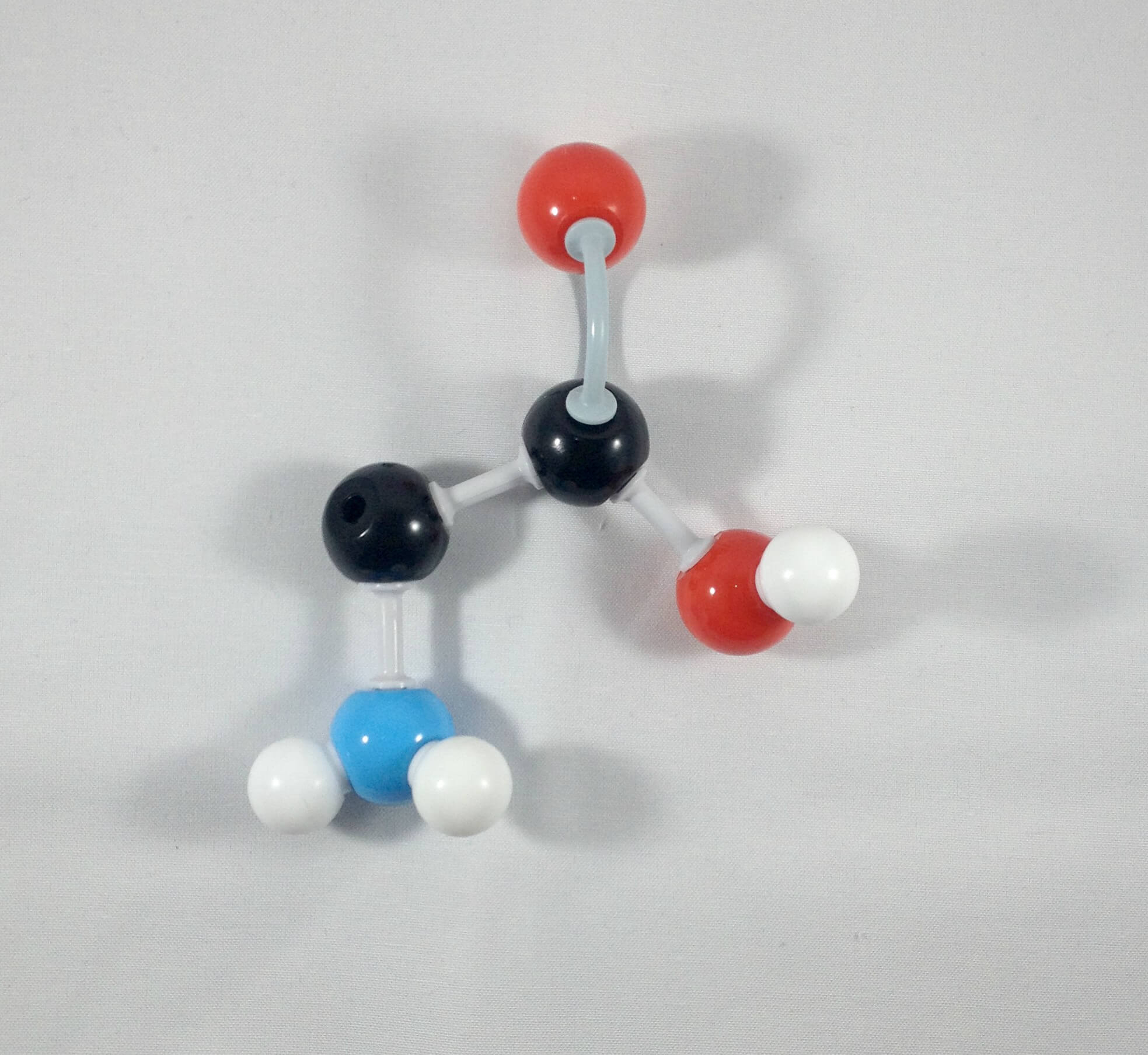
2. Detach the hydrogen atom and the beta (β) carbon containing the phenol side chain.
-
3
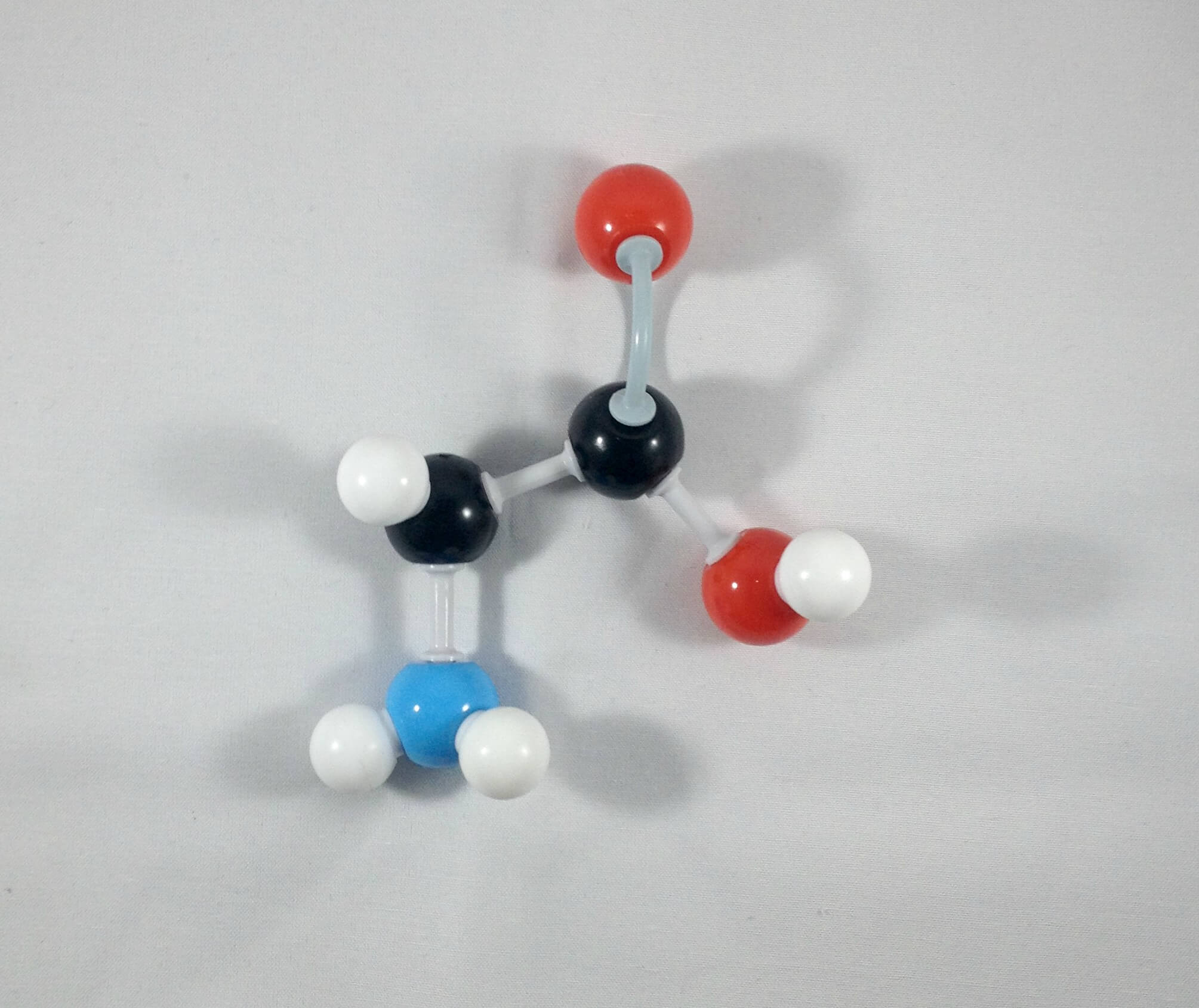
3. Place the hydrogen atom in front of the alpha (α) carbon.
-
4
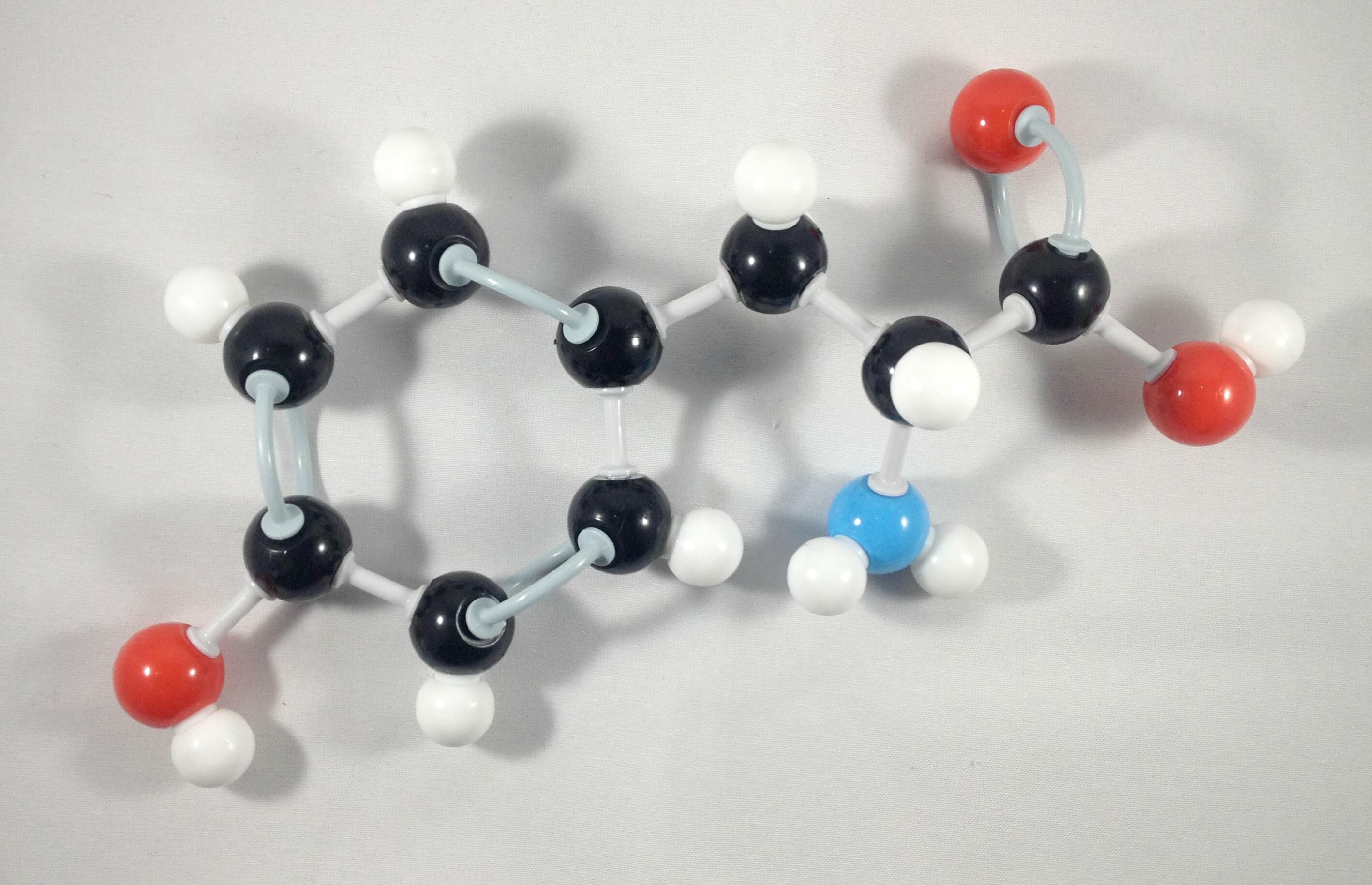
4. Then, attach the beta (β) carbon with the phenol functional group at the back side of alpha (α) carbon.
There we go! We now have 2 molecules of Tyrosine! See how these molecules seem to mirror each other!

L – Tyrosine

D – Tyrosine






















바카라사이트
카지노사이트
카지노게임사이트
온라인카지노
퀸즈슬롯
맥스카지노
비바카지노
카지노주소
바카라추천
온라인바카라게임
안전한 바카라사이트
바카라
카지노
퀸즈슬롯 카지노
바카라게임사이트
온라인바카라
밀리언클럽카지노
안전카지노사이트
바카라사이트추천
우리카지노계열
슬롯머신777
로얄카지노사이트
크레이지슬롯
온라인블랙잭
인터넷룰렛
카지노검증사이트
안전바카라사이트
모바일바카라
바카라 필승법
메리트카지노
바카라 노하우
바카라사이트
온라인카지노
카지노사이트
카지노게임사이트
퀸즈슬롯
맥스카지노
비바카지노
카지노주소
바카라추천
온라인바카라게임
안전한 바카라사이트
바카라
카지노
퀸즈슬롯 카지노
바카라게임사이트
온라인바카라
밀리언클럽카지노
안전카지노사이트
바카라사이트추천
우리카지노계열
슬롯머신777
로얄카지노사이트
크레이지슬롯
온라인블랙잭
인터넷룰렛
카지노검증사이트
안전바카라사이트
모바일바카라
바카라 필승법
메리트카지노
바카라 노하우
https://youube.me/
https://instagrme.com/
https://youubbe.me/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
바카라사이트
온라인바카라
실시간바카라
퀸즈슬롯
바카라게임
카지노주소
온라인카지노
온라인카지노사이트
바카라게임사이트
실시간바카라사이트
바카라
카지노
우리카지노
더킹카지노
샌즈카지노
예스카지노
코인카지노
더나인카지노
더존카지노
카지노사이트
골드카지노
에볼루션카지노
카지노 슬롯게임
baccarat
텍사스 홀덤 포카
blackjack
바카라사이트
온라인바카라
실시간바카라
퀸즈슬롯
바카라게임
카지노주소
온라인카지노
온라인카지노사이트
바카라게임사이트
실시간바카라사이트
바카라
카지노
우리카지노
더킹카지노
샌즈카지노
예스카지노
코인카지노
더나인카지노
더존카지노
카지노사이트
골드카지노
에볼루션카지노
카지노 슬롯게임
baccarat
텍사스 홀덤 포카
blackjack
https://youube.me/
https://gamja888.com/
https://youubbe.me/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
카지노사이트
슬롯사이트
온라인카지노
카지노주소
카지노검증사이트
안전한카지노사이트
슬롯카지노
바카라게임
카지노추천
비바카지노
퀸즈슬롯
카지노
바카라
안전한 바카라사이트
온라인슬롯
카지노사이트
바카라
바카라사이트
파라오카지노
제왕카지노
mgm카지노
더킹카지노
코인카지노
솔레어카지노
카지노게임
마이크로게이밍
아시아게이밍
타이산게이밍
오리엔탈게임
에볼루션게임
드래곤타이거
드림게이밍
비보게이밍
카지노사이트
슬롯사이트
온라인카지노
카지노주소
카지노검증사이트
안전한카지노사이트
슬롯카지노
바카라게임
카지노추천
비바카지노
퀸즈슬롯
카지노
바카라
안전한 바카라사이트
온라인슬롯
카지노사이트
바카라
바카라사이트
파라오카지노
제왕카지노
mgm카지노
더킹카지노
코인카지노
솔레어카지노
카지노게임
마이크로게이밍
아시아게이밍
타이산게이밍
오리엔탈게임
에볼루션게임
드래곤타이거
드림게이밍
비보게이밍
https://youube.me/
https://gamja888.com/
https://instagrme.com/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
온라인바카라
카지노사이트
바카라사이트
인터넷카지노
바카라게임사이트
퀸즈슬롯
카지노주소
비바카지노
카지노추천
카지노게임
온라인카지노사이트
카지노
바카라
온라인카지노
카지노게임사이트
카지노검증사이트
로얄카지노계열
슬롯머신사이트
맥스카지노
바카라게임사이트
카심바코리아 카지노
모바일카지노
실시간바카라
라이브카지노
온라인슬롯
바카라 이기는방법
안전카지노사이트
우리카지노사이트
샌즈카지노주소
바카라 게임규칙
바카라 게임방법
온라인바카라
카지노사이트
바카라사이트
인터넷카지노
바카라게임사이트
퀸즈슬롯
카지노주소
비바카지노
카지노추천
카지노게임
온라인카지노사이트
카지노
바카라
온라인카지노
카지노게임사이트
카지노검증사이트
로얄카지노계열
슬롯머신사이트
맥스카지노
바카라게임사이트
카심바코리아 카지노
모바일카지노
실시간바카라
라이브카지노
온라인슬롯
바카라 이기는방법
안전카지노사이트
우리카지노사이트
샌즈카지노주소
바카라 게임규칙
바카라 게임방법
https://gamja888.com/
https://instagrme.com/
https://youubbe.me/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
buy cialis and viagra online