Tryptophan: The molecule that calms your mind
Tryptophan is an essential amino acid that is a parent molecule of Serotonin and Melatonin – molecules that regulate your sleep-wake cycle. Melatonin, in particular, is a hormone that plays a vital role in our body’s circadian rhythm (or body clock) which is important in regulating our body’s sleep-wake activities. Peak levels of melatonin are produced at nighttime. Serotonin, on the other hand, is important in regulating our mood. In fact, this molecule is involved in the treatment of depression. Without Tryptophan, Melatonin and Serotonin are not produced.
How does Tryptophan look like in Chemistry?
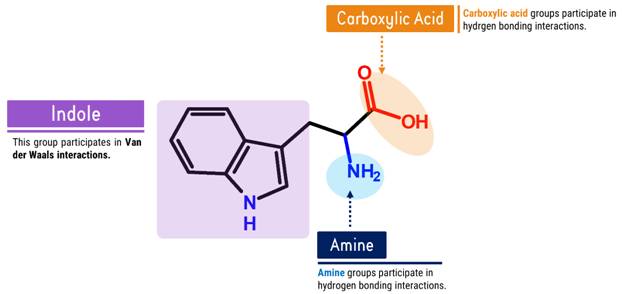
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create the Tryptophan Molecule! You’ll need:
- 11 Carbon atoms
- 2 Oxygen atoms
- 12 Hydrogen atoms
- 2 Nitrogen atoms
- 12 Small connectors (compact small bonds for hydrogen)
- 11 Medium Connectors
- 10 Long connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building With Our Amino Acid Skeleton portion!
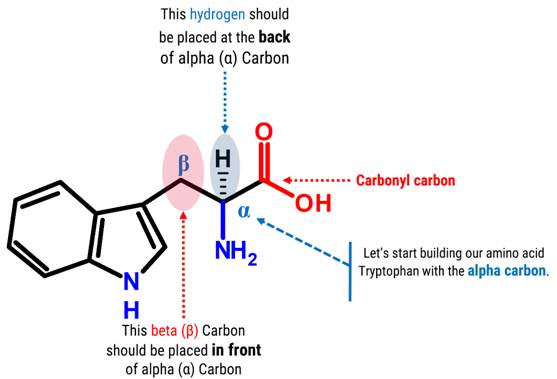
Note: We will build the skeleton portion of our amino acid starting with our chiral carbon(α Carbon).
Steps:
-
1

1. Get one carbon atom (α Carbon)then, place one hydrogen atom at the back side using one small connector.
-
2

2. Then, get another carbon atom (β Carbon)then place this in front of α Carbon using 1 medium connector. Add 2 hydrogen atoms on β Carbon using 2 small connectors.
-
3

3. Attach another carbon (Carbonyl Carbon) on α Carbon using 1 medium connector.
-
4
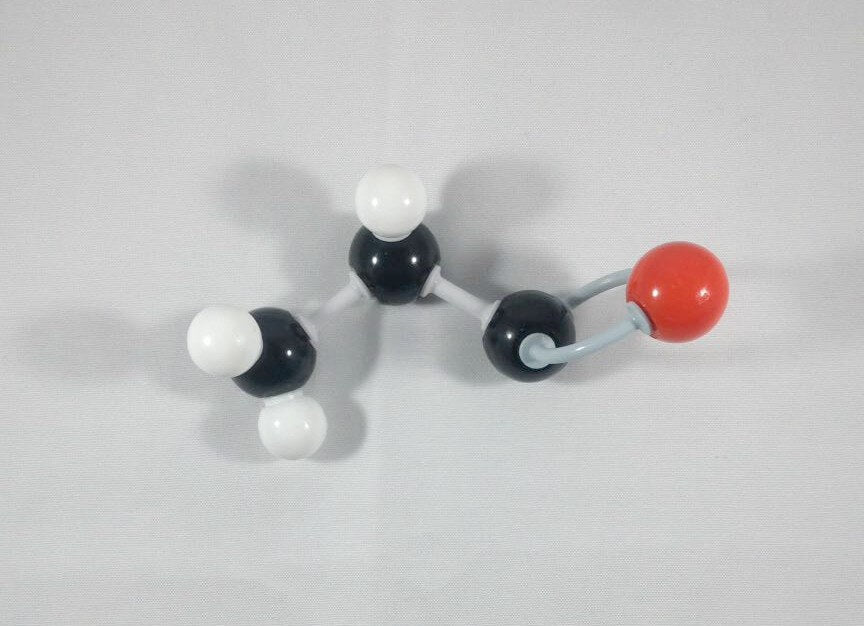
4. Get an Oxygen atom and attach this to the Carbonyl Carbon using 2 long connectors.
-
5

5. Get another Oxygen atom then attach this to the Carbonyl Carbon using a medium connector. Place a 1 hydrogen atom on this oxygen using one small connector.
-
6
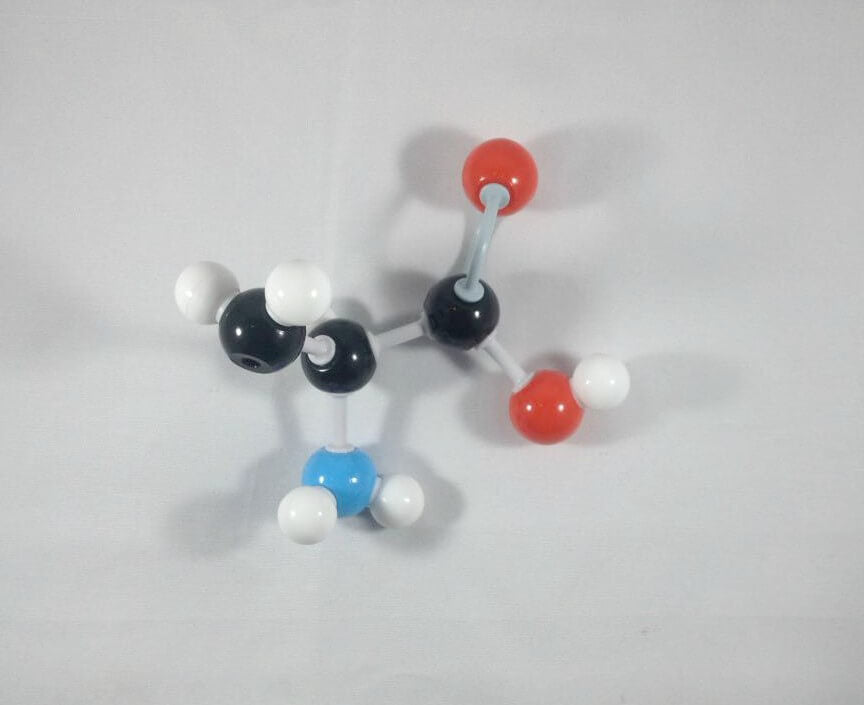
6. Then, get your Nitrogen atom and attach this to the α Carbon using one medium connector. Place 2 hydrogen atoms on this Nitrogen using 2 small connectors
-

Yay! We've just built our amino acid skeleton!
Note: Let’s now attach the 5-membered ring of our indole portion at the beta (β) carbon! Let’s start with Carbon 3. We will build this portion in a clockwise direction.
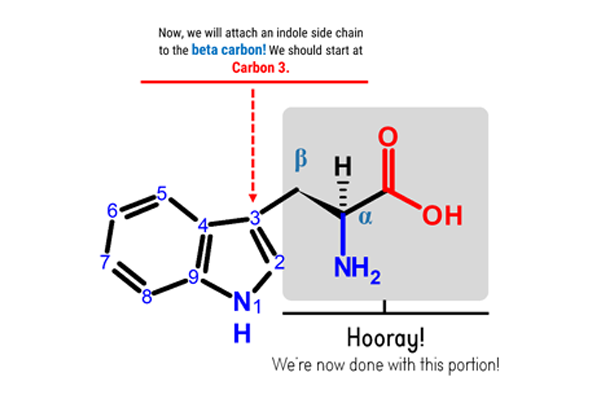
Steps:
-
1
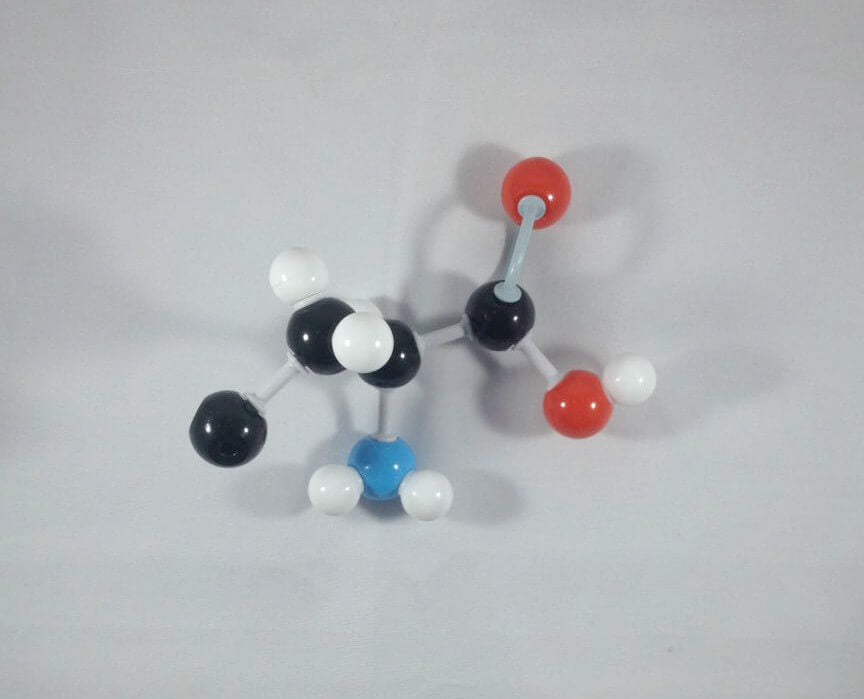
1. Get one Carbon atom (Carbon 3)then attach this using 1 medium connector. Add 1 hydrogen atom to Carbon 3using 1 small connector.
-
2
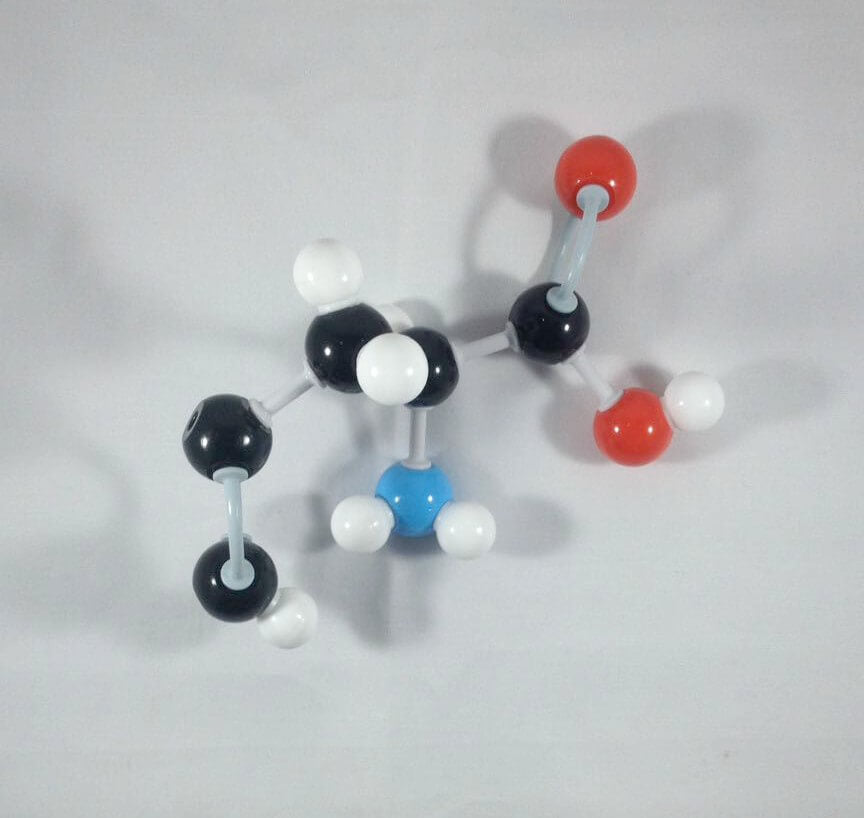
2. Attach another Carbon atom(Carbon 2)to Carbon 3 using 2 long connectors. Then, add 1 hydrogen atomto Carbon 2using 1 small connector.
-
3

3. Get a nitrogen atom (Nitrogen 1) then attach this to Carbon 2 using a medium connector. Add 1 hydrogen atomto this nitrogen using 1 small connector.
-
4
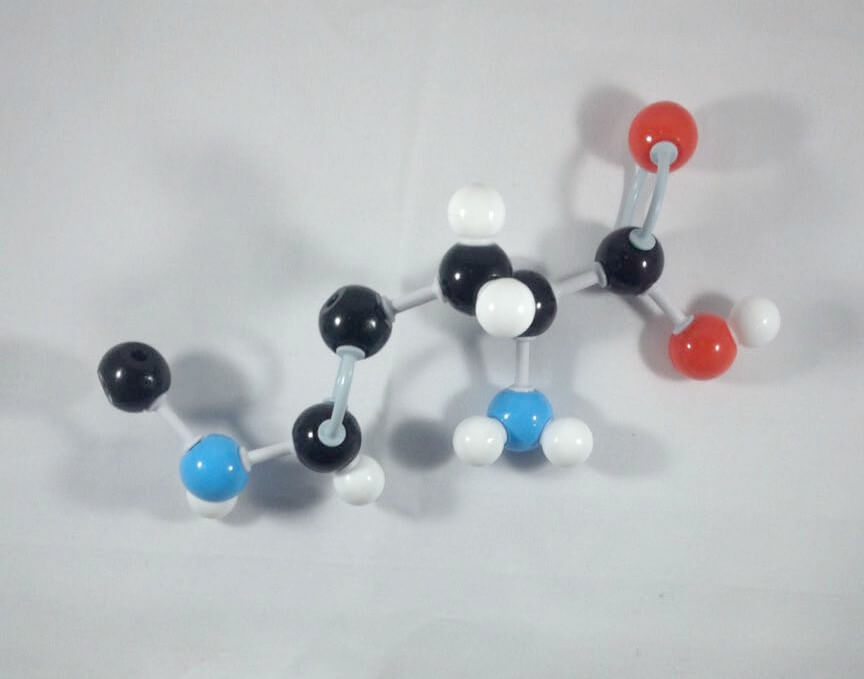
4. Attach a Carbon atom(Carbon 9) to Nitrogen 1 using 1 medium connector.
-
5
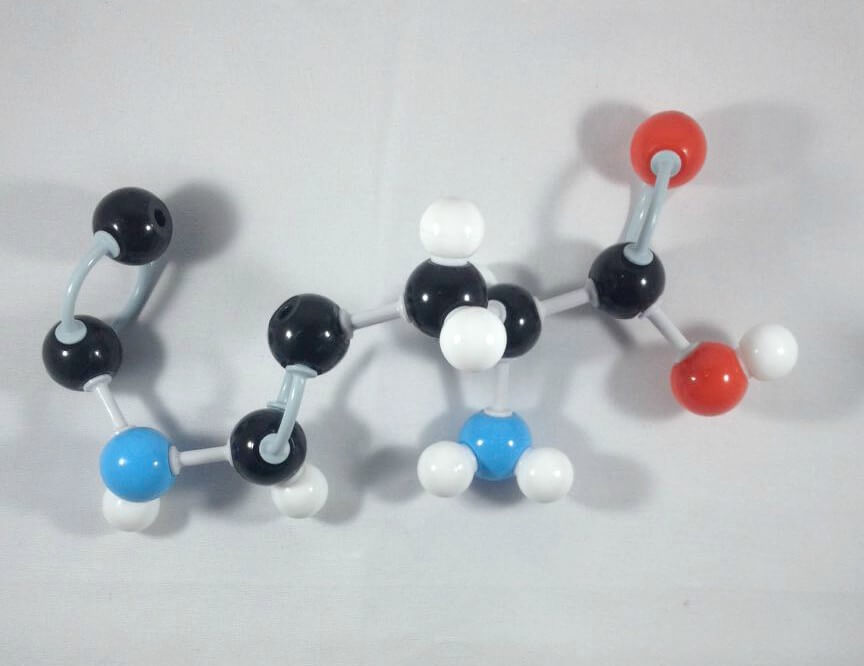
5. Get another Carbon atom (Carbon 4) then attach this to Carbon 9using 2 long connectors.
-
6
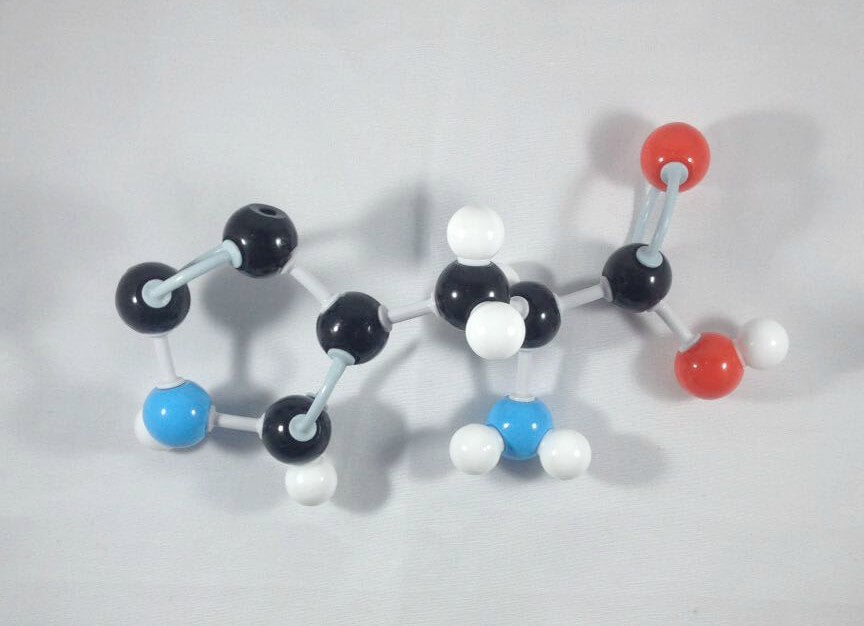
6. Join Carbon 3 and Carbon 4 together using a medium connector.
-

Hooray! We now have our 5-membered portion!
Note: Let’s build the 6-membered ring of our indole portion starting with Carbon 5, in a counter clockwise direction.
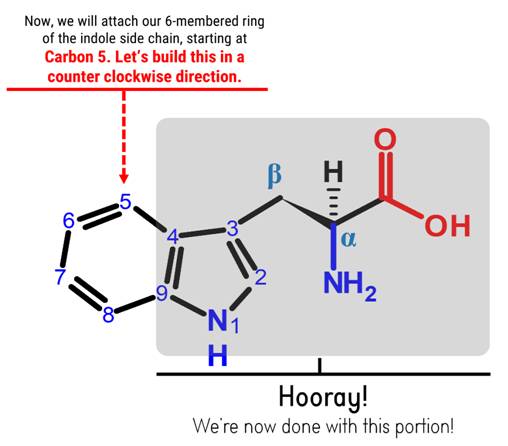
Steps:
-
1
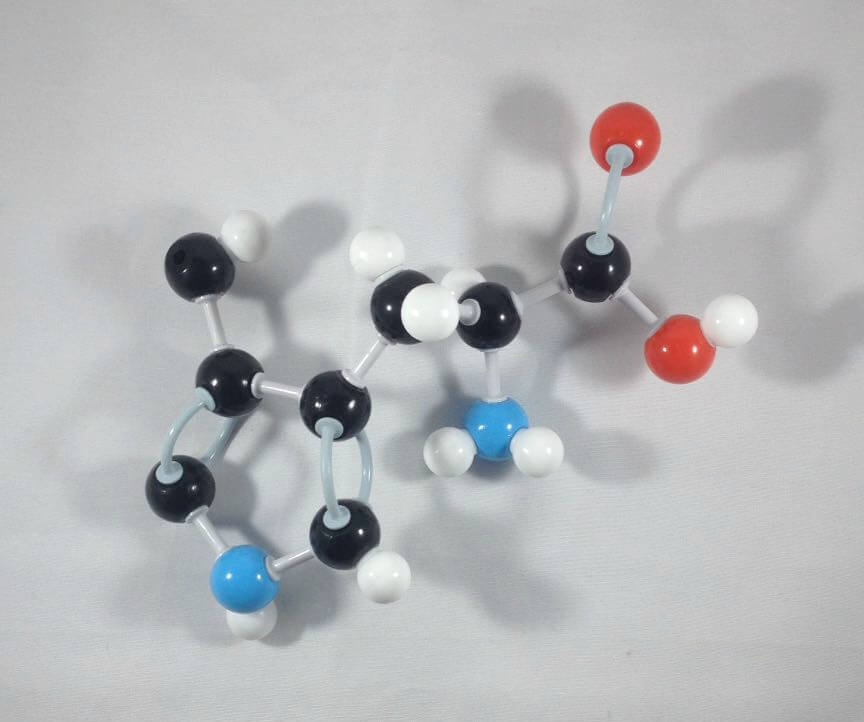
1. Get one Carbon atom (Carbon 5) then attach this to Carbon 4 using 1 medium connector. Add 1 hydrogen atom to Carbon 5 using 1 small connector.
-
2
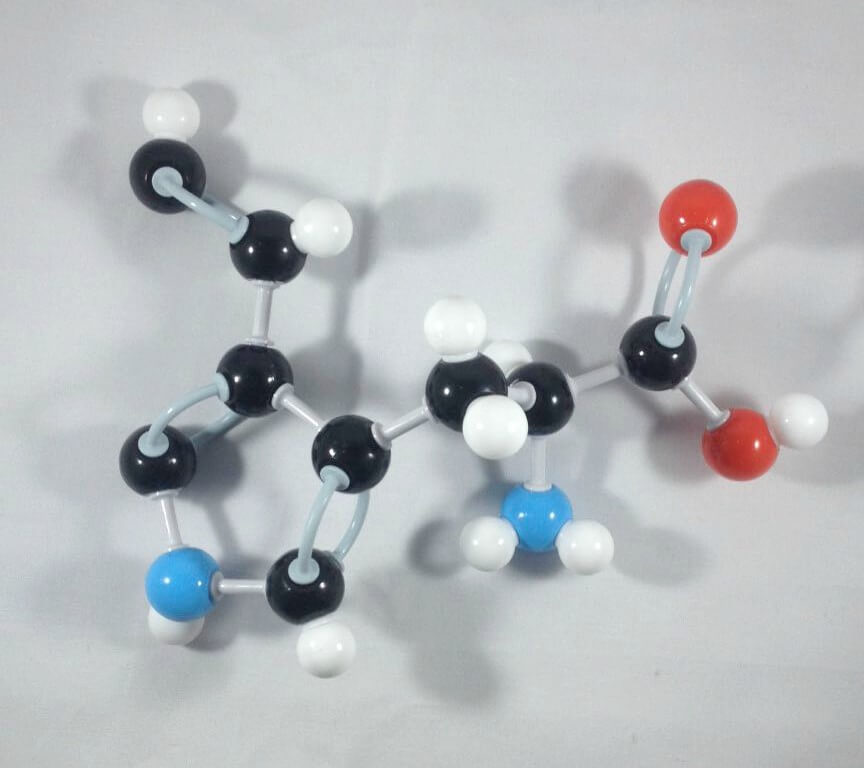
2. Attach another Carbon atom (Carbon 6)to Carbon 5 using 2 long connectors. Then, add 1 hydrogen atom to Carbon 6 using 1 small connector.
-
3
3. Get a Carbon atom (Carbon 7) then attach this to Carbon 6 using a medium connector. Add 1 hydrogen atom to Carbon 7 using 1 small connector.
-
4

4. Attach another Carbon atom (Carbon 8) to Carbon 7 using 2 long connectors. Add 1 hydrogen atom to Carbon 8 using 1 small connector.
-
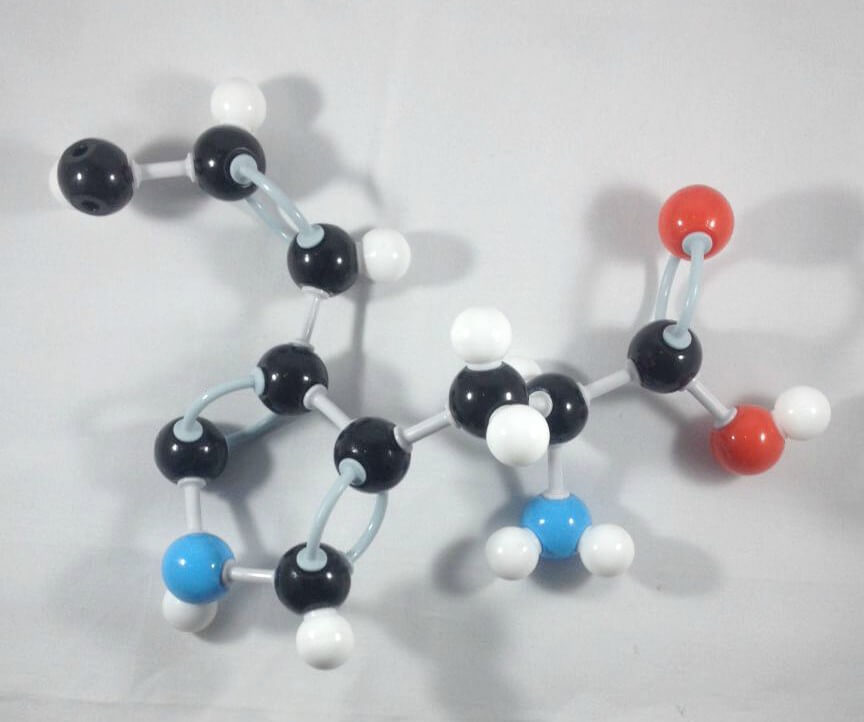
5
5. Finally, join Carbon 8 and Carbon 9 together using a medium connector.
-
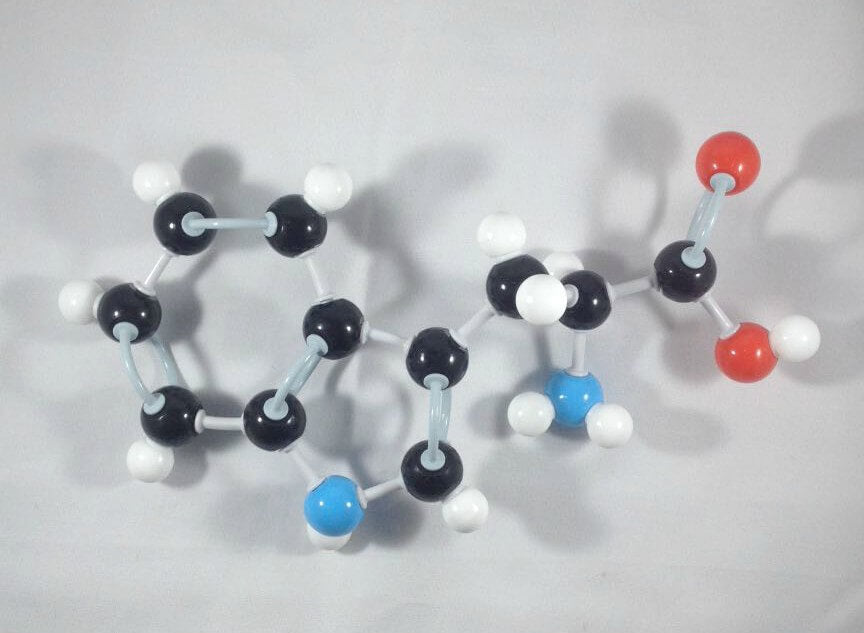
6

Yay! We’ve just built our L-Tryptophan molecule.
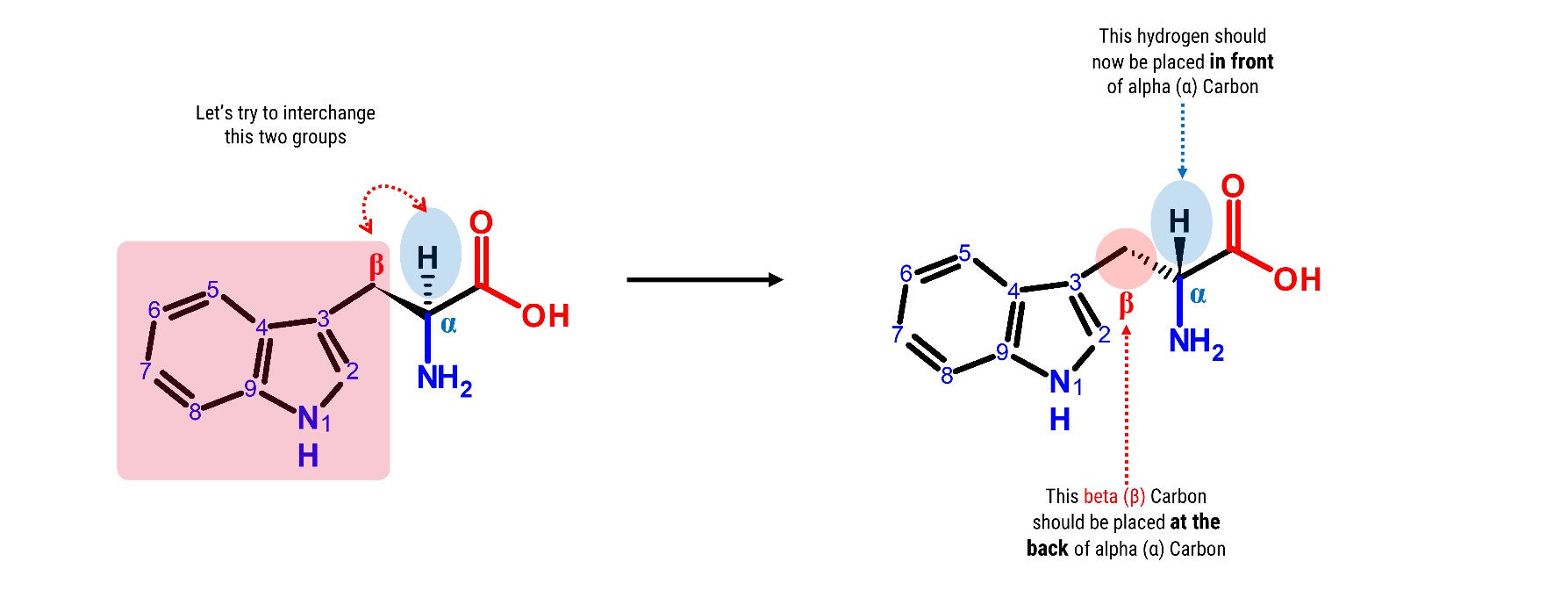
Now, try this! Let’s build another L-Tryptophan molecule by following the steps outlined above. Then let’s try to interchange the Hydrogen attached to the alpha (α) carbon and the beta (β) Carbon containing the indole functional group.
-
1
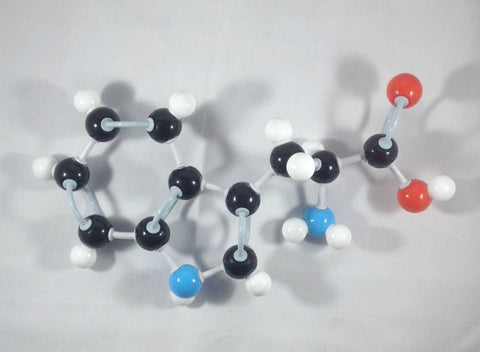
1. Build another tryptophan molecule following the steps outlined above.
-
2

2. Detach the hydrogen atom and the beta (β) carbon containing the indole side chain.
-
3
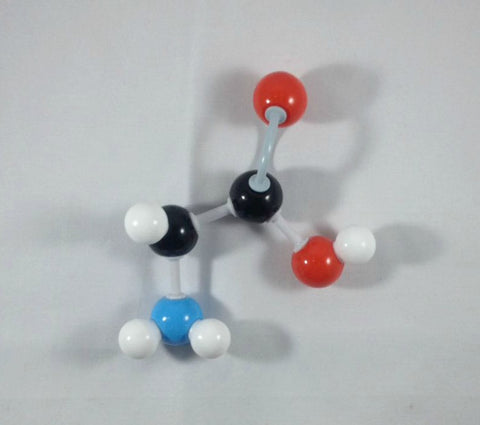
3. Place the hydrogen atom in front of the alpha (α) carbon.
-
4
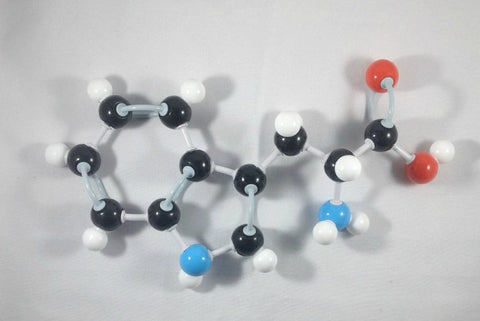
4. Then, attach the beta (β) carbon with the indole functional group at the back side of alpha (α) carbon.




























https://rb.gy/mixjhy
안전 카지노사이트_https://betop24.com/
온라인카지노 추천_https://betop24.com/
바카라사이트 추천_https://betop24.com/
파라오카지노_https://betop24.com/pharaoh-casino/
쿨카지노_https://betop24.com/cool-casino/
뉴헤븐카지노_https://betop24.com/nhcasino/
솔카지노_https://betop24.com/solcasino/
펀카지노_https://betop24.com/fun-casino/
헤라카지노_https://betop24.com/hera-casino/
J9카지노_https://betop24.com/j9-casino/
https://apaste.info/Ald3
https://bitbin.it/3uzmIXhm/
https://tinyurl.com/598thjcu
https://sites.google.com/view/betop24online/%ED%99%88
https://forms.gle/KZ6riHkPr4o4CtE56
https://linktr.ee/betop24
https://taplink.cc/betop24
https://sleek.bio/betop24
https://betop24.carrd.co/
https://solo.to/betop24
https://biolinky.co/betop24
https://paste.enginehub.org/IDSg9YSy_
https://pasteio.com/xVgdrVZYdk0p
https://apaste.info/nYTW
https://apaste.info/x5f0
https://pasteio.com/xsgR7oZ7rU9Q
http://bit.ly/3mSEOju
https://cutt.ly/E4furTX
https://www.google.co.zw/url?q=https://betop24.com/safe-casino-site%2F
https://www.adminer.org/redirect/?url=https://betop24.com/safe-casino-site/
https://sfwater.org/redirect.aspx?url=https://betop24.com/safe-casino-site/
https://web.archive.org/web/20180804180141/https://betop24.com/safe-casino-site/
https://baoviet.com.vn/Redirect.aspx?url=https://betop24.com/safe-casino-site/
https://sc.sie.gov.hk/TuniS/https://betop24.com/safe-casino-site/
https://www.ric.edu/Pages/link_out.aspx?target=https://betop24.com/safe-casino-site/
https://cse.google.com.pe/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.pk/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.np/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.jm/url?q=https://betop24.com/safe-casino-site/
https://cse.google.li/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.kh/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.co/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cm/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.gi/url?q=https://betop24.com/safe-casino-site/
https://cse.google.la/url?q=https://betop24.com/safe-casino-site/
https://cse.google.tk/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.mz/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.sb/url?q=https://betop24.com/safe-casino-site/
https://cse.google.sh/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.ly/url?q=https://betop24.com/safe-casino-site/
https://cse.google.fm/url?q=https://betop24.com/safe-casino-site/
https://cse.google.vu/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cf/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.tj/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cv/url?q=https://betop24.com/safe-casino-site/
https://cse.google.st/url?q=https://betop24.com/safe-casino-site/
https://cse.google.sr/url?q=https://betop24.com/safe-casino-site/
https://www.google.iq/url?q=https://betop24.com/safe-casino-site/
https://www.google.tk/url?q=https://betop24.com/safe-casino-site/
https://posts.google.com/url?q=https://betop24.com/safe-casino-site/
https://www.google.nu/url?q=https://betop24.com/safe-casino-site/
https://clients1.google.com/url?q=https://betop24.com/safe-casino-site/
https://asia.google.com/url?q=https://betop24.com/safe-casino-site/
https://www.google.com/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.cz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.hu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.vn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.vn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pe/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pe/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.by/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ec/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ec/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ba/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ng/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ng/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.cr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.cr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.kw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.is/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.dz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.tn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.tn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.iq/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.iq/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.bd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.kz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ge/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ni/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.la/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.jm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.cy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.qa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ps/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bo/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.li/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.bh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.lb/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.lb/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.tt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ci/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.hn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.sn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.sn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.mt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cat/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.fm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.as/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.om/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.om/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.mz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.bw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ms/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.bz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ag/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ag/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://app.feedblitz.com/f/f.fbz?track=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://archives.midweek.com/?URL=http%253A%252F%252Fhttps://betop24.com/safe-casino-site/
http://fooyoh.com/wcn.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/&keyid=42973
http://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://betop24.com/safe-casino-site/
http://sc.sie.gov.hk/TuniS/https://betop24.com/safe-casino-site/
http://www.aa.org/pages/en_US/disclaimer?u=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.aastocks.com/sc/changelang.aspx?lang=sc&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.blueletterbible.org/tools/redirect.cfm?Site=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.cineuropa.org/el.aspx?el=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.drinksmixer.com/redirect.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.justjared.com/flagcomment.php?el=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.kaskus.co.id/redirect?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.meetme.com/apps/redirect/?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.office.xerox.com/perl-bin/reseller_exit.pl?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.popcouncil.org/scripts/leaving.asp?URL=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.ric.edu/Pages/link_out.aspx?target=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.sinp.msu.ru/ru/ext_link?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.webclap.com/php/jump.php?url=https://betop24.com/safe-casino-site/
http://www2.sandbox.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www2.sandbox.google.co.kr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://cr.naver.com/rd?u=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://devicedoctor.com/driver-feedback.php?device=PCI%20bus&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://domain.opendns.com/https://betop24.com/safe-casino-site/
http://my.dek-d.com/username/link/link.php?out=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://panchodeaonori.sakura.ne.jp/feed/aonori/feed2js.php?src=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://refer.ash1.ccbill.com/cgi-bin/clicks.cgi?CA=933914&PA=1785830&HTML=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://refer.ccbill.com/cgi-bin/clicks.cgi/http:/?CA=928498&PA=1458253&HTML=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://sc.youth.gov.hk/TuniS/https://betop24.com/safe-casino-site/
http://wasearch.loc.gov/e2k/*/https://betop24.com/safe-casino-site/
http://wikimapia.org/external_link?url=https://betop24.com/safe-casino-site/
http://www.astro.wisc.edu/?URL=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.astro.wisc.edu/?URL=https://betop24.com/safe-casino-site/
http://www.cssdrive.com/?URL=https://betop24.com/safe-casino-site/
http://www.earth-policy.org/?URL=https://betop24.com/safe-casino-site/
http://www.fmvz.unam.mx/fmvz/biblioteca/revistas_electronicas/control/envioClics.php?cnH57w=1118&kqJm89=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.ric.edu/Pages/link_out.aspx?target=https://betop24.com/safe-casino-site/
http://www.shinobi.jp/etc/goto.html?http%3A%2F%2Fbetop24.com/safe-casino-site/kuttymovies-2020-kuttymovies-hd-tamil-movies-download
http://www.spiritfanfiction.com/link?l=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.webclap.com/php/jump.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.zerocarts.com/demo/index.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://advisor.wmtransfer.com/SiteDetails.aspx?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://blog.ss-blog.jp/pages/mobile/step/index?u=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://bukkit.org/proxy.php?link=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://client.paltalk.com/client/webapp/client/External.wmt?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.esri.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.rsa.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.ac/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ad/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ad/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ae/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.al/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.am/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.as/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.at/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.az/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ba/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bi/url?q=https://betop24.com/safe-casino-site/
https://cse.google.bi/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bj/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bs/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bt/url?q=https://betop24.com/safe-casino-site/
https://cse.google.bt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.by/url?q=https://betop24.com/safe-casino-site/
https://cse.google.by/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ca/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cat/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cd/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cd/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cf/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ch/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ci/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ao/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.bw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ck/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.cr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.id/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.in/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.jp/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ke/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.kr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ls/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ma/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.mz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.nz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.th/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.tz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ug/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.uz/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.uz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ve/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.vi/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.za/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.zm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.zw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.af/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ag/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ai/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.ai/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.au/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.bd/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.bh/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.bn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.bo/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.co/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.co/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.cu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.cy/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ec/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.eg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.et/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.et/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.fj/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.gh/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.gi/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.gt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.hk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.jm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.kh/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.kw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ly/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.mm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.mt/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.mt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.mx/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.my/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.na/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.nf/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ng/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ni/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.np/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.om/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.pa/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.pe/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.pg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ph/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.pr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.sa/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.sa/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.sg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.sv/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.tj/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.tr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.tw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.ua/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.uy/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.vc/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.de/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.dk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.dz/url?q=https://betop24.com/safe-casino-site/
https://cse.google.dz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ee/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.es/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.fi/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.fr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ga/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ga/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gp/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.gy/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.hn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.hr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ht/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.hu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ie/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.im/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.iq/url?q=https://betop24.com/safe-casino-site/
https://cse.google.is/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.it/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.je/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.jo/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.kg/url?q=https://betop24.com/safe-casino-site/
https://cse.google.kg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ki/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ki/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.kz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.la/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.li/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.lk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.lt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.lu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.lv/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.me/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mn/url?q=https://betop24.com/safe-casino-site/
https://cse.google.mn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ms/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mv/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.mw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ne/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.nl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.no/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.nr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.nu/url?q=https://betop24.com/safe-casino-site/
https://cse.google.nu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ps/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ro/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.rs/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ru/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.rw/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.sc/url?q=https://betop24.com/safe-casino-site/
https://cse.google.se/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.sh/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.si/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.sk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.sm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.sn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.so/url?q=https://betop24.com/safe-casino-site/
https://cse.google.sr/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.td/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.tg/url?q=https://betop24.com/safe-casino-site/
https://cse.google.tg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.tl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.tm/url?q=https://betop24.com/safe-casino-site/
https://cse.google.tm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.tn/url?q=https://betop24.com/safe-casino-site/
https://cse.google.to/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.tt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.vg/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.vu/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ws/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ws/url?sa=i&url=https://betop24.com/safe-casino-site/
https://ditu.google.com/url?q=https://betop24.com/safe-casino-site/
https://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://foro.infojardin.com/proxy.php?link=https://betop24.com/safe-casino-site/
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://galter.northwestern.edu/exit?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://id.telstra.com.au/register/crowdsupport?gotoURL=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ad/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ad/url?q=https://betop24.com/safe-casino-site/
https://images.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bs/url?q=https://betop24.com/safe-casino-site/
https://images.google.bt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bt/url?q=https://betop24.com/safe-casino-site/
https://images.google.by/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.by/url?q=https://betop24.com/safe-casino-site/
https://images.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cat/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.bw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.kr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ug/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ug/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.uz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.uz/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ec/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.et/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.et/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.gt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.jm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.jm/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.kh/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.lb/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ng/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.np/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.om/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.om/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.pe/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pe/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ph/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pk/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.pk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sa/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tj/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.uy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.vn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dm/url?q=https://betop24.com/safe-casino-site/
https://images.google.dz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dz/url?q=https://betop24.com/safe-casino-site/
https://images.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.iq/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.iq/url?q=https://betop24.com/safe-casino-site/
https://images.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kg/url?q=https://betop24.com/safe-casino-site/
https://images.google.lk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mn/url?q=https://betop24.com/safe-casino-site/
https://images.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.se/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tm/url?q=https://betop24.com/safe-casino-site/
https://images.google.tn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tn/url?q=https://betop24.com/safe-casino-site/
https://images.google.tt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ws/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ws/url?q=https://betop24.com/safe-casino-site/
https://legacy.aom.org/verifymember.asp?nextpage=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bs/url?q=https://betop24.com/safe-casino-site/
https://maps.google.bt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bt/url?q=https://betop24.com/safe-casino-site/
https://maps.google.by/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.cr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ke/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.kr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ug/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ec/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.et/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.et/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.gt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.jm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ng/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ni/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.om/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.om/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.pe/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ph/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ge/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.iq/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.iq/url?q=https://betop24.com/safe-casino-site/
https://maps.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.kg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.kg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.li/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.li/url?q=https://betop24.com/safe-casino-site/
https://maps.google.lk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mn/url?q=https://betop24.com/safe-casino-site/
https://maps.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.sc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.se/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.tn/url?q=https://betop24.com/safe-casino-site/
https://maps.google.tn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.vu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.vu/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ws/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ws/url?q=https://betop24.com/safe-casino-site/
https://optimize.viglink.com/page/pmv?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://plus.google.com/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://pw.mail.ru/forums/fredirect.php?url=https://betop24.com/safe-casino-site/
https://ref.gamer.com.tw/redir.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://sfwater.org/redirect.aspx?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://sfwater.org/redirect.aspx?url=https://betop24.com/safe-casino-site/
https://wfc2.wiredforchange.com/dia/track.jsp?v=2&c=hdorrh%2BHcDlQ%2BzUEnZU5qlfKZ1Cl53X6&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://wikimapia.org/external_link?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.adminer.org/redirect/?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ae/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.as/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.at/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.bg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.by/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ca/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.cl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.bw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.il/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.ke/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.kr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.ma/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.nz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.th/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.ve/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.za/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.au/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.bd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.co/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.do/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ec/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.eg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.hk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.my/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ng/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.np/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pe/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ph/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.sv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.uy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.vn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.cz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.dz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.fi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.gr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.hn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.is/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.kg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.kg/url?q=https://betop24.com/safe-casino-site/
https://www.google.kz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ro/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.se/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.si/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.tn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.tt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.transtats.bts.gov/exit.asp?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.transtats.bts.gov/exit.asp?url=https://betop24.com/safe-casino-site/
https://www.youtube.com/redirect?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://youtube.com/redirect?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://betop24.com/safe-casino-site/
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://betop24.com/safe-casino-site/&tab=feedback
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://betop24.com/safe-casino-site/&tab=rating
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://betop24.com/safe-casino-site/&tab=wminfo
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://betop24.com/safe-casino-site/
https://anonym.to/?https://betop24.com/safe-casino-site/
https://anonym.to/?http%3A%2F%2Fbetop24.com/safe-casino-site/
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=https://betop24.com/safe-casino-site/
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://bukkit.org/proxy.php?link=https://betop24.com/safe-casino-site/
https://bukkit.org/proxy.php?link=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cdrinfo.com/Sections/Ads/ReviewsAroundTheWebRedirector.aspx?TargetUrl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://ceskapozice.lidovky.cz/redir.aspx?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cgl.ethz.ch/disclaimer.php?dlurl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://chofu.keizai.biz/banner.php?type=text_banner&position=right&id=3&uri=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ac/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ad/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ae/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.al/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.am/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.as/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.az/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.bf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.bi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.bj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.bs/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.bt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.by/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.cd/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.cf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.cg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ci/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.cm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.ao/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.ck/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.il/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.in/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.jp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.kr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.ls/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.ma/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.mz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.nz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.th/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.tz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.uz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.ve/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.vi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.zm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.co.zw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.af/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.ag/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.ai/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.ar/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.bh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.bn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.bo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.bz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.cu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.cy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.eg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.fj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.gh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.gi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.hk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.jm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.kh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.kw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.ly/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.mm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.mt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.my/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.na/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.np/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.om/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.pe/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.pg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.pk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.pr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.qa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.sa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.sl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.tj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.tr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.tw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.ua/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.uy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.vn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.cv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.de/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.dj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.dm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.fm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ga/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.gg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.gl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.gm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.gp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.gy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ht/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ie/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.im/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.is/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.it/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.jo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.kg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ki/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.la/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.li/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.md/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.me/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.mg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.mn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ms/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.mv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.mw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ne/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.nr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.nu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.pn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ro/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.rw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.sc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.sh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.sm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.so/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.sr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.st/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.td/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.tg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.tl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.tm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.to/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.tt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.vg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.vu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.ws/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.cypress.com/external-link.jspa?url=https://betop24.com/safe-casino-site/
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.esri.com/external-link.jspa?url=https://betop24.com/safe-casino-site/
https://community.esri.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://community.rsa.com/external-link.jspa?url=https://betop24.com/safe-casino-site/
https://contacts.google.com/url?sa=t&source=web&rct=j&url=http%3A%2F%2Fbetop24.com/safe-casino-site/&ved=2ahUKEwip75vMsq7mAhUv06YKHYSzAN44rAIQFjAYegQIKRAB
https://cse.google.ba/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.by/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.cm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.co.id/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.co.in/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.co.jp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.co.ke/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.hk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.nf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.ng/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.pr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.tr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.tw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.com.vn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.it/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.ru/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.se/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.tt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://demo.html5xcss3.com/demo.php?url=https://betop24.com/safe-casino-site/
https://dereferer.org/?https://betop24.com/safe-casino-site/
https://drive.sandbox.google.com.br/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://engawa.kakaku.com/jump/?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://eventlog.centrum.cz/redir?data=aclick1c68565-349178t12&s=najistong&v=1&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://foro.infojardin.com/proxy.php?link=https://betop24.com/safe-casino-site/%2F
https://forum.solidworks.com/external-link.jspa?url=https://betop24.com/safe-casino-site/
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://forums-archive.eveonline.com/default.aspx?g=warning&l=http%3A%2F%2Fbetop24.com/safe-casino-site/&domain=https://betop24.com/safe-casino-site/
https://galter.northwestern.edu/exit?url=https://betop24.com/safe-casino-site/
https://galter.northwestern.edu/exit?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://gleam.io/zyxKd-INoWr2EMzH?l=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://hobby.idnes.cz/peruanske-palive-papricky-rocoto-dlz-/redir.aspx?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://id.telstra.com.au/register/crowdsupport?gotoURL=https://betop24.com/safe-casino-site/
https://images.google.ac/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ac/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ad/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ae/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ae/url?q=https://betop24.com/safe-casino-site/
https://images.google.al/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.al/url?q=https://betop24.com/safe-casino-site/
https://images.google.al/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.am/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.am/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.as/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.as/url?q=https://betop24.com/safe-casino-site/
https://images.google.as/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.at/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.at/url?q=https://betop24.com/safe-casino-site/
https://images.google.az/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.az/url?q=https://betop24.com/safe-casino-site/
https://images.google.az/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ba/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ba/url?q=https://betop24.com/safe-casino-site/
https://images.google.ba/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.be/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.be/url?q=https://betop24.com/safe-casino-site/
https://images.google.bf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bf/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bj/url?q=https://betop24.com/safe-casino-site/
https://images.google.bj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bs/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.bt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.by/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ca/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cat/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cat/url?q=https://betop24.com/safe-casino-site/
https://images.google.cd/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cd/url?q=https://betop24.com/safe-casino-site/
https://images.google.cd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cf/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cg/url?q=https://betop24.com/safe-casino-site/
https://images.google.cg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ch/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ch/url?q=https://betop24.com/safe-casino-site/
https://images.google.ci/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ci/url?q=https://betop24.com/safe-casino-site/
https://images.google.ci/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ao/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ao/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ao/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.bw/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ck/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ck/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ck/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.cr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.cr/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.id/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.id/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.il/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.in/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.jp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ke/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ke/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ke/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.kr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.kr/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ls/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ls/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ls/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ma/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ma/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.mz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.mz/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.mz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.nz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.th/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.tz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.tz/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.tz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ug/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.uk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ve/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.ve/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.vi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.vi/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.vi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.za/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.za/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.zm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.zm/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.zm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.zw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.zw/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.zw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.af/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.af/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.af/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ag/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ag/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ag/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ai/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ai/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ai/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ar/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ar/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ar/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.au/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bd/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bh/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.bh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bn/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.bn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bo/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.bo/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.co/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.co/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.cy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.cy/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.cy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.do/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.do/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ec/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.eg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.eg/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.et/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.fj/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.fj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gi/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.gt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gt/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.hk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.hk/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.jm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.kw/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.kw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.lb/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ly/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ly/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ly/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mm/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.mm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mt/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.mt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mx/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.mx/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.my/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.my/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.na/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.na/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.na/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.nf/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ng/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ni/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ni/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.np/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.np/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.om/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ph/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ph/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.pk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pr/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.pr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.py/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.py/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.qa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.qa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sg/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.sl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.sv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tw/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.ua/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ua/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.uy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.uy/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.vc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.vc/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.vn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.vn/url?q=https://betop24.com/safe-casino-site/
https://images.google.com/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.cv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cz/url?q=https://betop24.com/safe-casino-site/
https://images.google.de/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.de/url?q=https://betop24.com/safe-casino-site/
https://images.google.dj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dk/url?q=https://betop24.com/safe-casino-site/
https://images.google.dm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.dz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ee/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ee/url?q=https://betop24.com/safe-casino-site/
https://images.google.es/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.es/url?q=https://betop24.com/safe-casino-site/
https://images.google.fi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fi/url?q=https://betop24.com/safe-casino-site/
https://images.google.fm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fr/url?q=https://betop24.com/safe-casino-site/
https://images.google.ga/url?q=https://betop24.com/safe-casino-site/
https://images.google.ga/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ga/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ge/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ge/url?q=https://betop24.com/safe-casino-site/
https://images.google.ge/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gg/url?q=https://betop24.com/safe-casino-site/
https://images.google.gg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gm/url?q=https://betop24.com/safe-casino-site/
https://images.google.gm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gr/url?q=https://betop24.com/safe-casino-site/
https://images.google.gy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hn/url?q=https://betop24.com/safe-casino-site/
https://images.google.hn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hr/url?q=https://betop24.com/safe-casino-site/
https://images.google.ht/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ht/url?q=https://betop24.com/safe-casino-site/
https://images.google.ht/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.hu/url?q=https://betop24.com/safe-casino-site/
https://images.google.ie/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ie/url?q=https://betop24.com/safe-casino-site/
https://images.google.im/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.im/url?q=https://betop24.com/safe-casino-site/
https://images.google.im/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.iq/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.is/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.is/url?q=https://betop24.com/safe-casino-site/
https://images.google.it/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.it/url?q=https://betop24.com/safe-casino-site/
https://images.google.je/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.je/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.jo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.jo/url?q=https://betop24.com/safe-casino-site/
https://images.google.jo/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ki/url?q=https://betop24.com/safe-casino-site/
https://images.google.ki/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ki/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kz/url?q=https://betop24.com/safe-casino-site/
https://images.google.kz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.la/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.la/url?q=https://betop24.com/safe-casino-site/
https://images.google.la/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.li/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.li/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lk/url?q=https://betop24.com/safe-casino-site/
https://images.google.lu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lu/url?q=https://betop24.com/safe-casino-site/
https://images.google.lv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lv/url?q=https://betop24.com/safe-casino-site/
https://images.google.md/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.md/url?q=https://betop24.com/safe-casino-site/
https://images.google.md/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.me/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.me/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mg/url?q=https://betop24.com/safe-casino-site/
https://images.google.mg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mk/url?q=https://betop24.com/safe-casino-site/
https://images.google.mk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ms/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ms/url?q=https://betop24.com/safe-casino-site/
https://images.google.ms/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mu/url?q=https://betop24.com/safe-casino-site/
https://images.google.mu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mv/url?q=https://betop24.com/safe-casino-site/
https://images.google.mv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mw/url?q=https://betop24.com/safe-casino-site/
https://images.google.mw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ne/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ne/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.no/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.no/url?q=https://betop24.com/safe-casino-site/
https://images.google.no/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nr/url?q=https://betop24.com/safe-casino-site/
https://images.google.nr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nu/url?q=https://betop24.com/safe-casino-site/
https://images.google.nu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.nu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pn/url?q=https://betop24.com/safe-casino-site/
https://images.google.pn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ps/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ps/url?q=https://betop24.com/safe-casino-site/
https://images.google.ps/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.pt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ro/url?q=https://betop24.com/safe-casino-site/
https://images.google.rs/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.rs/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ru/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ru/url?q=https://betop24.com/safe-casino-site/
https://images.google.rw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.rw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sc/url?q=https://betop24.com/safe-casino-site/
https://images.google.sc/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.se/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.se/url?q=https://betop24.com/safe-casino-site/
https://images.google.sh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sh/url?q=https://betop24.com/safe-casino-site/
https://images.google.sh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.si/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.si/url?q=https://betop24.com/safe-casino-site/
https://images.google.sk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sk/url?q=https://betop24.com/safe-casino-site/
https://images.google.sm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sn/url?q=https://betop24.com/safe-casino-site/
https://images.google.sn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.so/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.so/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.sr/url?q=https://betop24.com/safe-casino-site/
https://images.google.sr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.st/url?q=https://betop24.com/safe-casino-site/
https://images.google.st/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.st/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.td/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.td/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tg/url?q=https://betop24.com/safe-casino-site/
https://images.google.tg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.to/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.tt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.vg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.vg/url?q=https://betop24.com/safe-casino-site/
https://images.google.vg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.vu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.vu/url?q=https://betop24.com/safe-casino-site/
https://images.google.vu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.ws/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://inquiry.princetonreview.com/away/?value=cconntwit&category=FS&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://ipv4.google.com/url?q=https://betop24.com/safe-casino-site/
https://it.paltalk.com/client/webapp/client/External.wmt?url=http://https://betop24.com/safe-casino-site/
https://jump.2ch.net/?https://betop24.com/safe-casino-site/
https://lcu.hlcommission.org/lcu/pages/auth/forgotPassword.aspx?Returnurl=http://https://betop24.com/safe-casino-site/
https://legacy.aom.org/verifymember.asp?nextpage=https://betop24.com/safe-casino-site/
https://lexsrv2.nlm.nih.gov/fdse/search/search.pl?Match=0&Realm=All&Terms=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://m.addthis.com/live/redirect/?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=https://betop24.com/safe-casino-site/
https://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=https://betop24.com/safe-casino-site/
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=http://https://betop24.com/safe-casino-site/
https://maps.google.ad/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ad/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ae/url?q=https://betop24.com/safe-casino-site/
https://maps.google.as/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.as/url?q=https://betop24.com/safe-casino-site/
https://maps.google.as/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.at/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.at/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ba/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ba/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.be/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.be/url?q=https://betop24.com/safe-casino-site/
https://maps.google.bf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bf/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.bi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bi/url?q=https://betop24.com/safe-casino-site/
https://maps.google.bj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bj/url?q=https://betop24.com/safe-casino-site/
https://maps.google.bj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bs/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bs/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.by/url?q=https://betop24.com/safe-casino-site/
https://maps.google.by/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ca/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cat/url?q=https://betop24.com/safe-casino-site/
https://maps.google.cat/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cf/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.cg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ch/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ch/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ci/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ci/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cl/url?q=https://betop24.com/safe-casino-site/
https://maps.google.cm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ao/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ao/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ao/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.bw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.bw/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.bw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ck/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ck/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ck/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.cr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.cr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.id/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.il/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.in/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.in/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ke/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.kr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.kr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ls/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ls/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ls/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.mz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.mz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.mz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.nz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.th/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.th/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.tz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.tz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.ug/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ug/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.uk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ve/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ve/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.vi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.vi/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.vi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.za/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.za/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.zm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.zm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.zm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.zw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.zw/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ag/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ag/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ag/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ai/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ai/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ai/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ar/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ar/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ar/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.au/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bd/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bd/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.bd/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bh/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.bh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bn/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.bn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.br/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.bz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.co/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.co/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.cu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.cu/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.cu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.do/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.do/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ec/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.eg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.eg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.fj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.fj/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.fj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gh/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.gh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gi/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.gi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.gt/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.hk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.hk/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.jm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.jm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.kh/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.kh/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.kh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.kw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.kw/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.kw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.lb/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ly/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ly/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ly/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.mm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mt/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.mt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mx/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mx/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.my/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.my/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.na/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.na/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.na/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ng/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ni/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ni/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.om/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pe/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pe/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.pg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ph/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.pr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.pr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.py/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.py/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.qa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.qa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sa/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sa/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.sg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.sl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.tw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.tw/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ua/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ua/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.ua/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.uy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.uy/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.uy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.vc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.vc/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.de/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.de/url?q=https://betop24.com/safe-casino-site/
https://maps.google.dj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dj/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dk/url?q=https://betop24.com/safe-casino-site/
https://maps.google.dm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.dm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ee/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ee/url?q=https://betop24.com/safe-casino-site/
https://maps.google.es/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.es/url?q=https://betop24.com/safe-casino-site/
https://maps.google.fi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fi/url?q=https://betop24.com/safe-casino-site/
https://maps.google.fm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ga/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ge/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ge/url?q=https://betop24.com/safe-casino-site/
https://maps.google.gg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.gg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gl/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.gm/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gp/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.gy/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hn/url?q=https://betop24.com/safe-casino-site/
https://maps.google.hr/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hr/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ht/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hu/url?q=https://betop24.com/safe-casino-site/
https://maps.google.hu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ie/url?q=https://betop24.com/safe-casino-site/
https://maps.google.im/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.im/url?q=https://betop24.com/safe-casino-site/
https://maps.google.im/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.iq/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.is/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.is/url?q=https://betop24.com/safe-casino-site/
https://maps.google.is/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.it/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.it/url?q=https://betop24.com/safe-casino-site/
https://maps.google.je/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.je/url?q=https://betop24.com/safe-casino-site/
https://maps.google.je/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.jo/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.jo/url?q=https://betop24.com/safe-casino-site/
https://maps.google.jo/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.kg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ki/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ki/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ki/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.kz/url?q=https://betop24.com/safe-casino-site/
https://maps.google.kz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.la/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.la/url?q=https://betop24.com/safe-casino-site/
https://maps.google.la/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.li/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lk/url?q=https://betop24.com/safe-casino-site/
https://maps.google.lt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lu/url?q=https://betop24.com/safe-casino-site/
https://maps.google.lv/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lv/url?q=https://betop24.com/safe-casino-site/
https://maps.google.mg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mg/url?q=https://betop24.com/safe-casino-site/
https://maps.google.mg/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mk/url?q=https://betop24.com/safe-casino-site/
https://maps.google.mk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.mn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ms/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ms/url?q=https://betop24.com/safe-casino-site/
http://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://betop24.com/safe-casino-site/
https://www.ladan.com.ua/link/go.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/%2F
https://www.webclap.com/php/jump.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/%2F
https://schwarzes-bw.de/wbb231/redir.php?url=https://betop24.com/safe-casino-site/
https://www.webclap.com/php/jump.php?url=https://betop24.com/safe-casino-site/
https://cse.google.com.cu/url?q=https://betop24.com/safe-casino-site/
http://www2.ogs.state.ny.us/help/urlstatusgo.html?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://go.infomine.com/?re=121&tg=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://link.harikonotora.net/?http%3A%2F%2Fbetop24.com/safe-casino-site/
http://text.usg.edu/tt/https://betop24.com/safe-casino-site/
http://uniportal.huaweidevice.com/uniportal/jsp/clearCrossDomainCookie.jsp?redirectUrl=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.fito.nnov.ru/go.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://www2.ogs.state.ny.us/help/urlstatusgo.html?url=https://betop24.com/safe-casino-site/
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&a_bid=9abb5269&desturl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://s5.histats.com/stats/r.php?869637&100&47794&urlr=&https://betop24.com/safe-casino-site/
https://15.pro.tok2.com/%7Ekoro/cutlinks/rank.php?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&a_bid=9abb5269&desturl=https://betop24.com/safe-casino-site/
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&abid=9abb5269&desturl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://channel.pixnet.net/newdirect.php?blog=cr19h3id&serial=0&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.cu/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://janeyleegrace.worldsecuresystems.com/redirect.aspx?destination=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.ba/url?q=https://betop24.com/safe-casino-site/
https://cse.google.be/url?q=https://betop24.com/safe-casino-site/
https://cse.google.bg/url?q=https://betop24.com/safe-casino-site/
https://cse.google.bs/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ca/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cg/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ch/url?q=https://betop24.com/safe-casino-site/
https://cse.google.cl/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.bw/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.cr/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.id/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.il/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.il/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.in/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.jp/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.ke/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.kr/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.ma/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.nz/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.th/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.ug/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.uk/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.uk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ve/url?q=https://betop24.com/safe-casino-site/
https://cse.google.co.za/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.af/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.au/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.bd/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.br/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.do/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.py/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.qa/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.sg/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.sv/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.tr/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.tw/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.ua/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.uy/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.vn/url?q=https://betop24.com/safe-casino-site/
https://cse.google.pt/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.rs/url?q=https://betop24.com/safe-casino-site/
https://cse.google.si/url?q=https://betop24.com/safe-casino-site/
https://cse.google.sk/url?q=https://betop24.com/safe-casino-site/
https://cse.google.tn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.vg/url?q=https%3A%2F%2Fhttps://betop24.com/safe-casino-site/
https://images.google.at/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.be/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.bg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ca/url?q=https://betop24.com/safe-casino-site/
https://images.google.ca/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ch/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.cl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.il/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.in/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.jp/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.nz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.th/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.uk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.co.za/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.au/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.br/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.br/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.cu/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.hk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.mx/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.my/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.sg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.tr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.tw/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.ua/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.com.vn/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.vn/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.cz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.de/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.dk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.es/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.fi/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.fr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.gr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.hu/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ie/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.nl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.pl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.pt/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ro/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ru/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.sk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.be/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ca/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ch/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cl/url?q=https://betop24.com/safe-casino-site/
https://maps.google.cl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site%2F
https://maps.google.co.il/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.in/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.jp/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.nz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.th/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.uk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.za/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.au/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.br/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.hk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.mx/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.my/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.sg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.tr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.tw/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com.tw/url?sa=t&url=https://betop24.com/safe-casino-site%2F
https://maps.google.com.ua/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.com/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.de/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.dk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.es/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.fi/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.fr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.gr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.hu/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ie/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.nl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.pl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.pt/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ro/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ru/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.sk/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.at/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.id/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.il/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.jp/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.kr/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.uk/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.za/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ar/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.br/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.mx/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.my/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.pe/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ph/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.tw/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ua/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.vn/url?q=https://betop24.com/safe-casino-site/
https://www.google.com/url?q=https://betop24.com/safe-casino-site/
https://www.google.de/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.hu/url?q=https://betop24.com/safe-casino-site/
https://www.google.ie/url?q=https://betop24.com/safe-casino-site/
https://www.google.it/url?q=https://betop24.com/safe-casino-site/
https://www.google.pt/url?q=https://betop24.com/safe-casino-site/
https://www.google.ro/url?q=https://betop24.com/safe-casino-site/
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=https://betop24.com/safe-casino-site/
https://www.google.ae/url?q=https://betop24.com/safe-casino-site/
https://www.google.az/url?q=https://betop24.com/safe-casino-site/
https://www.google.bs/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.bw/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.ke/url?q=https://betop24.com/safe-casino-site/
https://www.google.co.ve/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.bd/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.bh/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.cy/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ec/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ng/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.np/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.pk/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.qa/url?q=https://betop24.com/safe-casino-site/
https://www.google.lu/url?q=https://betop24.com/safe-casino-site/
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=https://betop24.com/safe-casino-site/
https://keywordspace.com/site-info/betop24.com
https://www.bombstat.com/domain/betop24.com
https://maps.google.com.br/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.jo/url?q=https://betop24.com/safe-casino-site/
https://www.google.com.ly/url?q=https://betop24.com/safe-casino-site/
https://cse.google.mu/url?q=https://betop24.com/safe-casino-site/
https://cse.google.ml/url?q=https://betop24.com/safe-casino-site/
https://www.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.uk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ch/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.be/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.tr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.mx/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.dk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.pt/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.pr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.lu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.co.ma/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.mn/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.com.kh/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.ug/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ly/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
http://tyonabi.sakura.ne.jp/link/cgi-bin/out.cgi?id=dorian362&cg=1&siteurl=https://betop24.com/safe-casino-site/
http://feeds.copyblogger.com/%7E/t/0/0/copyblogger/%7Ehttp%3A%2F%2Fbetop24.com/safe-casino-site/
http://www.scoop.co.nz/link-out?p=blog93&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.be/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.bf/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ck/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.bz/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.om/url?q=https://betop24.com/safe-casino-site/
https://cse.google.com.pk/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.sl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.com.vn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.dm/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ge/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.iq/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.pl/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.pn/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.so/url?sa=i&url=https://betop24.com/safe-casino-site/
https://images.google.co.cr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.pe/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.li/url?q=https://betop24.com/safe-casino-site/
https://lcu.hlcommission.org/lcu/pages/auth/forgotPassword.aspx?Returnurl=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.bo/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.lb/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.np/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.sa/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.de/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ee/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ie/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.nl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.tn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://t.me/iv?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://transtats.bts.gov/exit.asp?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.by/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.es/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.pl/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://www.google.sk/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://a.pr-cy.ru/https://betop24.com/safe-casino-site/
https://client.paltalk.com/client/webapp/client/External.wmt?url=https://betop24.com/safe-casino-site/
https://clients1.google.com.et/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.nf/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.com.vc/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.je/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.lk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://clients1.google.mk/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.co.kr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://cse.google.fr/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://doodle.com/r?url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://galter.northwestern.edu/exit?url=https://betop24.com/safe-casino-site/
https://images.google.bg/url?q=https://betop24.com/safe-casino-site/
https://images.google.bs/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cl/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.bw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.in/url?q=https://betop24.com/safe-casino-site/
https://images.google.co.ma/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.th/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.co.uz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bd/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.br/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.bz/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.cu/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ec/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.fj/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gh/url?q=https://betop24.com/safe-casino-site/
https://images.google.com.gi/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.gi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.ni/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.com.tw/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.cz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.gy/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.is/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.kz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.lt/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.me/url?q=https://betop24.com/safe-casino-site/
https://images.google.ro/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://images.google.rs/url?q=https://betop24.com/safe-casino-site/
https://images.google.sm/url?q=https://betop24.com/safe-casino-site/
https://images.google.to/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://ipv4.google.com/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ad/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ae/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.bi/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cat/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cd/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.cd/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ci/url?q=https://betop24.com/safe-casino-site/
https://maps.google.cm/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.id/url?q=https://betop24.com/safe-casino-site/
https://maps.google.co.jp/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.tz/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.co.zw/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ec/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.et/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.mm/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ng/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.np/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.np/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.pg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com.ph/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.sv/url?q=https://betop24.com/safe-casino-site/
https://maps.google.com.sv/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.com/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ga/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.gg/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.hn/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ht/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ht/url?q=https://betop24.com/safe-casino-site/
https://maps.google.ie/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.kz/url?q=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://maps.google.ms/url?sa=t&url=http%3A%2F%2Fbetop24.com/safe-casino-site/
https://sitereport.netcraft.com/?url=https://betop24.com/safe-casino-site%2F
https://ranking.crawler.com/SiteInfo.aspx?url=https://betop24.com/safe-casino-site/
https://valueanalyze.com/show.php?url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.uy/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.tw/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.tr/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.sa/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.py/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.pr/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.pk/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.pe/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.my/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.hk/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.gt/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.gh/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.com.ar/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.com.ag/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.zm/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.za/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.ve/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.uz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.ug/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.th/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.nz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.kr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.ke/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.il/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.cr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://clients1.google.co.ck/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.co.ck/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.co.bw/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.cm/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.cl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.ci/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.ch/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.ch/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.cg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.cd/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.by/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.bs/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.bi/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.bg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.be/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.be/url?sa=i&url=https://betop24.com/safe-casino-site/
https://cse.google.ba/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.az/url?sa=t&url=https://betop24.com/safe-casino-site/
https://cse.google.at/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ca/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.by/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.bs/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.bi/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.bg/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.bf/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.be/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ba/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ba/url?q=https://betop24.com/safe-casino-site/
https://images.google.az/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.at/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.as/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.am/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.al/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ae/url?sa=t&url=https://betop24.com/safe-casino-site/
https://images.google.ae/url?q=https://betop24.com/safe-casino-site/
https://images.google.ad/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.ke/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.jp/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.in/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.il/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.cr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.co.bw/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cm/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ci/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ch/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cd/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.cat/url?sa=t&url=https://betop24.com/safe-casino-site/
https://maps.google.ca/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.tz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.th/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.nz/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.ma/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.ls/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.kr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.ke/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.jp/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.in/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.il/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.id/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.cr/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.co.bw/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.cm/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.cl/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.ci/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.ch/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.cd/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.cat/url?sa=t&url=https://betop24.com/safe-casino-site/
https://www.google.ca/url?sa=t&url=https://betop24.com/safe-casino-site/
http://toolbarqueries.google.com.uy/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.tw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.tr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.sa/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.py/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.pr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.pk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.pe/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.my/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.hk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.gt/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://toolbarqueries.google.com.gh/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ar/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ag/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.zm/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.za/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ve/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.uz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ug/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.th/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.nz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.kr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ke/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.il/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.cr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ck/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ck/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.bw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cm/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ci/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ch/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ch/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cd/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.by/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bs/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bi/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.be/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.be/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ba/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.az/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.at/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.by/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bs/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bi/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bf/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ba/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ba/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.az/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.as/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.am/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.al/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ad/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ke/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.jp/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.il/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.cr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.bw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cm/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ci/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cd/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cat/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ca/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.tz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.th/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.nz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ma/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ls/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.kr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ke/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.jp/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.in/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.il/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.cr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.bw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cm/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ci/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ch/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cd/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cat/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ca/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.zw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://www.adminer.org/redirect/?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://sfwater.org/redirect.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://web.archive.org/web/20180804180141/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://baoviet.com.vn/Redirect.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://sc.sie.gov.hk/TuniS/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.ric.edu/Pages/link_out.aspx?target=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pe/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.np/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.jm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.li/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.kh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.co/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.gi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.la/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.mz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sb/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ly/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.fm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.vu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cf/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.st/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.iq/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.tk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://posts.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.nu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://asia.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.vn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pe/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pe/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.by/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ec/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ec/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ba/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ng/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ng/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.cr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.cr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.kw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.is/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.dz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.tn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.tn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.iq/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.iq/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.kz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ge/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ni/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.la/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.jm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.qa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ps/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bo/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.li/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.lb/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.lb/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.tt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ci/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.sn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.sn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.mt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cat/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.fm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.as/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.om/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.om/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.mz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.bw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ms/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ag/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ag/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://app.feedblitz.com/f/f.fbz?track=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://archives.midweek.com/?URL=http%253A%252F%252Fhttps://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://fooyoh.com/wcn.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&keyid=42973
http://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://sc.sie.gov.hk/TuniS/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.aa.org/pages/en_US/disclaimer?u=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.aastocks.com/sc/changelang.aspx?lang=sc&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.blueletterbible.org/tools/redirect.cfm?Site=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.cineuropa.org/el.aspx?el=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.drinksmixer.com/redirect.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.justjared.com/flagcomment.php?el=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.kaskus.co.id/redirect?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.meetme.com/apps/redirect/?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.office.xerox.com/perl-bin/reseller_exit.pl?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.popcouncil.org/scripts/leaving.asp?URL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.ric.edu/Pages/link_out.aspx?target=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.sinp.msu.ru/ru/ext_link?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.webclap.com/php/jump.php?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www2.sandbox.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www2.sandbox.google.co.kr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://cr.naver.com/rd?u=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://devicedoctor.com/driver-feedback.php?device=PCI%20bus&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://domain.opendns.com/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://my.dek-d.com/username/link/link.php?out=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://panchodeaonori.sakura.ne.jp/feed/aonori/feed2js.php?src=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://refer.ash1.ccbill.com/cgi-bin/clicks.cgi?CA=933914&PA=1785830&HTML=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://refer.ccbill.com/cgi-bin/clicks.cgi/http:/?CA=928498&PA=1458253&HTML=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://sc.youth.gov.hk/TuniS/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://wasearch.loc.gov/e2k/*/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://wikimapia.org/external_link?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.astro.wisc.edu/?URL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.astro.wisc.edu/?URL=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.cssdrive.com/?URL=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.earth-policy.org/?URL=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.fmvz.unam.mx/fmvz/biblioteca/revistas_electronicas/control/envioClics.php?cnH57w=1118&kqJm89=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.ric.edu/Pages/link_out.aspx?target=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.shinobi.jp/etc/goto.html?http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.htmlkuttymovies-2020-kuttymovies-hd-tamil-movies-download
http://www.spiritfanfiction.com/link?l=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.webclap.com/php/jump.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.zerocarts.com/demo/index.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://advisor.wmtransfer.com/SiteDetails.aspx?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://blog.ss-blog.jp/pages/mobile/step/index?u=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://bukkit.org/proxy.php?link=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://client.paltalk.com/client/webapp/client/External.wmt?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.esri.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.rsa.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ac/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ad/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ad/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ae/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.al/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.am/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.as/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.at/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.az/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ba/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bi/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bj/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bs/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.by/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.by/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ca/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cat/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cd/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cf/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ch/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ci/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ao/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.bw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ck/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.cr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.id/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.in/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.jp/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ke/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.kr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ls/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ma/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.mz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.nz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.th/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.tz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ug/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.uz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.uz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ve/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.vi/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.za/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.zm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.zw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.af/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ag/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ai/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ai/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.au/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bd/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bh/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bo/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.co/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.co/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.cu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.cy/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ec/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.eg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.et/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.et/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.fj/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.gh/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.gi/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.gt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.hk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.jm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.kh/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.kw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ly/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.mm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.mt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.mt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.mx/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.my/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.na/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.nf/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ng/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ni/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.np/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.om/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pa/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pe/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ph/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sa/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sa/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sv/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tj/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ua/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.uy/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.vc/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.de/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.dk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.dz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.dz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ee/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.es/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.fi/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.fr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ga/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ga/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gp/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.gy/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.hn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.hr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ht/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.hu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ie/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.im/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.iq/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.is/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.it/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.je/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.jo/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.kg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.kg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ki/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ki/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.kz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.la/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.li/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.lk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.lt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.lu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.lv/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.me/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ms/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mv/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ne/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.nl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.no/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.nr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.nu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.nu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ps/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ro/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.rs/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ru/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.rw/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sc/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.se/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sh/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.si/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.so/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sr/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.td/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.to/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.vg/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.vu/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ws/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ws/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://ditu.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://foro.infojardin.com/proxy.php?link=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://foro.infojardin.com/proxy.php?link=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://galter.northwestern.edu/exit?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://id.telstra.com.au/register/crowdsupport?gotoURL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ad/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ad/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.by/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.by/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cat/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.bw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.kr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ug/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ug/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ec/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.et/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.et/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.jm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.jm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.lb/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ng/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.np/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.om/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.om/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pe/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pe/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ph/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sa/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.uy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.iq/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.iq/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.se/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ws/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ws/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://legacy.aom.org/verifymember.asp?nextpage=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.by/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.cr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ke/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.kr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ug/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ec/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.et/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.et/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.jm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ng/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ni/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.om/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.om/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pe/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ph/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ge/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.iq/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.iq/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.li/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.li/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.sc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.se/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.tn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.tn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.vu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.vu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ws/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ws/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://optimize.viglink.com/page/pmv?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://plus.google.com/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://pw.mail.ru/forums/fredirect.php?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://ref.gamer.com.tw/redir.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://sfwater.org/redirect.aspx?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://sfwater.org/redirect.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://wfc2.wiredforchange.com/dia/track.jsp?v=2&c=hdorrh%2BHcDlQ%2BzUEnZU5qlfKZ1Cl53X6&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.adminer.org/redirect/?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ae/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.as/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.at/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.bg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.by/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ca/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.bw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.il/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ke/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.kr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ma/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.nz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.th/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ve/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.za/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.au/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.co/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.do/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ec/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.eg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.hk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.my/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ng/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.np/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pe/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ph/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.sv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.uy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.vn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.cz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.dz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.fi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.gr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.is/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.kg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.kg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.kz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ro/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.se/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.si/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.tn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.tt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.transtats.bts.gov/exit.asp?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.transtats.bts.gov/exit.asp?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.youtube.com/redirect?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://youtube.com/redirect?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&tab=feedback
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&tab=rating
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&tab=wminfo
https://advisor.wmtransfer.com/SiteDetails.aspx?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://anonym.to/?https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://anonym.to/?http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://blogs.rtve.es/libs/getfirma_footer_prod.php?blogurl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://bukkit.org/proxy.php?link=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://bukkit.org/proxy.php?link=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cdrinfo.com/Sections/Ads/ReviewsAroundTheWebRedirector.aspx?TargetUrl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://ceskapozice.lidovky.cz/redir.aspx?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cgl.ethz.ch/disclaimer.php?dlurl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://chofu.keizai.biz/banner.php?type=text_banner&position=right&id=3&uri=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ac/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ad/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ae/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.al/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.am/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.as/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.az/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.bf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.bi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.bj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.bs/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.bt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.by/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.cd/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.cf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.cg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ci/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.cm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ao/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ck/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.il/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.in/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.jp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.kr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ls/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ma/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.mz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.nz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.th/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.tz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.uz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.ve/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.vi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.zm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.co.zw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.af/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ag/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ai/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ar/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.bh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.bn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.bo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.bz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.cu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.cy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.eg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.fj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.gh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.gi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.hk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.jm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.kh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.kw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ly/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.mm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.mt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.my/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.na/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.np/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.om/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.pe/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.pg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.pk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.pr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.qa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.sa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.sl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.tj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.tr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.tw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.ua/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.uy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.vn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.cv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.de/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.dj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.dm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.fm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ga/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.gg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.gl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.gm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.gp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.gy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ht/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ie/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.im/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.is/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.it/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.jo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.kg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ki/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.la/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.li/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.md/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.me/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.mg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.mn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ms/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.mv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.mw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ne/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.nr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.nu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.pn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ro/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.rw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.sc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.sh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.sm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.so/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.sr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.st/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.td/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.tg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.tl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.tm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.to/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.tt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.vg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.vu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.ws/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.cypress.com/external-link.jspa?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.esri.com/external-link.jspa?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.esri.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://community.rsa.com/external-link.jspa?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://contacts.google.com/url?sa=t&source=web&rct=j&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&ved=2ahUKEwip75vMsq7mAhUv06YKHYSzAN44rAIQFjAYegQIKRAB
https://cse.google.ba/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.by/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.id/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.in/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.jp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ke/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.hk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.nf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ng/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.vn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.it/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ru/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.se/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://demo.html5xcss3.com/demo.php?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://dereferer.org/?https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://drive.sandbox.google.com.br/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://engawa.kakaku.com/jump/?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://eventlog.centrum.cz/redir?data=aclick1c68565-349178t12&s=najistong&v=1&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://foro.infojardin.com/proxy.php?link=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://forum.solidworks.com/external-link.jspa?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://forums-archive.eveonline.com/default.aspx?g=warning&l=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html&domain=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://galter.northwestern.edu/exit?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://galter.northwestern.edu/exit?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://gleam.io/zyxKd-INoWr2EMzH?l=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://hobby.idnes.cz/peruanske-palive-papricky-rocoto-dlz-/redir.aspx?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://id.telstra.com.au/register/crowdsupport?gotoURL=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ac/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ac/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ad/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ae/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.al/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.al/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.al/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.am/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.am/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.as/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.as/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.as/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.az/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.az/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.az/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ba/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ba/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ba/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bf/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bs/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.by/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cat/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cat/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cd/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cf/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ch/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ch/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ci/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ci/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ci/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ao/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ao/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ao/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.bw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ck/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ck/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ck/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.cr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.cr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.id/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.id/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.il/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.in/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.jp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ke/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ke/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ke/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.kr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.kr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ls/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ls/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ls/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ma/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ma/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.mz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.mz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.mz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.nz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.th/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.tz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.tz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.tz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ug/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ve/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ve/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.vi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.vi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.vi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.za/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.za/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.zw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.af/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.af/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.af/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ag/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ag/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ag/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ai/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ai/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ai/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ar/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ar/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ar/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.au/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bd/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bo/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bo/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.co/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.co/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cy/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.do/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.do/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ec/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.eg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.eg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.et/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.fj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.fj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.hk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.hk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.jm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.kw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.lb/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ly/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ly/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ly/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mx/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mx/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.my/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.my/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.na/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.na/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.na/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.nf/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ng/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ni/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ni/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.np/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.np/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.om/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ph/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ph/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.py/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.py/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.qa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.qa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ua/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ua/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.uy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.uy/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vc/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.de/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.de/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ee/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ee/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.es/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.es/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ga/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ga/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ga/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ge/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ge/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ge/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ht/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ht/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ht/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ie/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ie/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.im/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.im/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.im/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.iq/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.is/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.is/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.it/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.it/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.je/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.je/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.jo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.jo/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.jo/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ki/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ki/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ki/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.la/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.la/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.la/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.li/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.li/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.md/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.md/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.md/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.me/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.me/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ms/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ms/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ms/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ne/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ne/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.no/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.no/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.no/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ps/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ps/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ps/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ro/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.rs/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.rs/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ru/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ru/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.rw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.rw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sc/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sc/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.se/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.se/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.si/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.si/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.so/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.so/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.st/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.st/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.st/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.td/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.td/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.to/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.tt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.vu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ws/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://inquiry.princetonreview.com/away/?value=cconntwit&category=FS&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://ipv4.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://it.paltalk.com/client/webapp/client/External.wmt?url=http://https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://jump.2ch.net/?https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://lcu.hlcommission.org/lcu/pages/auth/forgotPassword.aspx?Returnurl=http://https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://legacy.aom.org/verifymember.asp?nextpage=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://lexsrv2.nlm.nih.gov/fdse/search/search.pl?Match=0&Realm=All&Terms=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.addthis.com/live/redirect/?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.odnoklassniki.ru/dk?st.cmd=outLinkWarning&st.rfn=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://m.ok.ru/dk?st.cmd=outLinkWarning&st.rfn=http://https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ad/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ad/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ae/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.as/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.as/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.as/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.at/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.at/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ba/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ba/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.be/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.be/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bf/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bs/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bs/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.by/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.by/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ca/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cat/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cat/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cf/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ci/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ci/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ao/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ao/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ao/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.bw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.bw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.bw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ck/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ck/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ck/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.cr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.cr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.il/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ke/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.kr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.kr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ls/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ls/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ls/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.mz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.mz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.mz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.nz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.th/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.th/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.tz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.tz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ug/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ug/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.uk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ve/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ve/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.vi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.vi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.vi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.za/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.za/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ag/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ag/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ag/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ai/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ai/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ai/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ar/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ar/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ar/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.au/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bd/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bd/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.br/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.co/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.co/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.cu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.cu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.cu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.do/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.do/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ec/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.eg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.eg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.fj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.fj/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.fj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.gt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.hk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.hk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.jm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.jm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kh/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.kw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.lb/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ly/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ly/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ly/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mx/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mx/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.my/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.my/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.na/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.na/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.na/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ng/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ni/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ni/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.om/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pe/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pe/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ph/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.py/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.py/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.qa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.qa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sa/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sa/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ua/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ua/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ua/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.uy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.uy/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.uy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.vc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.vc/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.de/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.de/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dj/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ee/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ee/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.es/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.es/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fi/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ga/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ge/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ge/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gl/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gm/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gp/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gy/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hr/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ht/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ie/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.im/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.im/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.im/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.iq/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.is/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.is/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.is/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.it/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.it/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.je/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.je/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.je/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.jo/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.jo/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.jo/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ki/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ki/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ki/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.la/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.la/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.la/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.li/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lv/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mg/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.mn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ms/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ms/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.ladan.com.ua/link/go.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://www.webclap.com/php/jump.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://schwarzes-bw.de/wbb231/redir.php?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.webclap.com/php/jump.php?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.cu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www2.ogs.state.ny.us/help/urlstatusgo.html?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://go.infomine.com/?re=121&tg=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://link.harikonotora.net/?http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://text.usg.edu/tt/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://uniportal.huaweidevice.com/uniportal/jsp/clearCrossDomainCookie.jsp?redirectUrl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.fito.nnov.ru/go.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www2.ogs.state.ny.us/help/urlstatusgo.html?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&a_bid=9abb5269&desturl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://s5.histats.com/stats/r.php?869637&100&47794&urlr=&https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://15.pro.tok2.com/%7Ekoro/cutlinks/rank.php?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&a_bid=9abb5269&desturl=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://affiliates.bookdepository.com/scripts/click.php?a_aid=Alexa1202&abid=9abb5269&desturl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://channel.pixnet.net/newdirect.php?blog=cr19h3id&serial=0&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cu/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://janeyleegrace.worldsecuresystems.com/redirect.aspx?destination=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ba/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.be/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ca/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ch/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.cl/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.bw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.cr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.id/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.il/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.il/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.in/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.jp/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ke/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.kr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ma/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.nz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.th/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ug/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.uk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.uk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ve/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.za/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.af/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.au/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.br/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.do/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.py/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.qa/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.tw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.ua/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.uy/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.vn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.pt/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.rs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.si/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.sk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.tn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.vg/url?q=https%3A%2F%2Fhttps://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.at/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.be/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ca/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ch/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.il/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.in/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.jp/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.nz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.th/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.za/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.au/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.br/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.br/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.hk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.mx/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.my/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.sg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ua/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.vn/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.de/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.dk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.es/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fi/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.hu/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ie/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.nl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.pt/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ro/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ru/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.be/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ca/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://maps.google.co.il/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.in/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.jp/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.nz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.th/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.uk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.za/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.au/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.br/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.hk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mx/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.my/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sg/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.tw/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html%2F
https://maps.google.com.ua/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cz/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.de/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.es/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fi/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gr/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hu/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ie/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.nl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pl/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.pt/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ro/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ru/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.sk/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.at/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.id/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.il/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.jp/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.kr/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.uk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.za/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ar/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.br/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.mx/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.my/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pe/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ph/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.tw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ua/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.vn/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.de/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.hu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ie/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.it/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.pt/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ro/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.ae/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.az/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.bs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.bw/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ke/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ve/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.bh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.cy/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ec/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ng/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.np/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pk/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.qa/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.lu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://keywordspace.com/site-info/betop24.com
https://www.bombstat.com/domain/betop24.com
https://maps.google.com.br/url?sa=t&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.jo/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.ly/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.mu/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ml/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.uk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ch/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.be/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.tr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.mx/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.dk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.pt/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.pr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.lu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.co.ma/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.mn/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.com.kh/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.ug/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ly/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://tyonabi.sakura.ne.jp/link/cgi-bin/out.cgi?id=dorian362&cg=1&siteurl=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://feeds.copyblogger.com/%7E/t/0/0/copyblogger/%7Ehttp%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
http://www.scoop.co.nz/link-out?p=blog93&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.be/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.bf/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.ck/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.bz/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.om/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.pk/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.sl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.com.vn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.dm/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.ge/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.iq/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.pl/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.pn/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.so/url?sa=i&url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.cr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.pe/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.li/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://lcu.hlcommission.org/lcu/pages/auth/forgotPassword.aspx?Returnurl=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.bo/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.lb/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.np/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sa/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.de/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ee/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ie/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.nl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.tn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://t.me/iv?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://transtats.bts.gov/exit.asp?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.by/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.es/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.pl/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://www.google.sk/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://a.pr-cy.ru/https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://client.paltalk.com/client/webapp/client/External.wmt?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.et/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.nf/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.com.vc/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.je/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.lk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://clients1.google.mk/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.co.kr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://cse.google.fr/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://doodle.com/r?url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://galter.northwestern.edu/exit?url=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bg/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.bs/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cl/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.bw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.in/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.ma/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.th/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.co.uz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.br/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.bz/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.cu/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ec/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.fj/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gh/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gi/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.gi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.ni/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.com.tw/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.cz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.gy/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.is/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.kz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.lt/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.me/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.ro/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.rs/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.sm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://images.google.to/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://ipv4.google.com/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ad/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ae/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.bi/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cat/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cd/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cd/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ci/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.cm/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.id/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.jp/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.tz/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.co.zw/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ec/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.et/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.mm/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ng/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.np/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.np/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.pg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.ph/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sv/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com.sv/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.com/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ga/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.gg/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.hn/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ht/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ht/url?q=https://1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ie/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.kz/url?q=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
https://maps.google.ms/url?sa=t&url=http%3A%2F%2F1arctic.blogspot.com/2023/03/online-club-invite-reward-guarantee-now.html
kylef1066@gmail.com 11/21/22
https://cutt.ly/8ZI7qrb
https://casinobulk.com/
https://casinobulk.com/ 카지노사이트
https://casinobulk.com/ 바카라사이트
https://casinobulk.com/ 온라인카지노
https://casinobulk.com/ 온라인바카라
https://casinobulk.com/ 온라인슬롯사이트
https://casinobulk.com/ 카지노사이트게임
https://casinobulk.com/ 카지노사이트검증
https://casinobulk.com/ 카지노사이트추천
https://casinobulk.com/ 안전카지노사이트
https://casinobulk.com/ 안전카지노사이트도메인
https://casinobulk.com/ 안전한 카지노사이트 추천
https://casinobulk.com/ 바카라사이트게임
https://casinobulk.com/ 바카라사이트검증
https://casinobulk.com/ 바카라사이트추천
https://casinobulk.com/ 안전바카라사이트
https://casinobulk.com/ 안전바카라사이트도
https://casinobulk.com/ 안전한 바카라사이트
http://toolbarqueries.google.com.uy/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.tw/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.tr/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.sa/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.py/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.pr/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.pk/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.pe/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.my/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.hk/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.gt/url?sa=t&url=https://casinobulk.com/
http://toolbarqueries.google.com.gh/url?sa=t&url=https://casinobulk.com/
https://clients1.google.com.ar/url?sa=t&url=https://casinobulk.com/
https://clients1.google.com.ag/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.zm/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.za/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.ve/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.uz/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.ug/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.th/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.nz/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.kr/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.ke/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.il/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.id/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.cr/url?sa=t&url=https://casinobulk.com/
https://clients1.google.co.ck/url?sa=t&url=https://casinobulk.com/
https://cse.google.co.ck/url?sa=t&url=https://casinobulk.com/
https://cse.google.co.bw/url?sa=t&url=https://casinobulk.com/
https://cse.google.cm/url?sa=t&url=https://casinobulk.com/
https://cse.google.cl/url?sa=t&url=https://casinobulk.com/
https://cse.google.ci/url?sa=t&url=https://casinobulk.com/
https://cse.google.ch/url?sa=t&url=https://casinobulk.com/
https://cse.google.ch/url?sa=i&url=https://casinobulk.com/
https://cse.google.cg/url?sa=t&url=https://casinobulk.com/
https://cse.google.cd/url?sa=t&url=https://casinobulk.com/
https://cse.google.by/url?sa=t&url=https://casinobulk.com/
https://cse.google.bs/url?sa=t&url=https://casinobulk.com/
https://cse.google.bi/url?sa=t&url=https://casinobulk.com/
https://cse.google.bg/url?sa=t&url=https://casinobulk.com/
https://cse.google.be/url?sa=t&url=https://casinobulk.com/
https://cse.google.be/url?sa=i&url=https://casinobulk.com/
https://cse.google.ba/url?sa=t&url=https://casinobulk.com/
https://cse.google.az/url?sa=t&url=https://casinobulk.com/
https://cse.google.at/url?sa=t&url=https://casinobulk.com/
https://images.google.ca/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.by/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.bs/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.bi/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.bg/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.bf/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.be/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.ba/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.ba/url?q=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.az/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.at/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.as/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.am/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.al/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.ae/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.ae/url?q=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://images.google.ad/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.ke/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.jp/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.in/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.il/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.id/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.cr/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.co.bw/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.cm/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.cl/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.ci/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.ch/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.cd/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.cat/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://maps.google.ca/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.tz/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.th/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.nz/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.ma/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.ls/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.kr/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.ke/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.jp/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.in/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.il/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.id/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.cr/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.co.bw/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.cm/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.cl/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.ci/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.ch/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.cd/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.cat/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
https://www.google.ca/url?sa=t&url=https://images.google.ba/url?q=https://blogskorcasino1.blogspot.com/2022/11/15-things-you-had-close-to-zero.html
otizjess6@gmail.com 10/21/22
https://bit.ly/36En2s8+
카지노사이트 https://ce-top10.com/
바카라사이트 https://ce-top10.com/
온라인카지노 https://ce-top10.com/
온라인바카라 https://ce-top10.com/
온라인슬롯사이트 https://ce-top10.com/
카지노사이트게임 https://ce-top10.com/
카지노사이트검증 https://ce-top10.com/
카지노사이트추천 https://ce-top10.com/
안전카지노사이트 https://ce-top10.com/
안전카지노사이트도메인 https://ce-top10.com/
안전한 카지노사이트 추천 https://ce-top10.com/
바카라사이트게임 https://ce-top10.com/
바카라사이트검증 https://ce-top10.com/
바카라사이트추천 https://ce-top10.com/
안전바카라사이트 https://ce-top10.com/
안전바카라사이트도메인 https://ce-top10.com/
안전한 바카라사이트 추천 https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.sn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.sh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.rw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ru/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.pt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ps/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.pl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.nl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ms/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.mg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.me/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.md/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.li/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.la/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.kz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.kg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.jo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.je/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.it/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.iq/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ht/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.hn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.gp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.gm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.gl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.gg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ge/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.fr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.fm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.dz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.dj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.cz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.vn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ua/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.tw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.sv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.sg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.qa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.py/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.pk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ph/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.pe/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.pa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.om/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ni/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ng/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.na/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.mz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.mx/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.mt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ly/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.lb/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.kw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.kh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.jm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.gi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.gh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.fj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.et/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.cy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.cu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.co/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.bz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.br/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.bo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.bn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.bh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.bd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.au/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.ag/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.com.af/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.uk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.tz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.ma/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.ls/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.jp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.in/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.cm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ci/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ch/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.cd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.cat/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ca/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.by/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.bs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.bi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.bf/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.be/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ba/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.az/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.as/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.am/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.al/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://www.google.ad/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://plus.google.com/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.tn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.sn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.sh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.rw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ru/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.pt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.pl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.nl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ms/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.mg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.li/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.la/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.kz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.kg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.jo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.je/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.it/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.iq/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ht/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.hn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.gm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.gl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.gg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ge/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.fr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.fm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.dz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.dj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.cz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ua/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.tw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.sv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.sg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.qa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.py/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ph/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.pe/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.pa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.om/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ni/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ng/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.na/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.mz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.mx/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.mt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ly/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.lb/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.kw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.kh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.jm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.gi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.gh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.fj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.et/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.cu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.co/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.bz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.br/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.bo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.bn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.bh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.bd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.au/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.com.ag/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.uk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.tz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.ls/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.jp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.in/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.cm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ci/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ch/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.cd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.cat/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ca/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.by/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.bs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.bi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.bf/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.be/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ba/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.as/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://maps.google.ad/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.tn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.sn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.sh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.rw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ru/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.pt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ps/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.pl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.nl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ms/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.mg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.me/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.md/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.li/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.la/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.kz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.kg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.jo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.je/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.it/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.iq/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ht/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.hn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.gp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.gm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.gl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.gg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ge/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.fr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.fm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.dz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.dm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.dj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.vn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.vc/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ua/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.tw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.sv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.sg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.qa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.py/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.pk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ph/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.pe/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.pa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.om/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.np/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ni/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ng/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.na/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.mz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.mx/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.mt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ly/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.lb/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.kw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.kh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.jm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.gi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.gh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.fj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.et/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.cy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.cu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.co/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.bz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.br/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.bo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.bn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.bh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.bd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.au/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.ag/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.com.af/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.zm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.za/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.uz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.uk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.tz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.th/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ma/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ls/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.kr/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.jp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.in/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.id/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.cr/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.ck/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ci/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ch/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.cat/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ca/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.by/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.bs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.bi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.bf/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.be/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ba/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ba/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.az/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.as/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.am/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.al/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ae/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://images.google.ad/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.vg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.to/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.tn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.tm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.sn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.sm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.sh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.sc/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.rw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.pt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ps/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.mw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ms/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.mn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.md/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.kz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.kg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.jo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ht/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.gm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.gl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.gg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ge/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.fm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.es/url?sa=i&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.dm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.dj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.vn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ua/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.sg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.pk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ph/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.pa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.om/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.np/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ng/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.mt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ly/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.lb/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.kw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.jm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.hk/url?sa=i&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.gi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.fj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.et/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.cu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.co/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.bz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.bh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.bd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.au/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.com.ag/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.zm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.uz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.jp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.id/url?sa=i&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.ck/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.cm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ci/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ch/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ch/url?sa=i&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.cg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.cd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.by/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.bs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.bi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.be/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.be/url?sa=i&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ba/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.az/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.am/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://cse.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.vg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.to/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.tn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.tm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.sn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.sm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.sh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.sc/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.rw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.pt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ps/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.mw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ms/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.mn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.md/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.kz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.kg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.jo/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ie/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ht/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.gm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.gl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.gg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ge/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.fm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.es/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.dm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.dj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.vn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.vc/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ua/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.tr/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.sg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.qa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.py/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.pk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ph/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.pa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.np/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ng/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.mt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ly/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.lb/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.kw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.jm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.gi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.fj/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.et/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.co/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.co/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.bz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.bh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.bd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.com.ag/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.zm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.uz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.ck/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.cm/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.cl/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ci/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ch/url?q=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.cg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.cd/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.by/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.bs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.bi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.be/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ba/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.az/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.am/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
https://clients1.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.sk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.si/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.se/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ru/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.rs/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ro/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.pl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.no/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.nl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.lv/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.lu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.lt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.lk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.it/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.is/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ie/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.hu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.hr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.gr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.fr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.fi/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.es/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ee/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.dk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.de/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.cz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.vn/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.uy/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.tw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.tr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.sa/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.py/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.pr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.pk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.pe/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.my/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.hk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.gt/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.gh/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.eg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.ec/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.do/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.cu/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.br/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.au/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.com.ar/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.za/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.ve/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.uk/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.ug/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.th/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.nz/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.kr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.ke/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.jp/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.in/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.il/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.id/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.cr/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.co.bw/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.cl/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ch/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ca/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.bg/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.at/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
http://toolbarqueries.google.ae/url?sa=t&url=https://gamblingdiscussion45456.blogspot.com/2022/10/superstitions-in-casinos-quick-overview.html
klayline4@gmail.com
10/20
https://bit.ly/3IrPU5E
카지노사이트 https://joinlive77.com/
바카라사이트 https://joinlive77.com/
온라인카지노 https://joinlive77.com/
온라인바카라 https://joinlive77.com/
온라인슬롯사이트 https://joinlive77.com/
카지노사이트게임 https://joinlive77.com/
카지노사이트검증 https://joinlive77.com/
카지노사이트추천 https://joinlive77.com/
안전카지노사이트 https://joinlive77.com/
안전카지노사이트도메인 https://joinlive77.com/
안전한 카지노사이트 추천 https://joinlive77.com/
바카라사이트게임 https://joinlive77.com/
바카라사이트검증 https://joinlive77.com/
바카라사이트추천 https://joinlive77.com/
안전바카라사이트 https://joinlive77.com/
안전바카라사이트도메인 https://joinlive77.com/
안전한 바카라사이트 추천 https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=https://joinlive77.com/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://story-bet.xyz/index.php/2022/10/19/how-to-play-slot-machines-step-by-step-instructions/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
https://www.google.tn/url?sa=t&url=http://casino-blog.click/
바카라사이트
카지노사이트
카지노게임사이트
온라인카지노
퀸즈슬롯
맥스카지노
비바카지노
카지노주소
바카라추천
온라인바카라게임
안전한 바카라사이트
바카라
카지노
퀸즈슬롯 카지노
바카라게임사이트
온라인바카라
밀리언클럽카지노
안전카지노사이트
바카라사이트추천
우리카지노계열
슬롯머신777
로얄카지노사이트
크레이지슬롯
온라인블랙잭
인터넷룰렛
카지노검증사이트
안전바카라사이트
모바일바카라
바카라 필승법
메리트카지노
바카라 노하우
바카라사이트
온라인카지노
카지노사이트
카지노게임사이트
퀸즈슬롯
맥스카지노
비바카지노
카지노주소
바카라추천
온라인바카라게임
안전한 바카라사이트
바카라
카지노
퀸즈슬롯 카지노
바카라게임사이트
온라인바카라
밀리언클럽카지노
안전카지노사이트
바카라사이트추천
우리카지노계열
슬롯머신777
로얄카지노사이트
크레이지슬롯
온라인블랙잭
인터넷룰렛
카지노검증사이트
안전바카라사이트
모바일바카라
바카라 필승법
메리트카지노
바카라 노하우
https://youube.me/
https://instagrme.com/
https://youubbe.me/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me