Glutamic Acid: Makes Food Taste Really Good!
Mono-sodium Glutamate, also known as MSG, is derived from the non-essential amino acid – Glutamic acid. MSG has been recognized to impart a distinct flavor in Asian cuisine we know as “umami” taste. The Japanese word “umami” literally means delicious. MSG is naturally present in some foods (such as ripened tomatoes) and was thought to synergistically react with other molecules and ultimately yields a combination of meaty and brothy savory taste. Despite the appealing use of MSG as seasoning, it is currently banned to some countries because of medical concerns in relatively large number individuals and safety issues associated with its production.
How does Glutamic Acid look like in Chemistry?
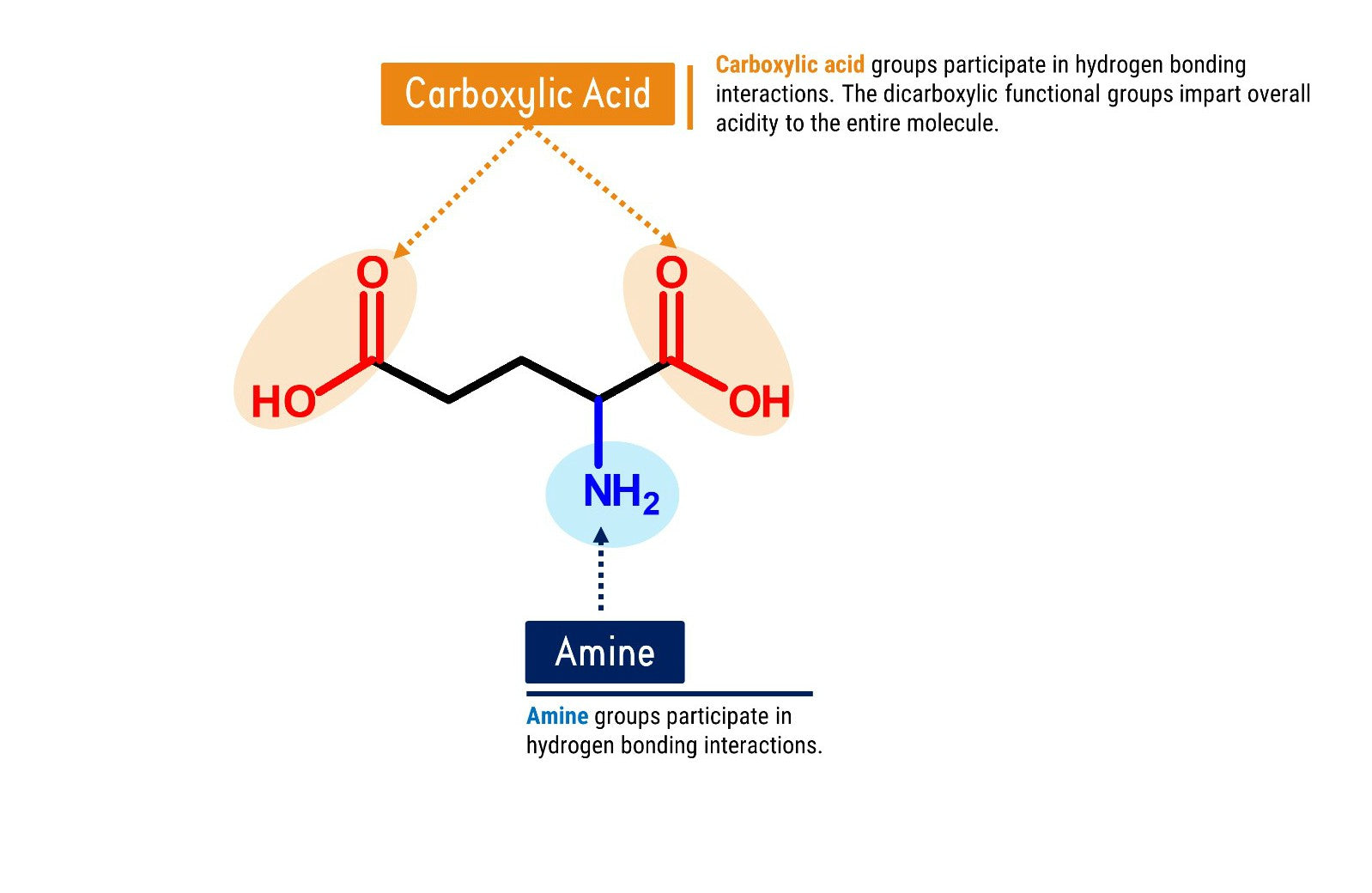
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create the Glutamic Acid Molecule! You’ll need:
- 5 Carbon atoms
- 4 Oxygen atoms
- 9 Hydrogen atoms
- 1 Nitrogen atom
- 9 Small connectors (compact small bonds for hydrogen)
- 7 Medium Connectors
- 4 Long connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building With Our Amino Acid Skeleton portion
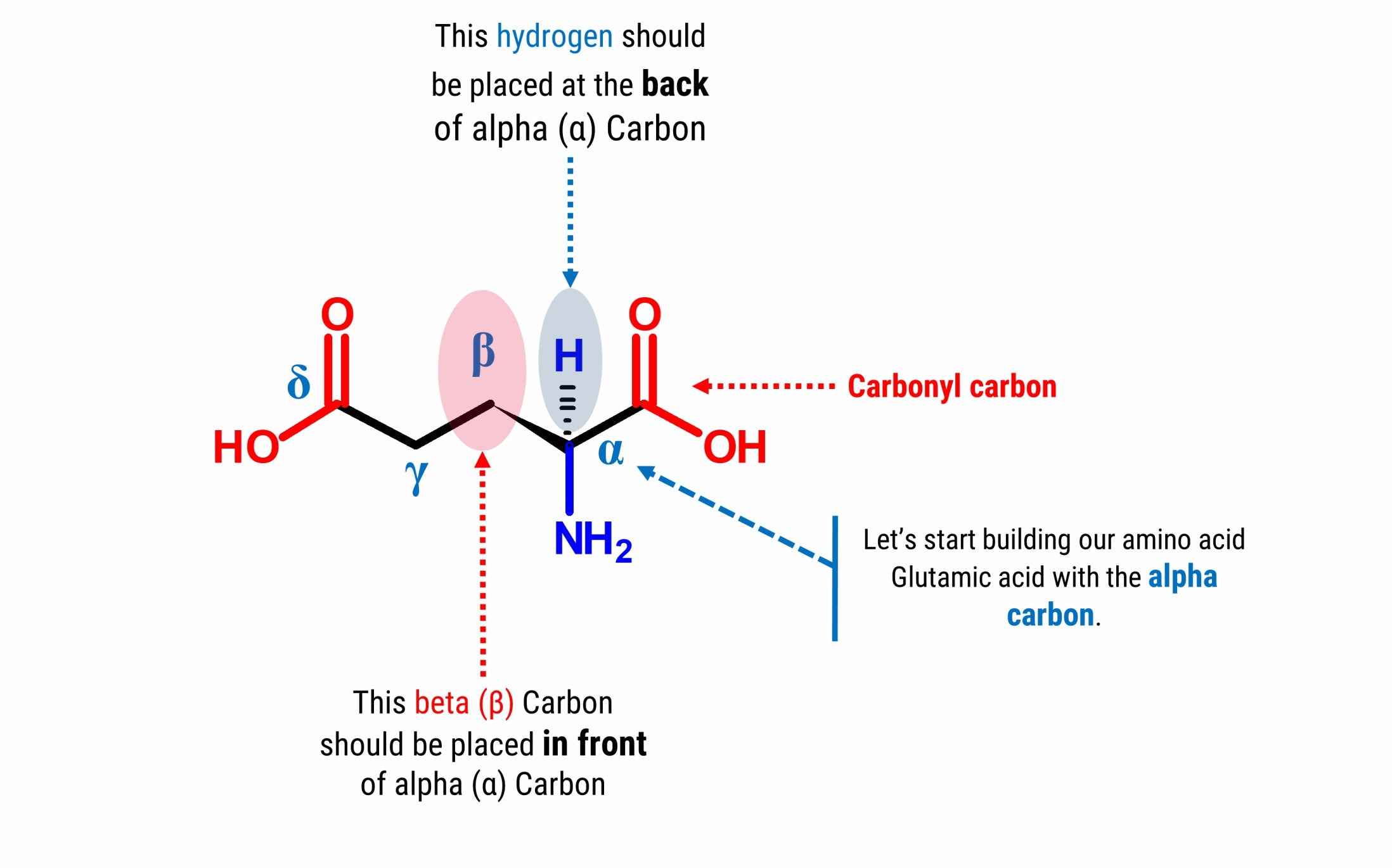
Note: We will build the skeleton portion of our amino acid starting with our chiral carbon(α Carbon).
Steps:
-
1

1. Get one carbon atom (α Carbon)then, place one hydrogen atom at the back side using one small connector.
-
2

2. Then, get another carbon atom (β Carbon)then place this in front of α Carbon using 1 medium connector. Add 2 hydrogen atoms on β Carbon using 2 small connectors.
-
3

3. Attach another carbon (Carbonyl Carbon)on α Carbon using 1 medium connector.
-
4
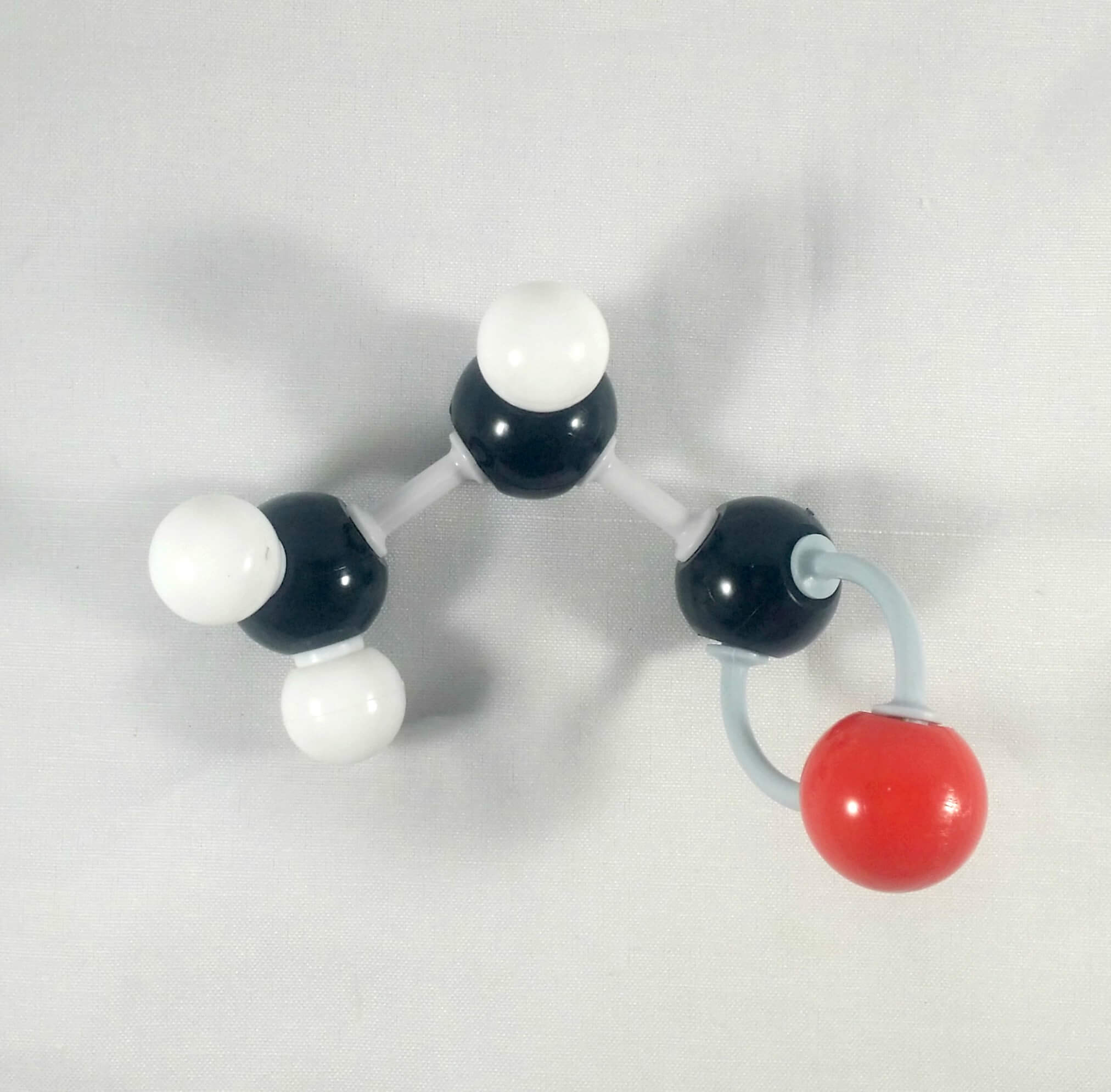
4. Get an Oxygen atom and attach this to the Carbonyl Carbonusing 2 long connectors.
-
5
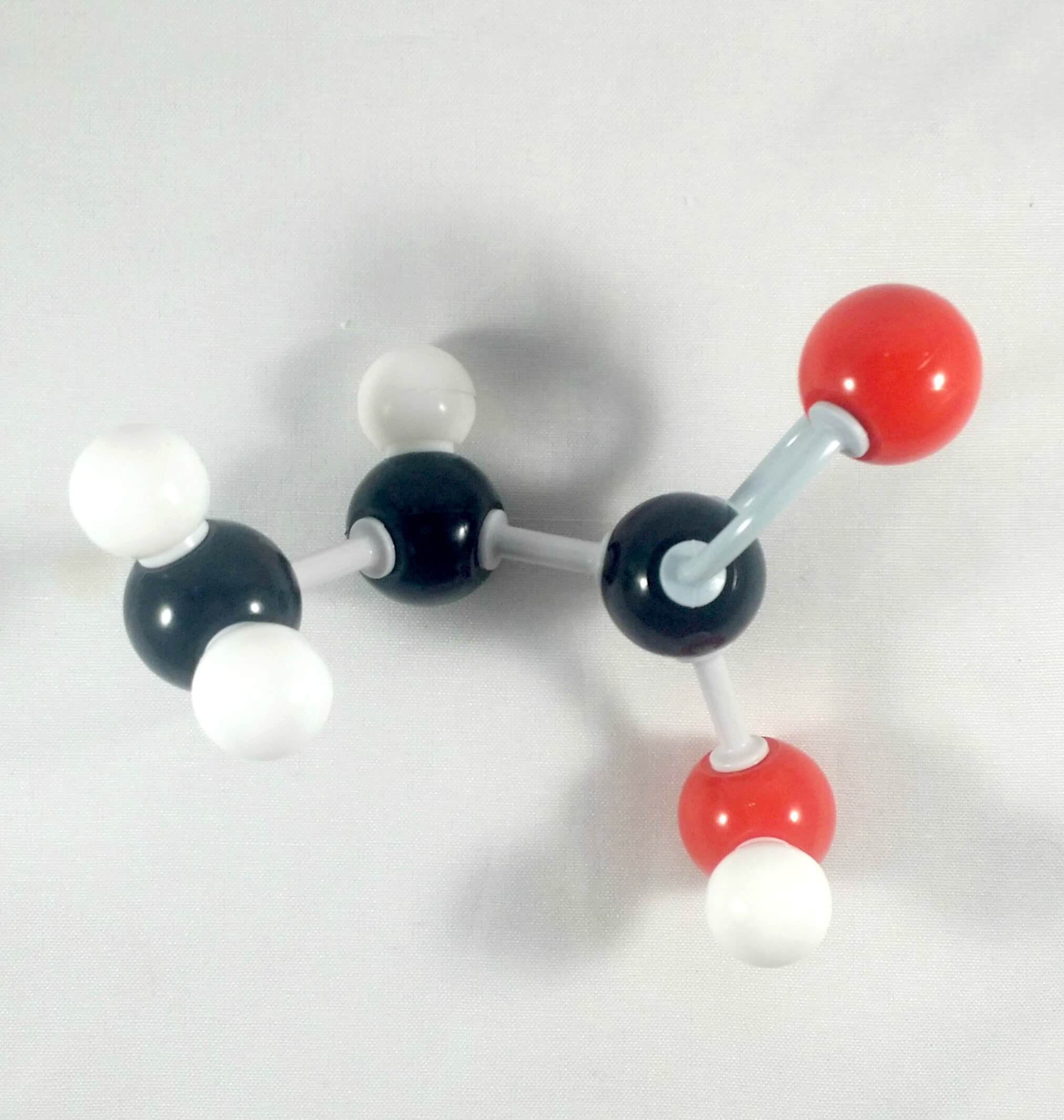
5. Get another Oxygen atom then attach this to the Carbonyl Carbon using a medium connector. Place a 1 hydrogen atom on this oxygen using one small connector
-
6
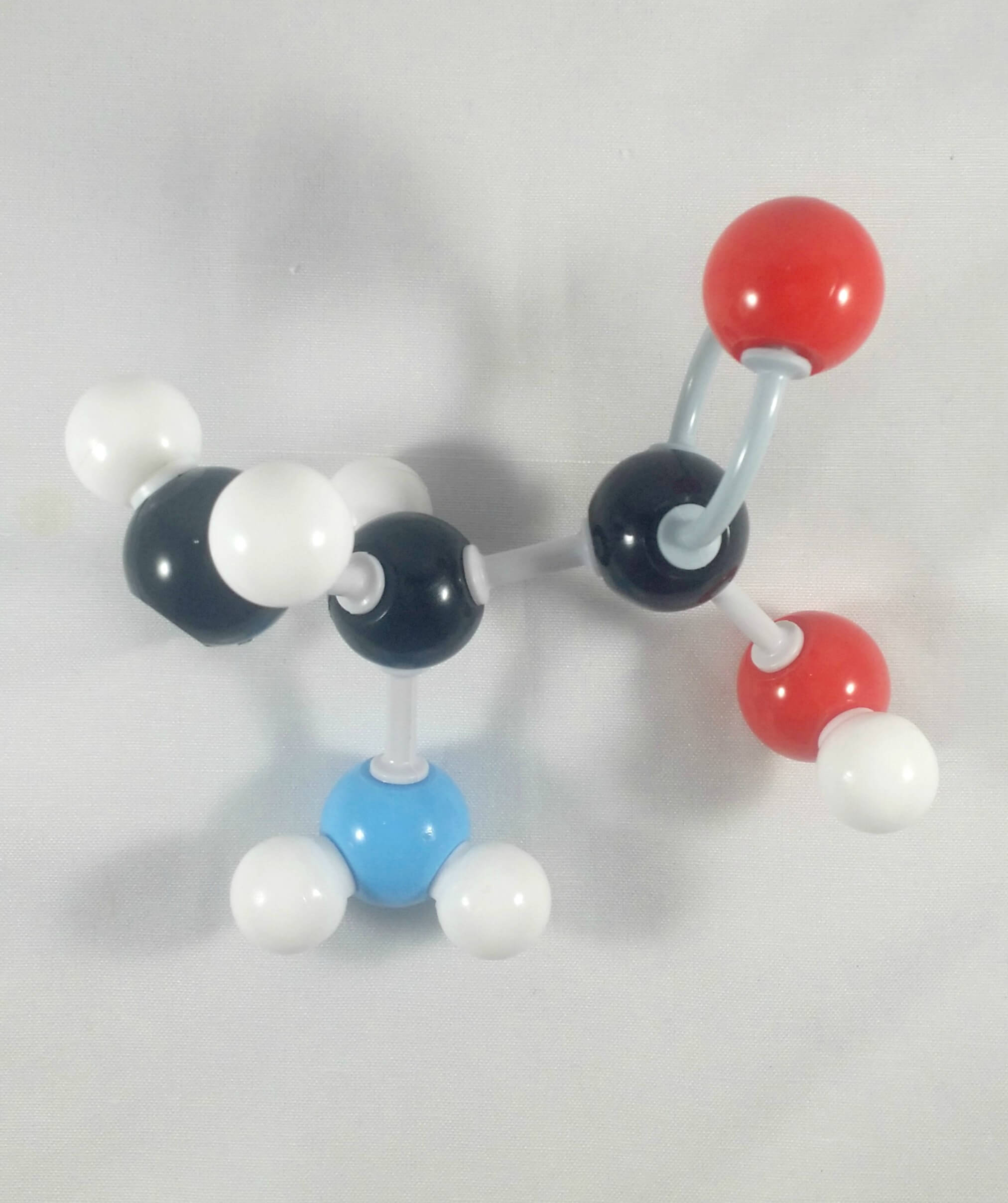
6. Then, get your Nitrogen atom and attach this to the α Carbon using one medium connector. Place 2 hydrogen atoms on this Nitrogen using 2 small connectors.
-

Yay! We've just built our amino acid skeleton!
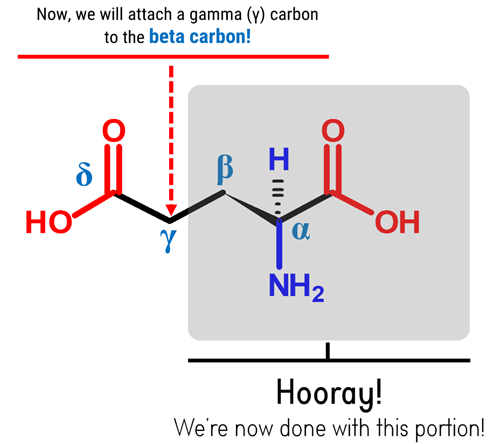
Note: Let’s now attach the side chain containing the carboxyl groupat the beta (β) carbon – starting off with the Gamma (γ) Carbon.
-
1

1. Get one Carbon atom (Gamma or γ Carbon)then attach this to the beta (β) carbon using 1 medium connector. Add 2 hydrogen atoms to the gamma carbon using 2 small connectors.
-
2
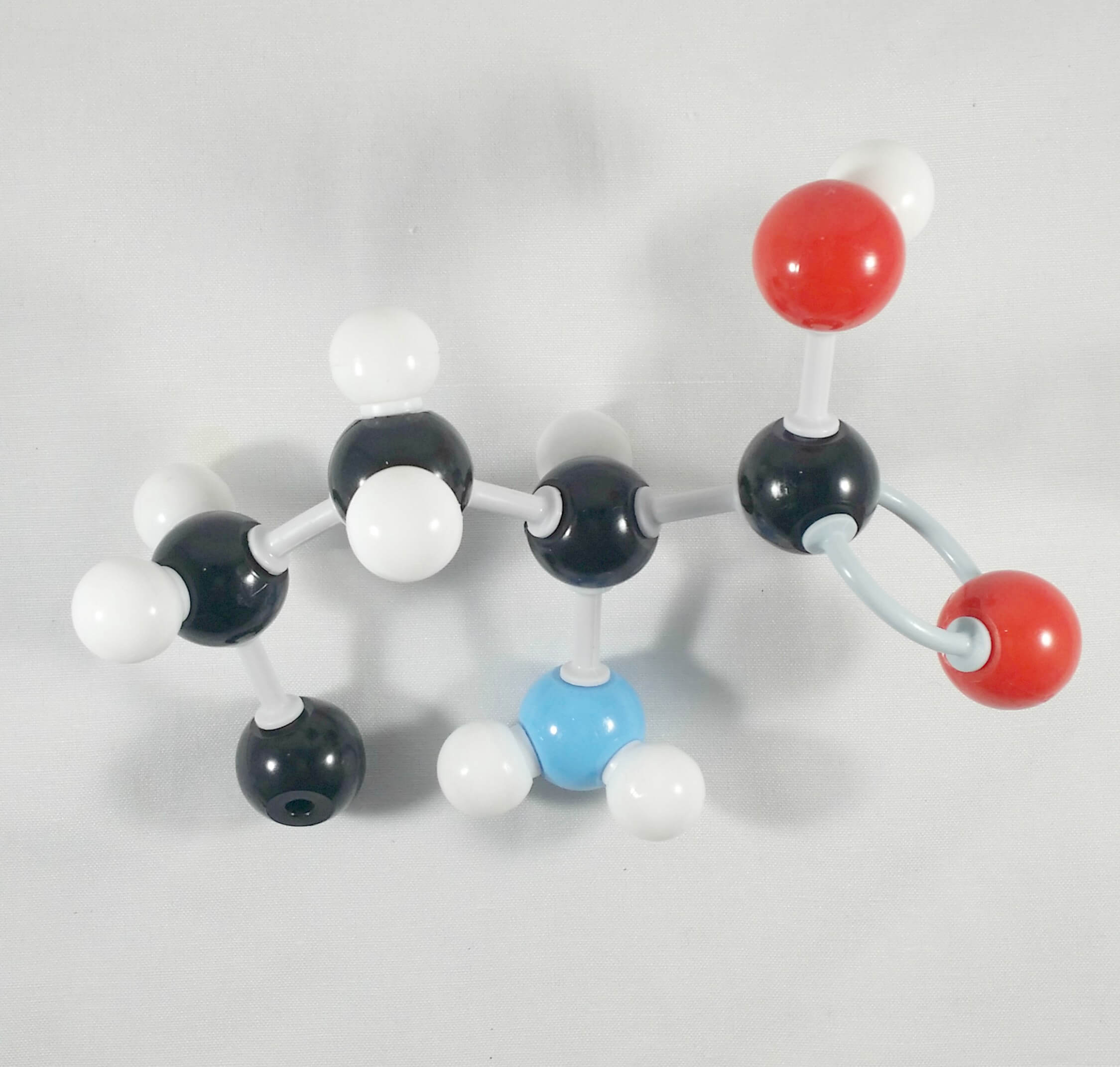
2. Attach another carbon atom (Delta or δ Carbon)to the Gamma (γ) carbon using a medium connector.
-
3
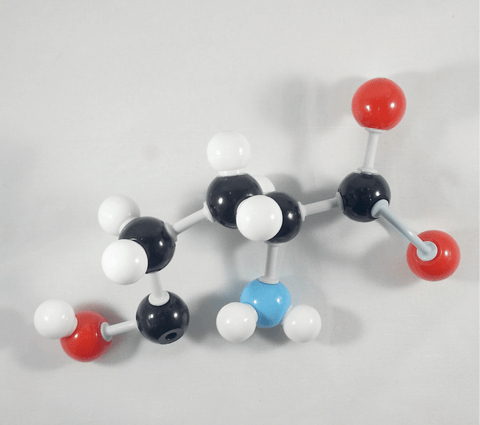
3. Get your Oxygen atom and attach this to the delta(or δ) carbon using 1 medium connector. Place a 1 hydrogen atom on this oxygen using one small connector.
-
4
4. Get another Oxygen atom then attach this again to the delta(or δ) carbon using 2 long connectors.
-

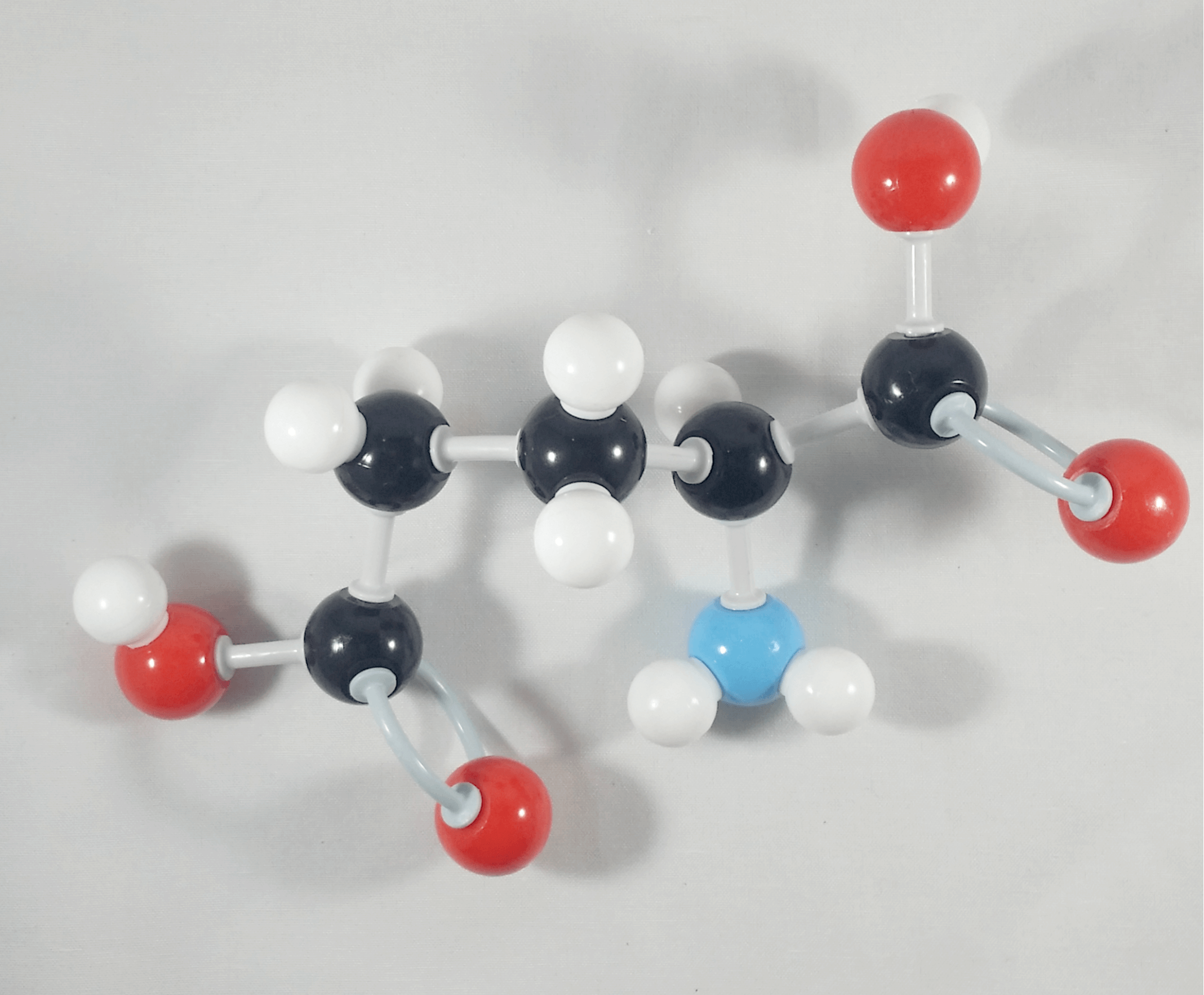
Hooray! We now have our L-Glutamic acid molecule!
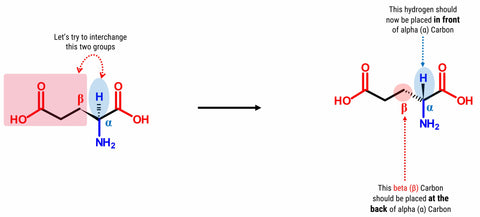
Now, try this! Let’s build another Glutamic Acid molecule by following the steps outlined above. Then let’s try to interchange the Hydrogen attached to the alpha (α) carbon and the beta (β) Carbon containing the carboxylic acid functional group.
-
1

1. Build another glutamic acid molecule following the steps outlined above.
-
2
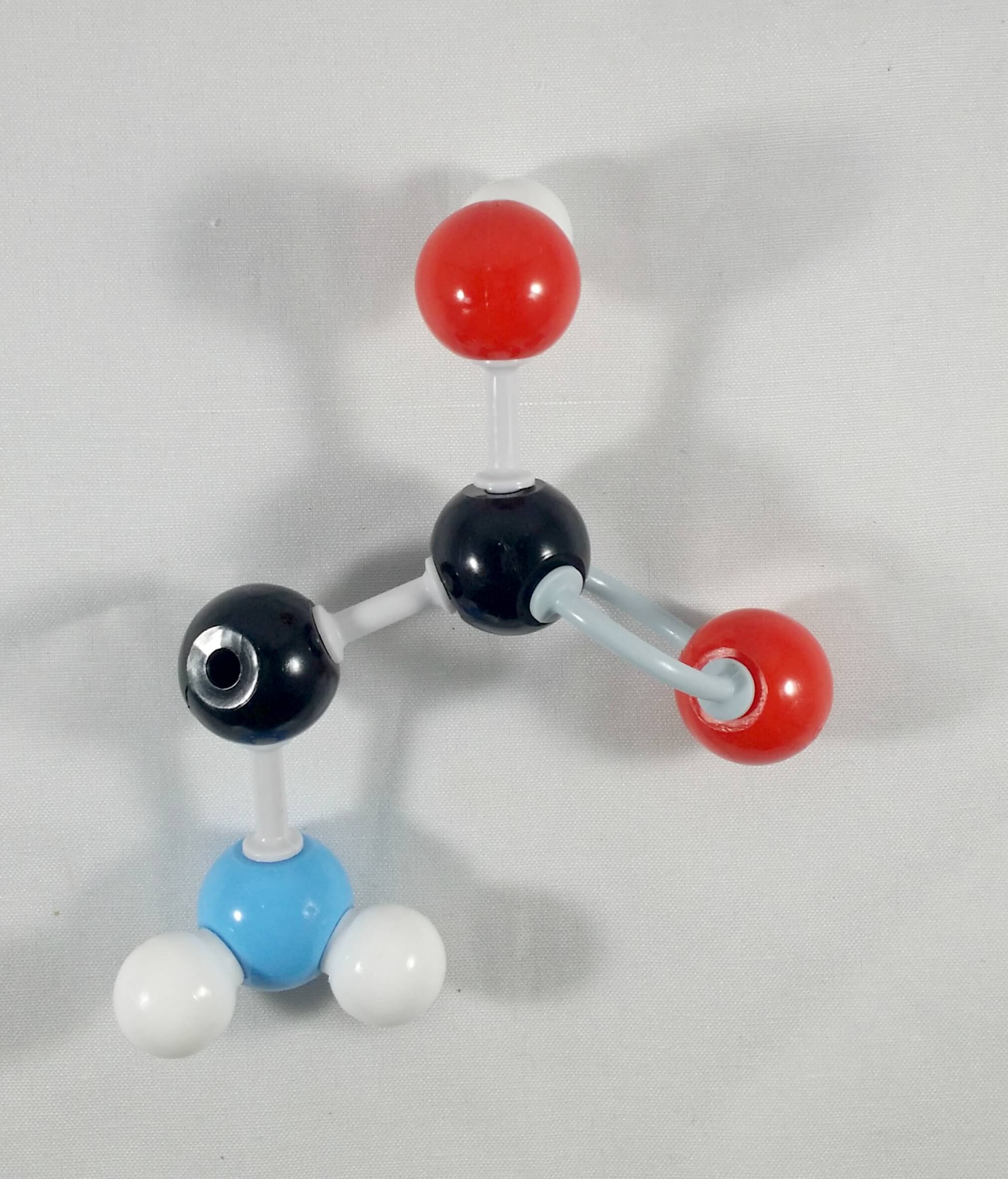
2. Detach the hydrogen atom and the beta (β) carbon containing the carboxyl side chain.
-
3

3. Place the hydrogen atom in front of the alpha (α) carbon.
-
4
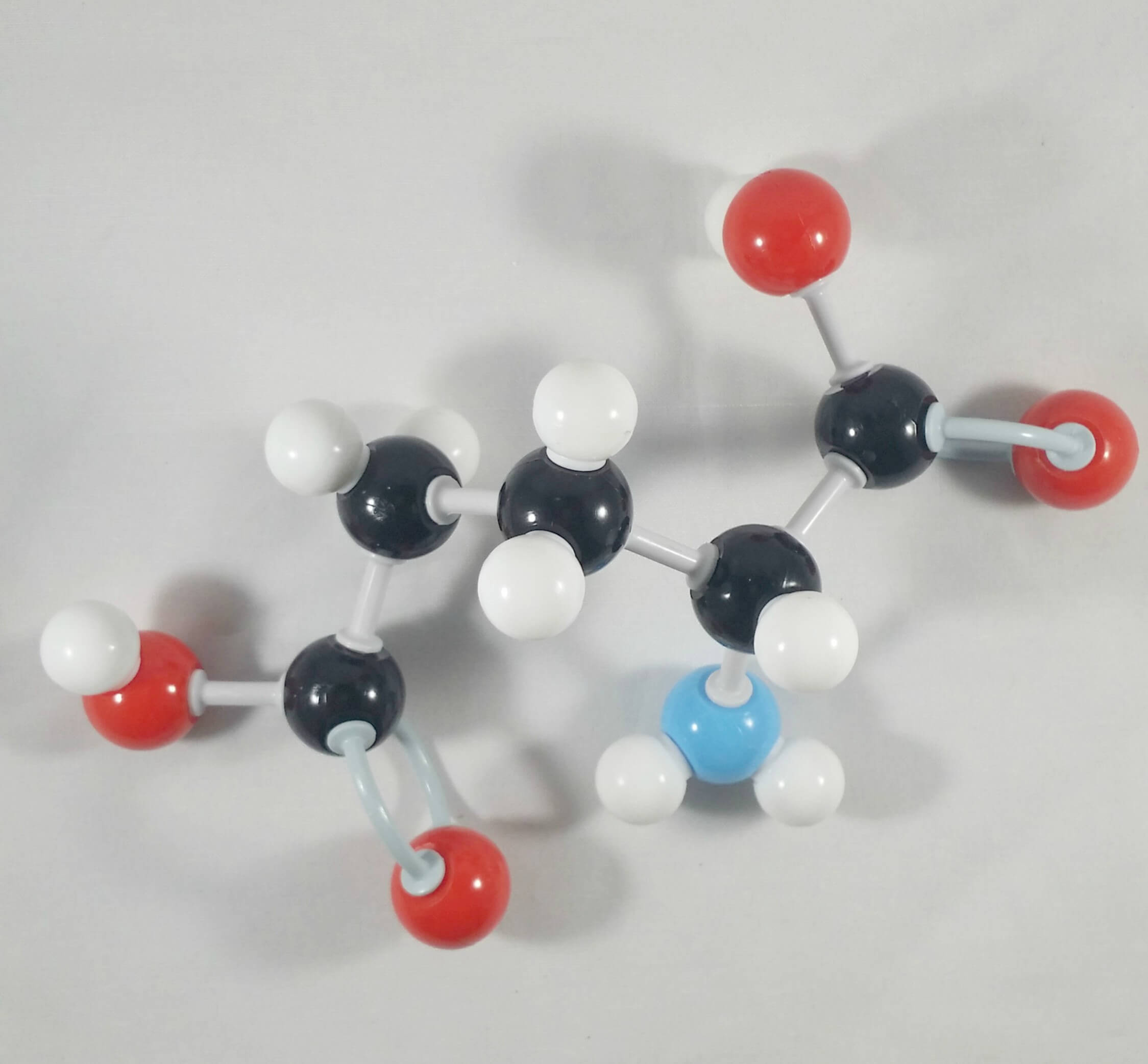
4. Then, attach the beta (β) carbon with the carboxylic acid functional group at the back side of alpha (α) carbon.
There we go! We now have 2 molecules of Glutamic Acid! See how these molecules seem to mirror each other!

L –Glutamic Acid

D –Glutamic Acid




















해외배팅사이트 추천 https://spo337.com/
해외스포츠배팅사이트 추천 https://spo337.com/
해외배팅사이트에이전시 추천 https://spo337.com/
머니라인247 https://spo337.com/ml247/
황룡카지노 https://spo337.com/gdcasino/
아시안커넥트 https://spo337.com/asianconnect/
스보벳 https://spo337.com/sbobet/
피나클 https://spo337.com/pinbet88/
맥스벳 https://spo337.com/maxbet/
파워볼 https://spo337.com/category/powerball/
BTI Sports https://spo337.com/btisports/
https://rb.gy/4y0o7
https://sites.google.com/view/spo337/
https://bit.ly/45dAsE6
https://cutt.ly/dwbyj5Dq
https://ln.run/Cl2oj
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/iv97s
https://cutt.ly/2wbyki06
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/
https://ln.run/VribE
https://sites.google.com/view/hdcasino/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/3vh3j
https://cutt.ly/Cwbykk2t
https://sites.google.com/view/hdcasino/
https://ln.run/6Bw_0
https://sites.google.com/view/moneyline247/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/03f4u
https://sites.google.com/view/moneyline247/
https://duckduckgo.com/?q=https%3A%2F%2Fspo337.com&t=h_&ia=web
mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
materinstvo.ru/forward?link=http%3A%2F%2Fspo337.com%2F
http://computer-chess.org/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
https://enchantedcottageshop.com/shop/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
https://buist-keatch.org/sphider/include/click_counter.php?url=http%3A%2F%2Fspo337.com%2F
www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
rel.chubu-gu.ac.jp/soumokuji/cgi-bin/go.cgi?http%3A%2F%2Fspo337.com%2F
fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
www.veloxbox.us/link/?h=http%3A%2F%2Fspo337.com%2F
www.adult-plus.com/ys/rank.php?mode=link&id=592&url=http%3A%2F%2Fspo337.com%2F
mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
sparktime.justclick.ru/lms/api-login/?hash=MO18szcRUQdzpT%2FrstSCW5K8Gz6ts1NvTJLVa34vf1A%3D&authBhvr=1&email=videotrend24%40mail.ru&expire=1585462818&lms%5BrememberMe%5D=1&targetPath=http%3A%2F%2Fspo337.com%2F
http://vcteens.com/cgi-bin/at3/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.bquest.org/Links/Redirect.aspx?ID=164&url=http%3A%2F%2Fspo337.com%2F
www.stipendije.info/phpAdsNew/adclick.php?bannerid=129&zoneid=1&source=&dest=http%3A%2F%2Fspo337.com%2F
today.od.ua/redirect.php?url=http%3A%2F%2Fspo337.com%2F
search.kcm.co.kr/jump.php?url=http%3A%2F%2Fspo337.com%2F
laskma.megastart-slot.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
www.mir-stalkera.ru/go?http%3A%2F%2Fspo337.com%2F
duhocphap.edu.vn/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.millerovo161.ru/go?http%3A%2F%2Fspo337.com%2F
www.naturaltranssexuals.com/cgi-bin/a2/out.cgi?id=97&l=toplist&u=http%3A%2F%2Fspo337.com%2F
https://amanaimages.com/lsgate/?lstid=pM6b0jdQgVM-Y9ibFgTe6Zv1N0oD2nYuMA&lsurl=http%3A%2F%2Fspo337.com%2F
planszowkiap.pl/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://covenantpeoplesministry.org/cpm/wp/sermons/?show&url=http%3A%2F%2Fspo337.com%2F
https://mightypeople.asia/link.php?id=M0ZGNHFISkd2bFh0RmlwSFU4bDN4QT09&destination=http%3A%2F%2Fspo337.com%2F
www.chinaleatheroid.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
i.s0580.cn/module/adsview/content/?action=click&bid=5&aid=163&url=http%3A%2F%2Fspo337.com%2F&variable=&source=https%3A%2F%2Fcutepix.info%2Fsex%2Friley-reyes.php
jsv3.recruitics.com/redirect?rx_cid=506&rx_jobId=39569207&rx_url=http%3A%2F%2Fspo337.com%2F
www.supermoto8.com/sidebanner/62?href=http%3A%2F%2Fspo337.com%2F
http://www.youtube.de/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.es/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ca/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.nl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ch/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.be/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.se/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.dk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.no/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.gr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.at/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.bg/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.sk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.rs/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.si/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.hr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ee/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lu/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.tn/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.ke/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.cr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.kz/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cat/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ge/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F&gl=ml
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=DE
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=IT
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AR
https://www.youtube.at/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ch/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.fr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.es/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.jp/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.co.uk/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ru/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.pl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.gr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.nl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ca/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.cz/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AU
https://www.youtube.com.tw/redirect?q=http%3A%2F%2Fspo337.com%2F
www.mexicolore.co.uk/click.php?url=http%3A%2F%2Fspo337.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=http%3A%2F%2Fspo337.com%2F
www.invisalign-doctor.in/api/redirect?url=http%3A%2F%2Fspo337.com%2F
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=http%3A%2F%2Fspo337.com%2F
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=http%3A%2F%2Fspo337.com%2F
trackingapp4.embluejet.com/Mod_Campaigns/tracking.asp?idem=31069343&em=larauz@untref.edu.ar&ca=73143&ci=0&me=72706&of=581028&adirecta=0&url=http%3A%2F%2Fspo337.com%2F
smils.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=http%3A%2F%2Fspo337.com/
cipresso.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.vacacionartravel.com/DTCSpot/public/banner_redirect.aspx?idca=286&ids=75665176&cp=167&idcli=0&ci=2&p=http%3A%2F%2Fspo337.com%2F
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=http%3A%2F%2Fspo337.com%2F
www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=http%3A%2F%2Fspo337.com%2F
www.cccowe.org/lang.php?lang=en&url=http%3A%2F%2Fspo337.com%2F
www.joeshouse.org/booking?link=http%3A%2F%2Fspo337.com%2F&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://okane-antena.com/redirect/index/fid100269/?u=http%3A%2F%2Fspo337.com%2F
www.gmwebsite.com/web/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
swra.backagent.net/ext/rdr/?http%3A%2F%2Fspo337.com%2F
www.mytokachi.jp/index.php?type=click&mode=sbm&code=2981&url=http%3A%2F%2Fspo337.com%2F
www.wqketang.com/logout?goto=http%3A%2F%2Fspo337.com%2F
access.bridges.com/externalRedirector.do?url=http%3A%2F%2Fspo337.com%2F
www.dolgin.net/zen_dolgin/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
bot.buymeapie.com/recipe?url=http%3A%2F%2Fspo337.com%2F
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=http%3A%2F%2Fspo337.com%2F
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
http://blackwhitepleasure.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.knet-web.net/m/pRedirect.php?uID=2&iID=259&iURL=http%3A%2F%2Fspo337.com%2F
azlan.techdata.com/InTouch/GUIBnrT3/BnrTrackerPublic.aspx?CountryCode=18&BannerLangCulture=nl-nl&URL=http%3A%2F%2Fspo337.com%2F&Target=2&BannerId=41919&Zoneid=281&Parameters=&cos=Azlan
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44__zoneid=1__cb=7743e8d201__oadest=http%3A%2F%2Fspo337.com%2F
www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=http%3A%2F%2Fspo337.com%2F
www.lzmfjj.com/Go.asp?URL=http%3A%2F%2Fspo337.com%2F
http://alexmovs.com/cgi-bin/atx/out.cgi?id=148&tag=topatx&trade=http%3A%2F%2Fspo337.com%2F
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2__bannerid=40__zoneid=16__cb=fc1d72225c__oadest=http%3A%2F%2Fspo337.com%2F
psylive.ru/success.aspx?id=0&goto=spo337.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=http%3A%2F%2Fspo337.com%2F
www.mybunnies.net/te3/out.php?u=http%3A%2F%2Fspo337.com%2F
lubaczowskie.pl/rdir/?l=http%3A%2F%2Fspo337.com%2F&lid=1315
www.kowaisite.com/bin/out.cgi?id=kyouhuna&url=http%3A%2F%2Fspo337.com%2F
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=http%3A%2F%2Fspo337.com%2F
www.wave24.net/cgi-bin/linkrank/out.cgi?id=106248&cg=1&url=spo337.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
zh-hk.guitarians.com/home/redirect/ubid/1015?r=http%3A%2F%2Fspo337.com%2F
www.cumshoter.com/cgi-bin/at3/out.cgi?id=98&tag=top&trade=http%3A%2F%2Fspo337.com%2F
shp.hu/hpc_uj/click.php?ml=5&url=http%3A%2F%2Fspo337.com%2F
lrnews.ru/xgo.php?url=http%3A%2F%2Fspo337.com%2F
www.dive-international.net/places/redirect.php?b=797&web=www.http%3A%2F%2Fspo337.com%2F
www.themza.com/redirect.php?r=http%3A%2F%2Fspo337.com%2F
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://http%3A%2F%2Fspo337.com%2F
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=http%3A%2F%2Fspo337.com%2F
spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
forest.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
reefcentral.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=http%3A%2F%2Fspo337.com%2F
https://trackdaytoday.com/redirect-out?url=http%3A%2F%2Fspo337.com%2F
https://bigjobslittlejobs.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=4__zoneid=4__cb=68dbdae1d1__oadest=http%3A%2F%2Fspo337.com%2F
www.obertaeva.com/include/get.php?go=http%3A%2F%2Fspo337.com%2F
www.quanmama.com/t/goto.aspx?url=http%3A%2F%2Fspo337.com%2F
quartiernetz-friesenberg.ch/links-go.php?to=http%3A%2F%2Fspo337.com%2F
http://anorexicpornmovies.com/cgi-bin/atc/out.cgi?id=20&u=http%3A%2F%2Fspo337.com%2F
www.maultalk.com/url.php?to=http%3A%2F%2Fspo337.com%2F
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=http%3A%2F%2Fspo337.com%2F
www.e-expo.net/category/click_url.html?url=http%3A%2F%2Fspo337.com%2F
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=http%3A%2F%2Fspo337.com%2F
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=http%3A%2F%2Fspo337.com%2F
http://getdatasheet.com/url.php?url=http%3A%2F%2Fspo337.com%2F
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=http%3A%2F%2Fspo337.com%2F
www.aldolarcher.com/tools/esstat/esdown.asp?File=http%3A%2F%2Fspo337.com%2F
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&no_chat=1&domain=http%3A%2F%2Fspo337.com%2F
cs.payeasy.com.tw/click?url=http%3A%2F%2Fspo337.com%2F
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=http%3A%2F%2Fspo337.com%2F
http://tswzjs.com/go.asp?url=http%3A%2F%2Fspo337.com%2F
asstomouth.guru/out.php?url=http%3A%2F%2Fspo337.com%2F
advtest.exibart.com/adv/adv.php?id_banner=7201&link=http%3A%2F%2Fspo337.com%2F
https://thairesidents.com/l.php?b=85&p=2,5&l=http%3A%2F%2Fspo337.com%2F
www.latestnigeriannews.com/link_channel.php?channel=http%3A%2F%2Fspo337.com%2F
www.haogaoyao.com/proad/default.aspx?url=http%3A%2F%2Fspo337.com%2F
globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
citysafari.nl/Home/setCulture?language=en&returnUrl=http%3A%2F%2Fspo337.com%2F
es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
lorena-kuhni.kz/redirect?link=http%3A%2F%2Fspo337.com%2F
www.webshoptrustmark.fr/Change/en?returnUrl=http%3A%2F%2Fspo337.com%2F
https://processon.com/setting/locale?language=zh&back=http%3A%2F%2Fspo337.com%2F
c.yam.com/srh/wsh/r.c?http%3A%2F%2Fspo337.com%2F
www.jolletorget.no/J/l.php?l=http%3A%2F%2Fspo337.com%2F
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=http%3A%2F%2Fspo337.com%2F
www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
www.sparetimeteaching.dk/forward.php?link=http%3A%2F%2Fspo337.com%2F
https://paspn.net/default.asp?p=90&gmaction=40&linkid=52&linkurl=http%3A%2F%2Fspo337.com%2F
www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
www.poddebiczak.pl/?action=set-desktop&url=http%3A%2F%2Fspo337.com%2F
ant53.ru/file/link.php?url=http%3A%2F%2Fspo337.com%2F
www.docin.com/jsp_cn/mobile/tip/android_v1.jsp?forward=http%3A%2F%2Fspo337.com%2F
old.magictower.ru/cgi-bin/redir/redir.pl?http%3A%2F%2Fspo337.com%2F
go.flx1.com/click?id=1&m=11&pl=113&dmcm=16782&euid=16603484876&out=http%3A%2F%2Fspo337.com%2F
moba-hgh.de/link2http.php?href=http%3A%2F%2Fspo337.com%2F
https://gazetablic.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=34__zoneid=26__cb=0e0dfef92b__oadest=http%3A%2F%2Fspo337.com%2F
watchvideo.co/go.php?url=http%3A%2F%2Fspo337.com%2F
www.todoku.info/gpt/rank.cgi?mode=link&id=29649&url=http%3A%2F%2Fspo337.com%2F
www.fotochki.com/redirect.php?go=http%3A%2F%2Fspo337.com%2F
bayerwald.tips/plugins/bannerverwaltung/bannerredirect.php?bannerid=1&url=http%3A%2F%2Fspo337.com%2F
ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=http%3A%2F%2Fspo337.com%2F
www.widgetinfo.net/read.php?sym=FRA_LM&url=http%3A%2F%2Fspo337.com%2F
arctic.nyheter24.se/rdb/nyheter24_eed6ad4b451f2fb8193922f832bc91ed/5?url=http%3A%2F%2Fspo337.com%2F
www.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
school.mosreg.ru/soc/moderation/abuse.aspx?link=http%3A%2F%2Fspo337.com%2F
affiliates.kanojotoys.com/affiliate/scripts/click.php?a_aid=widdi77&desturl=http%3A%2F%2Fspo337.com%2F
www.gotomypctech.com/affiliates/scripts/click.php?a_aid=ed983915&a_bid=&desturl=http%3A%2F%2Fspo337.com%2F
www.my-sms.ru/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F&rel=external
www.genderpsychology.com/http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
www.360wichita.com/amp-banner-tracking?adid=192059&url=http%3A%2F%2Fspo337.com%2F
www.tsv-bad-blankenburg.de/cms/page/mod/url/url.php?eid=16&urlpf=http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://zerlong.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
catalog.flexcom.ru/go?z=36047&i=55&u=http%3A%2F%2Fspo337.com%2F
www.wellvit.nl/response/forward/c1e41491e30c5af3c20f80a2af44e440.php?link=0&target=http%3A%2F%2Fspo337.com%2F
www.ident.de/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=76__zoneid=2__cb=8a18c95a9e__oadest=http%3A%2F%2Fspo337.com%2F
www.fertilab.net/background_manager.aspx?ajxName=link_banner&id_banner=50&url=http%3A%2F%2Fspo337.com%2F
shop-uk.fmworld.com/Queue/Index?url=http%3A%2F%2Fspo337.com%2F
www.weddinginlove.com/redirect/?url=http%3A%2F%2Fspo337.com%2F
nieuws.rvent.nl/bitmailer/statistics/mailstatclick/42261?link=http%3A%2F%2Fspo337.com%2F
https://jobanticipation.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobanticipation.com
www.xsbaseball.com/tracker/index.html?t=ad&pool_id=3&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
eos.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
b.sm.su/click.php?bannerid=56&zoneid=10&source=&dest=http%3A%2F%2Fspo337.com%2F
https://bethlehem-alive.com/abnrs/countguideclicks.cfm?targeturl=http%3A%2F%2Fspo337.com%2F&businessid=29579
www.bookmark-favoriten.com/?goto=http%3A%2F%2Fspo337.com%2F
shop.yuliyababich.eu/RU/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
www.depmode.com/go.php?http%3A%2F%2Fspo337.com%2F
https://urgankardesler.com/anasayfa/yonlen?link=http%3A%2F%2Fspo337.com%2F
www.metalindex.ru/netcat/modules/redir/?&site=http%3A%2F%2Fspo337.com%2F
www.rprofi.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://darudar.org/external/?link=http%3A%2F%2Fspo337.com%2F
www.postsabuy.com/autopost4/page/generate/?link=http%3A%2F%2Fspo337.com%2F&list=PL9d7lAncfCDSkF4UPyhzO59Uh8cOoD-8q&fb_node=942812362464093&picture&name=%E0%B9%82%E0%B8%9B%E0%B8%A3%E0%B9%81%E0%B8%81%E0%B8%A3%E0%B8%A1%E0%B9%82%E0%B8%9E%E0%B8%AA%E0%B8%82%E0%B8%B2%E0%B8%A2%E0%B8%AA%E0%B8%B4%E0%B8%99%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%AD%E0%B8%AD%E0%B8%99%E0%B9%84%E0%B8%A5%E0%B8%99%E0%B9%8C+&caption=%E0%B9%80%E0%B8%A5%E0%B8%82%E0%B8%B2%E0%B8%AA%E0%B9%88%E0%B8%A7%E0%B8%99%E0%B8%95%E0%B8%B1%E0%B8%A7+%E0%B8%97%E0%B8%B5%E0%B8%84%E0%B8%B8%E0%B8%93%E0%B8%A5%E0%B8%B7%E0%B8%A1%E0%B9%84%E0%B8%A1%E0%B9%88%E0%B8%A5%E0%B8%87+Line+%40postsabuy&description=%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%B2%E0%B8%96%E0%B8%B9%E0%B8%81%E0%B8%97%E0%B8%B5%E0%B9%88%E0%B8%AA%E0%B8%B8%E0%B8%94%E0%B9%83%E0%B8%99+3+%E0%B9%82%E0%B8%A5%E0%B8%81+%E0%B8%AD%E0%B8%B4%E0%B8%AD%E0%B8%B4
www.perimeter.org/track.pdf?url=http%3A%2F%2Fspo337.com%2F
forum.darievna.ru/go.php?http%3A%2F%2Fspo337.com%2F
techlab.rarus.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.spiritualforums.com/vb/redir.php?link=http%3A%2F%2Fspo337.com%2F
www.review-mag.com/cdn/www/delivery/view.php?ct=1&oaparams=2__bannerid=268__zoneid=1__cb=8c1317f219__oadest=http%3A%2F%2Fspo337.com%2F
belantara.or.id/lang/s/ID?url=http%3A%2F%2Fspo337.com%2F
ele-market.ru/consumer.php?url=http%3A%2F%2Fspo337.com%2F
https://www.хорошие-сайты.рф/r.php?r=http%3A%2F%2Fspo337.com%2F
https://jobsflagger.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://gpoltava.com/away/?go=http%3A%2F%2Fspo337.com%2F
www.cardexchange.com/index.php/tools/packages/tony_mailing_list/services/?mode=link&mlm=62&mlu=0&u=2&url=http%3A%2F%2Fspo337.com%2F
services.nfpa.org/Authentication/GetSSOSession.aspx?return=http%3A%2F%2Fspo337.com%2F
http://spaceup.org/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
yarko-zhivi.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
365sekretov.ru/redirect.php?action=url&goto=http%3A%2F%2Fspo337.com%2F%20
www.sgdrivingtest.com/redirect.php?page=http%3A%2F%2Fspo337.com%2F
www.jxren.com/news/link/link.asp?id=7&url=http%3A%2F%2Fspo337.com%2F
particularcareers.co.uk/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://bsaonline.com/MunicipalDirectory/SelectUnit?unitId=411&returnUrl=http%3A%2F%2Fspo337.com%2F&sitetransition=true
www.elit-apartament.ru/go?http%3A%2F%2Fspo337.com%2F
sendai.japansf.net/rank.cgi?mode=link&id=1216&url=http%3A%2F%2Fspo337.com%2F
www.bmwfanatics.ru/goto.php?l=http%3A%2F%2Fspo337.com%2F
www.saabsportugal.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
cms.sive.it/Jump.aspx?gotourl=http%3A%2F%2Fspo337.com%2F
largusladaclub.ru/go/url=https:/http%3A%2F%2Fspo337.com%2F
https://kekeeimpex.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=GBP&cRate=77.86247
mycounter.com.ua/go.php?http%3A%2F%2Fspo337.com%2F
l2base.su/go?http%3A%2F%2Fspo337.com%2F
https://freeseotool.org/url/?q=http%3A%2F%2Fspo337.com%2F
www.duomodicagliari.it/reg_link.php?link_ext=http%3A%2F%2Fspo337.com%2F&prov=1
assine.hostnet.com.br/cadastro/?rep=17&url=http%3A%2F%2Fspo337.com%2F
www.dvnlp.de/profile/gruppe/redirect/5?url=http%3A%2F%2Fspo337.com%2F
http://hotmaturegirlfriends.com/cl.php?porno=MzB4MzB4NjEw&url=http%3A%2F%2Fspo337.com%2F
www.smkn5pontianak.sch.id/redirect/?alamat=http%3A%2F%2Fspo337.com%2F
www.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
bilometro.brksedu.com.br/tracking?url=http%3A%2F%2Fspo337.com%2F&zorigem=hotsite-blackfriday
www.omschweiz.ch/select-your-country?publicUrl=http%3A%2F%2Fspo337.com%2F
https://anacolle.net/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.cubamusic.com/Home/ChangeLanguage?lang=es-ES&returnUrl=http%3A%2F%2Fspo337.com%2F
expoclub.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.deypenburgschecourant.nl/reklame/www/delivery/ck.php?oaparams=2__bannerid=44__zoneid=11__cb=078c2a52ea__oadest=http%3A%2F%2Fspo337.com%2F
tramplintk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
cnc.extranet.gencat.cat/treball_cnc/AppJava/FileDownload.do?pdf=http%3A%2F%2Fspo337.com%2F&codi_cnv=9998045
www.gamecollections.co.uk/search/redirect.php?retailer=127&deeplink=http%3A%2F%2Fspo337.com%2F
bearcong.no1.sexy/hobby-delicious/rank.cgi?mode=link&id=19&url=http%3A%2F%2Fspo337.com%2F
dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
www.surinenglish.com/backend/conectar.php?url=http%3A%2F%2Fspo337.com%2F
www.reference-cannabis.com/interface/sortie.php?adresse=http%3A%2F%2Fspo337.com%2F
login.0×69416d.co.uk/sso/logout?tenantId=tnl&gotoUrl=http%3A%2F%2Fspo337.com%2F&domain=0×69416d.co.uk
blog.link-usa.jp/emi?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://damki.net/go/?http%3A%2F%2Fspo337.com%2F
opensesame.wellymulia.zaxaa.com/b/66851136?s=1&redir=http%3A%2F%2Fspo337.com%2F
ism3.infinityprosports.com/ismdata/2009100601/std-sitebuilder/sites/200901/www/en/tracker/index.html?t=ad&pool_id=1&ad_id=112&url=http%3A%2F%2Fspo337.com%2F
apps.cancaonova.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=149__zoneid=20__cb=87d2c6208d__oadest=http%3A%2F%2Fspo337.com%2F
www.lespritjardin.be/?advp_click_bimage_id=19&url=http%3A%2F%2Fspo337.com%2F&shortcode_id=10
www.dresscircle-net.com/psr/rank.cgi?mode=link&id=14&url=http%3A%2F%2Fspo337.com%2F
www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
fms.csonlineschool.com.au/changecurrency/1?returnurl=http%3A%2F%2Fspo337.com%2F
www.v-archive.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.jagat.co.jp/analysis/analysis.php?url=http%3A%2F%2Fspo337.com%2F
https://vse-doski.com/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.karatetournaments.net/link7.asp?LRURL=http%3A%2F%2Fspo337.com%2F&LRTYP=O
simracing.su/go/?http%3A%2F%2Fspo337.com%2F
click.cheshi.com/go.php?proid=218&clickid=1393306648&url=http%3A%2F%2Fspo337.com%2F
fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
flypoet.toptenticketing.com/index.php?url=http%3A%2F%2Fspo337.com%2F
www.horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
d-click.fiemg.com.br/u/18081/131/75411/137_0/82cb7/?url=http%3A%2F%2Fspo337.com%2F
www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
www.packmage.net/uc/goto/?url=http%3A%2F%2Fspo337.com%2F
my.9991.com/login_for_index_0327.php?action=logout&forward=http%3A%2F%2Fspo337.com%2F
www.sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=986&redirect=http%3A%2F%2Fspo337.com%2F
www.naturum.co.jp/ad/linkshare/?siteID=p_L785d6UQY-V4Fh4Rxs7wNzOPgtzv95Tg&lsurl=http%3A%2F%2Fspo337.com%2F
www.d-e-a.eu/newsletter/redirect.php?link=http%3A%2F%2Fspo337.com%2F
www.bom.ai/goweburl?go=http%3A%2F%2Fspo337.com%2F
enews2.sfera.net/newsletter/redirect.php?id=sabricattani@gmail.com_0000006566_144&link=http%3A%2F%2Fspo337.com%2F
underwater.com.au/redirect_url/id/7509/?redirect=http%3A%2F%2Fspo337.com%2F
https://eqsoftwares.com/languages/setlanguage?languagesign=en&redirect=http%3A%2F%2Fspo337.com%2F
www.jagdambasarees.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=MYR&cRate=14.554
www.priegeltje.nl/gastenboek/go.php?url=http%3A%2F%2Fspo337.com%2F
www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.rz114.cn/url.html?url=http%3A%2F%2Fspo337.com%2F
www.greatdealsindia.com/redirects/infibeam.aspx?url=http%3A%2F%2Fspo337.com%2F
rs.345kei.net/rank.php?id=37&mode=link&url=http%3A%2F%2Fspo337.com%2F
webapp.jgz.la/?c=scene&a=link&id=8665466&url=http%3A%2F%2Fspo337.com%2F
www.erotiikkalelut.com/url.php?link=http%3A%2F%2Fspo337.com%2F
vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
https://fachowiec.com/zliczanie-bannera?id=24&url=http%3A%2F%2Fspo337.com%2F
ieea.ir/includes/change_lang.php?lang=en&goto=http%3A%2F%2Fspo337.com%2F
scribe.mmonline.io/click?evt_nm=Clicked+Registration+Completion&evt_typ=clickEmail&app_id=m4marry&eml_sub=Registration+Successful&usr_did=4348702&cpg_sc=NA&cpg_md=email&cpg_nm=&cpg_cnt=&cpg_tm=NA&link_txt=Live+Chat&em_type=Notification&url=http%3A%2F%2Fspo337.com%2F
polo-v1.feathr.co/v1/analytics/crumb?flvr=email_link_click&rdr=http%3A%2F%2Fspo337.com%2F
sintesi.provincia.mantova.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F
https://careerchivy.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
shinsekai.type.org/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
https://maned.com/scripts/lm/lm.php?tk=CQkJZWNuZXdzQGluZm90b2RheS5jb20JW05ld3NdIE1FSSBBbm5vdW5jZXMgUGFydG5lcnNoaXAgV2l0aCBUd2l4bCBNZWRpYQkxNjcyCVBSIE1lZGlhIENvbnRhY3RzCTI1OQljbGljawl5ZXMJbm8=&url=http%3A%2F%2Fspo337.com%2F
www.etaigou.com/turn2.php?ad_id=276&link=http%3A%2F%2Fspo337.com%2F
crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
www.tagirov.org/out.php?url=http%3A%2F%2Fspo337.com%2F
https://startlist.club/MSF/Language/Set?languageIsoCode=en&returnUrl=http%3A%2F%2Fspo337.com%2F
www.tetsumania.net/search/rank.cgi?mode=link&id=947&url=http%3A%2F%2Fspo337.com%2F
https://imperial-info.net/link?idp=125&url=http%3A%2F%2Fspo337.com%2F
www.brainlanguage-sa.com/setcookie.php?lang=en&file=http%3A%2F%2Fspo337.com%2F
www.asensetranslations.com/modules/babel/redirect.php?newlang=en_US&newurl=http%3A%2F%2Fspo337.com%2F
gameshock.jeez.jp/rank.cgi?mode=link&id=307&url=http%3A%2F%2Fspo337.com%2F
https://tripyar.com/go.php?http%3A%2F%2Fspo337.com%2F
www.floridafilmofficeinc.com/?goto=http%3A%2F%2Fspo337.com%2F
tsvc1.teachiworld.com/bin/checker?mode=4&module=11&mailidx=19130&dmidx=0&emidx=0&service=0&cidx=&etime=20120328060000&seqidx=3&objidx=22&encoding=0&url=http%3A%2F%2Fspo337.com%2F
rechner.atikon.at/lbg.at/newsletter/linktracking?subscriber=&delivery=38116&url=http%3A%2F%2Fspo337.com%2F
www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
go.eniro.dk/lg/ni/cat-2611/http:/http%3A%2F%2Fspo337.com%2F
www.fisherly.com/redirect?type=website&ref=listing_detail&url=http%3A%2F%2Fspo337.com%2F
bio-pack.ru/bitrix/redirect.php?goto=http://http%3A%2F%2Fspo337.com%2F
fid.com.ua/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.modernipanelak.cz/?b=618282165&redirect=http%3A%2F%2Fspo337.com%2F
h5.hbifeng.com/index.php?c=scene&a=link&id=14240604&url=http%3A%2F%2Fspo337.com%2F
www.bkdc.ru/bitrix/redirect.php?event1=news_out&event2=32reg.roszdravnadzor.ru/&event3=A0A0B5A09180D0%A09582A0BBA1A085%D0E2A084D0D1C2D0%A085+A0A0B5A182B0A0%C2D0D0D096+A1A0BBA0B180D0%A09795+A0A0B0A09582A1%D1D0D0D0A182B5+A0A091A08695A0%D1D0A6A185A0A085%D0D1D0D082A1A085%D0D0D1D0A095B1A0%C2D0D0D091&goto=http%3A%2F%2Fspo337.com%2F
record.affiliatelounge.com/WS-jvV39_rv4IdwksK4s0mNd7ZgqdRLk/7/?deeplink=http%3A%2F%2Fspo337.com%2F
d-click.artenaescola.org.br/u/3806/290/32826/1416_0/53052/?url=http%3A%2F%2Fspo337.com%2F
www.morroccoaffiliate.com/aff.php?id=883&url=http%3A%2F%2Fspo337.com%2F
www.flooble.com/cgi-bin/clicker.pl?id=grabbadl&url=http%3A%2F%2Fspo337.com%2F
www.sportsbook.ag/ctr/acctmgt/pl/openLink.ctr?ctrPage=http%3A%2F%2Fspo337.com%2F
www.ra2d.com/directory/redirect.asp?id=596&url=http%3A%2F%2Fspo337.com%2F
www.anorexicnudes.net/cgi-bin/atc/out.cgi?u=http%3A%2F%2Fspo337.com%2F
www.zjjiajiao.com.cn/ad/adredir.asp?url=http%3A%2F%2Fspo337.com%2F
https://hakobo.com/wp/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
t.agrantsem.com/tt.aspx?cus=216&eid=1&p=216-2-71016b553a1fa2c9.3b14d1d7ea8d5f86&d=http%3A%2F%2Fspo337.com%2F
moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.cheek.co.jp/location/location.php?id=keibaseminar&url=http%3A%2F%2Fspo337.com%2F
www.quantixtickets3.com/php-bin-8/kill_session_and_redirect.php?redirect=http%3A%2F%2Fspo337.com%2F
www.photokonkurs.com/cgi-bin/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.nbda.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.thislife.net/cgi-bin/webcams/out.cgi?id=playgirl&url=http%3A%2F%2Fspo337.com%2F
https://www.dans-web.nu/klick.php?url=http%3A%2F%2Fspo337.com%2F
https://voobrajulya.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.nafta-him.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.kyoto-osaka.com/search/rank.cgi?mode=link&id=9143&url=http%3A%2F%2Fspo337.com%2F
www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
red.ribbon.to/~zkcsearch/zkc-search/rank.cgi?mode=link&id=156&url=http%3A%2F%2Fspo337.com%2F
akademik.tkyd.org/Home/SetCulture?culture=en-US&returnUrl=http%3A%2F%2Fspo337.com%2F
www.rencai8.com/web/jump_to_ad_url.php?id=642&url=http%3A%2F%2Fspo337.com%2F
https://www.baby22.com.tw/Web/turn.php?ad_id=160&link=http%3A%2F%2Fspo337.com%2F
https://www.kichink.com/home/issafari?uri=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=1032500&url=http%3A%2F%2Fspo337.com%2F
5cfxm.hxrs6.servertrust.com/v/affiliate/setCookie.asp?catId=1180&return=http%3A%2F%2Fspo337.com%2F
http://3dcreature.com/cgi-bin/at3/out.cgi?id=187&trade=http%3A%2F%2Fspo337.com%2F
https://nowlifestyle.com/redir.php?k=9a4e080456dabe5eebc8863cde7b1b48&url=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
www.acutenet.co.jp/cgi-bin/lcount/lcounter.cgi?link=http%3A%2F%2Fspo337.com%2F
http://news-matome.com/method.php?method=1&url=http%3A%2F%2Fspo337.com%2F
https://lifecollection.top/site/gourl?url=http%3A%2F%2Fspo337.com%2F
https://www.toscanapiu.com/web/lang.php?lang=DEU&oldlang=ENG&url=http%3A%2F%2Fspo337.com%2F
speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.sainttropeztourisme.com/en/bannieres/redirection/index.html?id=649&lien=http%3A%2F%2Fspo337.com%2F
www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
www.interracialhall.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
https://www.tumimusic.com/link.php?url=http%3A%2F%2Fspo337.com%2F
www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
mail.resen.gov.mk/redir.hsp?url=http%3A%2F%2Fspo337.com%2F
https://www.pompengids.net/followlink.php?id=495&link=http%3A%2F%2Fspo337.com%2F
https://www.hirforras.net/scripts/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://lens-club.ru/link?go=http%3A%2F%2Fspo337.com%2F
https://t.raptorsmartadvisor.com/.lty?url=http%3A%2F%2Fspo337.com%2F&loyalty_id=14481&member_id=b01bbee6-4592-4345-a0ee-5d71ed6f1929
https://evoautoshop.com/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://beautycottageshop.com/change.php?lang=cn&url=http%3A%2F%2Fspo337.com%2F
http://1000love.net/lovelove/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.ffw-ellar.de/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://www.contactlenshouse.com/currency.asp?c=CAD&r=http%3A%2F%2Fspo337.com%2F
https://sogo.i2i.jp/link_go.php?url=http%3A%2F%2Fspo337.com%2F
https://ceb.bg/catalog/statistic/?id=61&location=http%3A%2F%2Fspo337.com%2F
https://malehealth.ie/redirect/?age=40&part=waist&illness=obesity&refer=http%3A%2F%2Fspo337.com%2F
https://magicode.me/affiliate/go?url=http%3A%2F%2Fspo337.com%2F
https://jobregistry.net/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobregistry.net&rgp_m=title13&et=4495
https://www.goatzz.com/adredirect.aspx?adType=SiteAd&ItemID=9595&ReturnURL=http%3A%2F%2Fspo337.com%2F
https://miyagi.lawyer-search.tv/details/linkchk.aspx?type=o&url=http%3A%2F%2Fspo337.com%2F
https://www.snwebcastcenter.com/event/page/count_download_time.php?url=http%3A%2F%2Fspo337.com%2F
https://go.onelink.me/v1xd?pid=Patch&c=Mobile%20Footer&af_web_dp=http%3A%2F%2Fspo337.com%2F
https://www.weather.net/cgi-bin/redir?http%3A%2F%2Fspo337.com%2F
https://gaggedtop.com/cgi-bin/top/out.cgi?ses=sBZFnVYGjF&id=206&url=http%3A%2F%2Fspo337.com%2F
https://e-bike-test.net/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://www.luckyplants.com/cgi-bin/toplist/out.cgi?id=rmontero&url=http%3A%2F%2Fspo337.com%2F
https://webankety.cz/dalsi.aspx?site=http%3A%2F%2Fspo337.com%2F
https://runkeeper.com/apps/authorize?redirect_uri=http%3A%2F%2Fspo337.com%2F
https://www.emiratesvoice.com/footer/comment_like_dislike_ajax/?code=like&commentid=127&redirect=http%3A%2F%2Fspo337.com%2F
https://envios.uces.edu.ar/control/click.mod.php?id_envio=8147&email=gramariani@gmail.com&url=http%3A%2F%2Fspo337.com%2F
https://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://www.ab-search.com/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.studyrama.be/tracking.php?origine=ficheform5683&lien=http://http%3A%2F%2Fspo337.com%2F
https://shop.merchtable.com/users/authorize?return_url=http%3A%2F%2Fspo337.com%2F
https://nudewwedivas.forumcommunity.net/m/ext.php?url=http%3A%2F%2Fspo337.com%2F
https://cztt.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://mientaynet.com/advclick.php?o=textlink&u=15&l=http%3A%2F%2Fspo337.com%2F
https://atlanticleague.com/tracker/index.html?t=ad&pool_id=11&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
https://www.podcastone.com/site/rd?satype=40&said=4&aaid=email&camid=-4999600036534929178&url=http%3A%2F%2Fspo337.com%2F
https://www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://monarchbeachmembers.play18.com/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
https://wayi.com.tw/wayi_center.aspx?flag=banner&url=http%3A%2F%2Fspo337.com%2F&idno=443
https://d.agkn.com/pixel/2389/?che=2979434297&col=22204979,1565515,238211572,435508400,111277757&l1=http%3A%2F%2Fspo337.com%2F
https://mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
https://bemidji.bigdealsmedia.net/include/sort.php?return_url=http%3A%2F%2Fspo337.com%2F&sort=a:3:{s:9:%E2%80%9Ddirection%E2%80%9D;s:3:%E2%80%9DASC%E2%80%9D;s:5:%E2%80%9Dfield%E2%80%9D;s:15:%E2%80%9DItems.PriceList%E2%80%9D;s:5:%E2%80%9Dlabel%E2%80%9D;s:9:%E2%80%9Dvalue_asc%E2%80%9D;}
https://hslda.org/content/a/LinkTracker.aspx?id=4015475&appeal=385&package=36&uri=http%3A%2F%2Fspo337.com%2F
https://panarmenian.net/eng/tofv?tourl=http%3A%2F%2Fspo337.com%2F
https://lavoro.provincia.como.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=935
https://www.dodeley.com/?action=show_ad&url=http%3A%2F%2Fspo337.com%2F
https://www.ignicaodigital.com.br/affiliate/?idev_id=270&u=http%3A%2F%2Fspo337.com%2F
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://m.wedkuje.pl/mobile/redirect.php?redir=http%3A%2F%2Fspo337.com%2F
https://area51.to/go/out.php?s=100&l=site&u=http%3A%2F%2Fspo337.com%2F
https://games4ever.3dn.ru/go?http%3A%2F%2Fspo337.com%2F
https://de.reasonable.shop/SetCurrency.aspx?currency=CNY&returnurl=http%3A%2F%2Fspo337.com%2F
https://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
https://mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.shop-bell.com/out.php?id=kibocase&category=ladies&url=http%3A%2F%2Fspo337.com%2F
https://m.addthis.com/live/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
https://horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
https://soom.cz/projects/get2mail/redir.php?id=c2e52da9ad&url=http%3A%2F%2Fspo337.com%2F
https://www.iaai.com/VehicleInspection/InspectionProvidersUrl?name=AA%20Transit%20Pros%20Inspection%20Service&url=http%3A%2F%2Fspo337.com%2F
https://cingjing.fun-taiwan.com/AdRedirector.aspx?padid=303&target=http%3A%2F%2Fspo337.com%2F
https://rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
https://campaign.unitwise.com/click?emid=31452&emsid=ee720b9f-a315-47ce-9552-fd5ee4c1c5fa&url=http%3A%2F%2Fspo337.com%2F
https://www.swipeclock.com/sc/cookie.asp?sitealias=79419397&redirect=http%3A%2F%2Fspo337.com%2F
https://www.gutscheinaffe.de/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://home.uceusa.com/Redirect.aspx?r=http%3A%2F%2Fspo337.com%2F
https://www.seankenney.com/include/jump.php?num=http%3A%2F%2Fspo337.com%2F
https://runningcheese.com/go?url=http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
https://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
https://crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
http://dstats.net/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://union.591.com.tw/stats/event/redirect?e=eyJpdiI6IjdUd1B5Z2FPTmNWQzBmZk1LblR2R0E9PSIsInZhbHVlIjoiQTI4TnVKMzdjMkxrUjcrSWlkcXdzbjRQeGRtZ0ZGbXdNSWxkSkVieENwNjQ1cHF5aDZmWmFobU92ZGVyUk5jRTlxVnI2TG5pb0dJVHZSUUlHcXFTbGo3UDliYWU5UE5MSjlMY0xOQnFmbVRQSFNoZDRGd2dqVDZXZEU4WFoyajJ0S0JITlQ2XC9SXC9jRklPekdmcnFGb09vRllqNHVtTHlYT284ZmN3d0ozOHFkclRYYnU5UlY2NTFXSGRheW5SbGxJb3BmYjQ2Mm9TWUFCTEJuXC9iT25nYkg4QXpOd2pHVlBWTWxWXC91aWRQMVhKQmVJXC9qMW9IdlZaVVlBdWlCYW4rS0JualhSMElFeVZYN3NnUW1qcUdxcWUrSlFROFhKbWttdkdvMUJ3aWVRa2I3MVV5TXpER3doa2ZuekFWNWd3OGpuQ1VSczFDREVKaklaUks0TTRIY2pUeXYrQmdZYUFTK1F4RWpTY0RRaW5Nc0krdVJ2N2VUT1wvSUxVVWVKN3hnQU92QmlCbjQyMUpRdTZKVWJcL0RCSVFOcWl0azl4V2pBazBHWmVhdWptZGREVXh0VkRNWWxkQmFSYXhBRmZtMHA5dTlxMzIzQzBVaWRKMEFqSG0wbGkxME01RDBaaElTaU5QKzIxbSswaUltS0FYSzViZlFmZjZ1XC9Yclg0U2VKdHFCc0pTNndcL09FWklUdjlQM2dcL2RuN0szQ3plWmcyYWdpQzJDQ2NIcWROczVua3dIM1Q3OXpJY3Z0XC93MVk3SHUyODZHU3Z5aHFVbWEwRFU1ZFdyMGt0YWpsb3BkQitsZER5aWk4YWMrZWYzSFNHNERhOGdDeUJWeEtoSm9wQ0hQb2EzOHZ3eHFGVTQ2Mk1QSEZERzlXZWxRRTJldjJkdnZUM0ZwaW1KcEVVc3ZXWjRHaTZWRDJOK0YxR3d4bXhMR3BhWmZBNkJ6eUYxQjR4ODVxc0d0YkFpYU8yZ2tuWGdzelBpU3dFUjJVYUVtYUlpZllSUTVpaHZMbjhySFp4VEpQR3EyYnRLTmdcLzRvKzQwRmtGNUdWWnQ0VjFpcTNPc0JubEdWenFiajRLRFg5a2dRZFJOZ1wvaUEwVHR3ZnYzakpYVmVtT294aFk1TXBUZ3FmVnF2dnNSVWJ5VEE0WGZpV3o3Y0k2SjJhM2RDK2hoQ0FvV2YrSW9QWnhuZG5QN1hlOEFaTVZrcFZ3c0pXVHhtNkRTUkpmenpibG8zdTM0cGF6Q3oxTEJsdDdiOUgwWXFOUkNHWjlEbTBFYzdIRUcyalYrcW4wYklFbnlGYlZJUG00R1VDQTZLZEVJRklIbFVNZFdpS3RkeCt5bVpTNUkrOXE3dDlxWmZ2bitjSGlSeE9wZTg5Yk9wS0V6N1wvd1EzUTNVenNtbjlvQUJhdGsxMzNkZTdjTU1LNkd4THJMYTBGUEJ4elEycFNTNGZabEJnalhJc0pYZit1c1wvWDBzSm1JMzRad3F3PT0iLCJtYWMiOiI4MjNhNDJlYTMwOTlmY2VlYzgxNmU1N2JiM2QzODk5YjI5MDFhYThhMDBkYzNhODljOTRmMTMzMzk0YTM5OGIzIn0=&source=BANNER.2913&url=http%3A%2F%2Fspo337.com%2F
https://fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
https://pdcn.co/e/http%3A%2F%2Fspo337.com%2F
https://api.webconnex.com/v1/postmaster/track/click/4f8036d14ee545798599c8921fbfcd22/db005310dba511e89fb606f49a4ee876?url=http%3A%2F%2Fspo337.com%2F
https://www.lecake.com/stat/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://www.im-harz.com/counter/counter.php?url=http%3A%2F%2Fspo337.com%2F
https://pzz.to/click?uid=8571&target_url=http%3A%2F%2Fspo337.com%2F
https://www.ricacorp.com/Ricapih09/redirect.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.tremblant.ca/Shared/LanguageSwitcher/ChangeCulture?culture=en&url=http%3A%2F%2Fspo337.com%2F
https://www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
https://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://igert2011.videohall.com/to_client?target=http%3A%2F%2Fspo337.com%2F
https://sete.gr/app_plugins/newsletterstudio/pages/tracking/trackclick.aspx?nid=218221206039243162226109144018149003034132019130&e=000220142174231130224127060133189018075115154134&url=http%3A%2F%2Fspo337.com%2F
https://ovatu.com/e/c?url=http%3A%2F%2Fspo337.com%2F
https://primepartners.globalprime.com/afs/wcome.php?c=427|0|1&e=GP204519&url=http%3A%2F%2Fspo337.com%2F
https://ltp.org/home/setlocale?locale=en&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.morhipo.com/shared/partnercookie?k=gort&url=http%3A%2F%2Fspo337.com%2F
https://www.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bg/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://maps.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://za.zalo.me/v3/verifyv2/pc?token=OcNsmjfpL0XY2F3BtHzNRs4A-hhQ5q5sPXtbk3O&continue=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://scanmail.trustwave.com/?c=8510&d=4qa02KqxZJadHuhFUvy7ZCUfI_2L10yeH0EeBz7FGQ&u=http%3A%2F%2Fspo337.com%2F
https://images.google.co.kr/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://animal.doctorsfile.jp/redirect/?ct=doctor&id=178583&url=http%3A%2F%2Fspo337.com%2F
https://hr.pecom.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://cse.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://jump2.bdimg.com/mo/q/checkurl?url=http%3A%2F%2Fspo337.com%2F
https://severeweather.wmo.int/cgi-bin/goto?where=http%3A%2F%2Fspo337.com%2F
https://beam.jpn.org/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=414788&url=http%3A%2F%2Fspo337.com%2F
https://wtk.db.com/777554543598768/optout?redirect=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.lv/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://m.caijing.com.cn/member/logout?referer=http%3A%2F%2Fspo337.com%2F
https://jamesattorney.agilecrm.com/click?u=http%3A%2F%2Fspo337.com%2F
https://wikimapia.org/external_link?url=http%3A%2F%2Fspo337.com%2F
https://rsv.nta.co.jp/affiliate/set/af100101.aspx?site_id=66108024&redi_url=http%3A%2F%2Fspo337.com%2F
https://adengine.old.rt.ru/go.jsp?to=http%3A%2F%2Fspo337.com%2F
https://thediplomat.com/ads/books/ad.php?i=4&r=http%3A%2F%2Fspo337.com%2F
https://mitsui-shopping-park.com/lalaport/iwata/redirect.html?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.es/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.it/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.br/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.au/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.be/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.at/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.se/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ch/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.my/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.co/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ie/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ph/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.no/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pe/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ae/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ee/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.eg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.si/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ec/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.qa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.li/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.uy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ba/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.is/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.az/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ng/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.np/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.by/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.as/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.do/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.jo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ai/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ni/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.la/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.jm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ps/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.af/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.fj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.lb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ms/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ci/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.py/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.am/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ad/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ly/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.na/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.me/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.je/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ge/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ht/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.im/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ml/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ac/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.st/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.td/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.nf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ne/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mn/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://www.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://asia.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://www.macro.ua/out.php?link=http%3A%2F%2Fspo337.com%2F
http://www.aurki.com/jarioa/redirect?id_feed=510&url=http%3A%2F%2Fspo337.com%2F
http://www.cnainterpreta.it/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.site-navi.net/sponavi/rank.cgi?mode=link&id=890&url=http%3A%2F%2Fspo337.com%2F
https://www.mattias.nu/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
http://teenstgp.us/cgi-bin/out.cgi?u=http%3A%2F%2Fspo337.com%2F
http://congovibes.com/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.humaniplex.com/jscs.html?hj=y&ru=http%3A%2F%2Fspo337.com%2F
http://www.qlt-online.de/cgi-bin/click/clicknlog.pl?link=http%3A%2F%2Fspo337.com%2F
http://ww.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
http://fosteringsuccessmichigan.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fotos24.org/url?q=http%3A%2F%2Fspo337.com%2F
http://fouillez-tout.com/cgi-bin/redirurl.cgi?http%3A%2F%2Fspo337.com%2F
http://frag-den-doc.de/index.php?s=verlassen&url=http%3A%2F%2Fspo337.com%2F
http://freethailand.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://freshcannedfrozen.ca/?URL=http%3A%2F%2Fspo337.com%2F
http://funkhouse.de/url?q=http%3A%2F%2Fspo337.com%2F
http://ga.naaar.nl/link/?url=http%3A%2F%2Fspo337.com%2F
http://gb.poetzelsberger.org/show.php?c453c4=http%3A%2F%2Fspo337.com%2F
http://getmethecd.com/?URL=http%3A%2F%2Fspo337.com%2F
http://goldankauf-oberberg.de/out.php?link=http%3A%2F%2Fspo337.com%2F
http://distributors.hrsprings.com/?URL=http%3A%2F%2Fspo337.com%2F
http://dorf-v8.de/url?q=http%3A%2F%2Fspo337.com%2F
http://doverwx.com/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://dr-guitar.de/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://drdrum.biz/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://ds-media.info/url?q=http%3A%2F%2Fspo337.com%2F
http://excitingperformances.com/?URL=http%3A%2F%2Fspo337.com%2F
http://familie-huettler.de/link.php?link=http%3A%2F%2Fspo337.com%2F
http://forum.vizslancs.hu/lnks.php?uid=net&url=http%3A%2F%2Fspo337.com%2F
http://forum.wonaruto.com/redirection.php?redirection=http%3A%2F%2Fspo337.com%2F
https://www.fairsandfestivals.net/?URL=http%3A%2F%2Fspo337.com%2F
http://chatx2.whocares.jp/redir.jsp?u=http%3A%2F%2Fspo337.com%2F
http://chuanroi.com/Ajax/dl.aspx?u=http%3A%2F%2Fspo337.com%2F
http://club.dcrjs.com/link.php?url=http%3A%2F%2Fspo337.com%2F
http://conny-grote.de/url?q=http%3A%2F%2Fspo337.com%2F
http://crazyfrag91.free.fr/?URL=http%3A%2F%2Fspo337.com%2F
http://crewe.de/url?q=http%3A%2F%2Fspo337.com%2F
http://cwa4100.org/uebimiau/redir.php?http%3A%2F%2Fspo337.com%2F
http://d-quintet.com/i/index.cgi?id=1&mode=redirect&no=494&ref_eid=33&url=http%3A%2F%2Fspo337.com%2F
http://data.allie.dbcls.jp/fct/rdfdesc/usage.vsp?g=http%3A%2F%2Fspo337.com%2F
http://data.linkedevents.org/describe/?url=http%3A%2F%2Fspo337.com%2F
http://dayviews.com/externalLinkRedirect.php?url=http%3A%2F%2Fspo337.com%2F
http://db.cbservices.org/cbs.nsf/forward?openform&http%3A%2F%2Fspo337.com%2F
http://simvol-veri.ru/xp/?goto=http%3A%2F%2Fspo337.com%2F
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=http%3A%2F%2Fspo337.com%2F
https://stroim100.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://kakaku-navi.net/items/detail.php?url=http%3A%2F%2Fspo337.com%2F
http://www.paladiny.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://tido.al/vazhdo.php?url=http%3A%2F%2Fspo337.com%2F
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=http%3A%2F%2Fspo337.com%2F
https://www.usjournal.com/go.php?campusID=190&url=http%3A%2F%2Fspo337.com%2F
https://temptationsaga.com/buy.php?url=http%3A%2F%2Fspo337.com%2F
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=http%3A%2F%2Fspo337.com%2F
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=http%3A%2F%2Fspo337.com%2F
https://spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
https://www.info-realty.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.sermemole.com/public/serbook/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hradycz.cz/redir.php?b=445&t=http%3A%2F%2Fspo337.com%2F
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=http%3A%2F%2Fspo337.com%2F
https://uk.kindofbook.com/redirect.php/?red=http%3A%2F%2Fspo337.com%2F
http://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
http://auyttrv.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bartos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://cllfather.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littlejohnny.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://semesmemos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://faultypirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mmurugesamnfo.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://katihfmaxtron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ikkemandar.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://googlejfgdlenewstoday.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrecadominicana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://anupam-bestprice89.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrepublica.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allinspirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://weallfreieds.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://shanmegurad.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://manualmfuctional.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://verybeayurifull.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prasat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://meri.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prabu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://easehipranaam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://nidatyi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://krodyit.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://naine.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://breajwasi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://beithe.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allthingnbeyondblog.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://usafunworldt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://financialallorner.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mjghouthernmatron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://chandancomputers.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingshopping.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptritam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tech-universes.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littleboy.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://correctinspiration.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://esujkamien.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ujkaeltnx.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ausnangck2809.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://supermu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://buyfcyclingteam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tumari.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hariomm.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://lejano.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://jotumko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bestandroid.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://discoverable.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://puriagatratt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://azizlemon.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptrfsiitan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://boardgame-breking.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://flowwergulab.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingcreater-uk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://skinnyskin.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://amil.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mohan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://dilip.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://avinasg.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://virat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hsadyttk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ashu-quality.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tinko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://konkaruna.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://udavhav.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://matura.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://patana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bopal.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gwl.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://delhi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gopi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kripa.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://charanraj.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pream.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://sang.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pasas.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kanchan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://assadaaka.nl/?URL=http%3A%2F%2Fspo337.com%2F
http://atchs.jp/jump?u=http%3A%2F%2Fspo337.com%2F
http://away3d.com/?URL=http%3A%2F%2Fspo337.com%2F
http://blackberryvietnam.net/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://boogiewoogie.com/?URL=http%3A%2F%2Fspo337.com%2F
http://bsumzug.de/url?q=http%3A%2F%2Fspo337.com%2F
http://business.eatonton.com/list?globaltemplate=http%3A%2F%2Fspo337.com%2F
http://archives.midweek.com/?URL=http%3A%2F%2Fspo337.com%2F
http://armdrag.com/mb/get.php?url=http%3A%2F%2Fspo337.com%2F
http://asai-kota.com/acc/acc.cgi?REDIRECT=http%3A%2F%2Fspo337.com%2F
http://ass-media.de/wbb2/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://cdiabetes.com/redirects/offer.php?URL=http%3A%2F%2Fspo337.com%2F
https://padletcdn.com/cgi/fetch?disposition=attachment&url=http%3A%2F%2Fspo337.com%2F
http://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
http://centuryofaction.org/?URL=http%3A%2F%2Fspo337.com%2F
http://Somewh.a.T.dfqw@www.newsdiffs.org/article-history/www.findabeautyschool.com/map.aspx?url=http%3A%2F%2Fspo337.com%2F
http://actontv.org/?URL=http%3A%2F%2Fspo337.com%2F
http://bigbarganz.com/g/?http%3A%2F%2Fspo337.com%2F
http://andreasgraef.de/url?q=http%3A%2F%2Fspo337.com%2F
http://app.espace.cool/ClientApi/SubscribeToCalendar/1039?url=http%3A%2F%2Fspo337.com%2F
http://archive.paulrucker.com/?URL=http%3A%2F%2Fspo337.com%2F
http://baseballpodcasts.net/Feed2JS/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
http://bbs.mottoki.com/index?bbs=hpsenden&act=link&page=94&linkk=http%3A%2F%2Fspo337.com%2F
http://bernhardbabel.com/url?q=http%3A%2F%2Fspo337.com%2F
http://albertaaerialapplicators.com/?URL=http%3A%2F%2Fspo337.com%2F
http://alexanderroth.de/url?q=http%3A%2F%2Fspo337.com%2F
http://alt.baunetzwissen.de/rd/nl-track/?obj=bnw&cg1=redirect&cg2=wissen-newsletter:beton&cg3=partner&datum=2017-01&titel=partnerklick-beton&firma=informationszentrumbeton&link=http%3A%2F%2Fspo337.com%2F
https://duckduckgo.com/?q=https%3A%2F%2Fspo1top.com&t=h_&ia=web
https://www.google.com/search?q=https%3A%2F%2Fspo1top.com&sca_esv=567933587&sxsrf=AM9HkKkLNk4hFHvgRG-dTHcW476TZakh3Q%3A1695527512326&ei=WLIPZZPIE66t4-EPwYOS4AE&ved=0ahUKEwiT1NCYrMKBAxWu1jgGHcGBBBwQ4dUDCBA&uact=5&oq=https%3A%2F%2Fspo1top.com&gs_lp=Egxnd3Mtd2l6LXNlcnAiE2h0dHBzOi8vc3BvMXRvcC5jb20yBBAjGCdI2x9QkAtY2xpwAXgBkAEAmAGHAaABygeqAQMwLji4AQPIAQD4AQHCAgoQABhHGNYEGLAD4gMEGAAgQYgGAZAGBg&sclient=gws-wiz-serp
http://client.paltalk.com/client/webapp/client/External.wmt?url=http%3A%2F%2Fspo337.com%2F
http://community.nxp.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
http://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://ceskapozice.lidovky.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mitsui-shopping-park.com/lalaport/ebina/redirect.html?url=http%3A%2F%2Fspo337.com%2F
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=http%3A%2F%2Fspo337.com%2F
http://fishbiz.seagrant.uaf.edu/click-thru.html?url=http%3A%2F%2Fspo337.com%2F
http://kupiauto.zr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://ipx.bcove.me/?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.td/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
https://home.guanzhuang.org/link.php?url=http%3A%2F%2Fspo337.com%2F
http://dvd24online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://twindish-electronics.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.beigebraunapartment.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.mix-choice.com/yomi/rank.cgi?mode=link&id=391&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
http://www.1soft-tennis.com/search/rank.cgi?mode=link&id=17&url=http%3A%2F%2Fspo337.com%2F
http://big-data-fr.com/linkedin.php?lien=http%3A%2F%2Fspo337.com%2F
http://reg.kost.ru/cgi-bin/go?http%3A%2F%2Fspo337.com%2F
http://www.kirstenulrich.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.inkwell.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.co.je/url?q=http%3A%2F%2Fspo337.com%2F
https://n1653.funny.ge/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://clients1.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
http://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
http://www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.nickl-architects.com/url?q=http%3A%2F%2Fspo337.com%2F
https://www.ocbin.com/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lobenhausen.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
http://redirect.me/?http%3A%2F%2Fspo337.com%2F
http://kank.o.oo7.jp/cgi-bin/ys4/rank.cgi?mode=link&id=569&url=http%3A%2F%2Fspo337.com%2F
http://ruslog.com/forum/noreg.php?http%3A%2F%2Fspo337.com%2F
http://forum.ahigh.ru/away.htm?link=http%3A%2F%2Fspo337.com%2F
http://www.wildner-medien.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.tifosy.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.co.ck/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://ads.icorp.ro/others/STS/?t=CeNortjKxUjK0NDFVsgZcMBADAm4-&g=http%3A%2F%2Fspo337.com%2F
http://deai-ranking.org/search/rank.cgi?mode=link&id=28&url=http%3A%2F%2Fspo337.com%2F
https://izispicy.com/go.php?url=http%3A%2F%2Fspo337.com%2F
http://demo.vieclamcantho.vn/baohiemthatnghiep/Redirect.aspx?sms=90bb20bb20tbb20thc3%B4ng&link=http%3A%2F%2Fspo337.com%2F
http://www.city-fs.de/url?q=http%3A%2F%2Fspo337.com%2F
http://p.profmagic.com/urllink.php?url=http%3A%2F%2Fspo337.com%2F
https://www.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ee/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://www.arndt-am-abend.de/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
http://maxnetworks.org/searchlink/rank.cgi?mode=link&id=321&url=http%3A%2F%2Fspo337.com%2F
https://image.google.com.ng/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
https://www.kenzai-navi.com/location/location_topbanner.php?bs=238&m=899&href=http%3A%2F%2Fspo337.com%2F
https://www.anybeats.jp/jump/?http%3A%2F%2Fspo337.com%2F
http://url-collector.appspot.com/positiveVotes?topic=Hate%20speech&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.be/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://top.hange.jp/linkdispatch/dispatch?targetUrl=http%3A%2F%2Fspo337.com%2F
http://www.link.gokinjyo-eikaiwa.com/rank.cgi?mode=link&id=5&url=http%3A%2F%2Fspo337.com%2F
http://www.air-dive.com/au/mt4i.cgi?mode=redirect&ref_eid=697&url=http%3A%2F%2Fspo337.com%2F
http://okozukai.j-web.jp/j-web/okozukai/ys4/rank.cgi?mode=link&id=6817&url=http%3A%2F%2Fspo337.com%2F
https://sfmission.com/rss/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
https://www.watersportstaff.co.uk/extern.aspx?src=http%3A%2F%2Fspo337.com%2F&cu=60096&page=1&t=1&s=42"
http://siamcafe.net/board/go/go.php?http%3A%2F%2Fspo337.com%2F
http://www.whitening-navi.info/cgi/search-smartphone/rank.cgi?mode=link&id=1431&url=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channeldescription&q=http%3A%2F%2Fspo337.com%2F
http://www.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://go.e-frontier.co.jp/rd2.php?uri=http%3A%2F%2Fspo337.com%2F
http://adchem.net/Click.aspx?url=http%3A%2F%2Fspo337.com%2F
http://www.reddotmedia.de/url?q=http%3A%2F%2Fspo337.com%2F
https://10ways.com/fbredir.php?orig=http%3A%2F%2Fspo337.com%2F
https://emailtrackerapi.leadforensics.com/api/URLOpen?EmailSentRecordID=17006&URL=http%3A%2F%2Fspo337.com%2F
http://search.haga-f.net/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://7ba.ru/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.51queqiao.net/link.php?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://rsyosetsu.bookmarks.jp/ys4/rank.cgi?mode=link&id=3519&url=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://underwood.ru/away.html?url=http%3A%2F%2Fspo337.com%2F
https://unicom.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.unifin.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://uogorod.ru/feed/520?redirect=http%3A%2F%2Fspo337.com%2F
http://uvbnb.ru/go?http%3A%2F%2Fspo337.com%2F
https://skibaza.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://smartservices.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.topkam.ru/gtu/?url=http%3A%2F%2Fspo337.com%2F
https://torggrad.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://torgi-rybinsk.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://tpprt.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.al/url?q=http%3A%2F%2Fspo337.com%2F
http://toolbarqueries.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.capitalbikepark.se/bok/go.php?url=http%3A%2F%2Fspo337.com%2F
http://cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
https://joomlinks.org/?url=http%3A%2F%2Fspo337.com%2F
https://managementportal.de/modules/mod_jw_srfr/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://www.green-cross.pro/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://m.adlf.jp/jump.php?l=http%3A%2F%2Fspo337.com%2F
https://www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
http://www.mac52ipod.cn/urlredirect.php?go=http%3A%2F%2Fspo337.com%2F
http://chrison.net/ct.ashx?id=dad2849a-8862-4a6d-b842-ef628cbfd3c3&url=http%3A%2F%2Fspo337.com%2F
http://datasheetcatalog.biz/url.php?url=http%3A%2F%2Fspo337.com%2F
http://minlove.biz/out.html?id=nhmode&go=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/win/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://request-response.com/blog/ct.ashx?url=http%3A%2F%2Fspo337.com%2F
https://turbazar.ru/url/index?url=http%3A%2F%2Fspo337.com%2F
http://members.asoa.org/sso/logout.aspx?returnurl=http%3A%2F%2Fspo337.com%2F
http://login.mediafort.ru/autologin/mail/?code=14844×02ef859015x290299&url=http%3A%2F%2Fspo337.com%2F
https://www.sports-central.org/cgi-bin/axs/ax.pl?http%3A%2F%2Fspo337.com%2F
http://dot.boss.sk/o/rs.php?ssid=1&szid=4&dsid=12&dzid=32&url=http%3A%2F%2Fspo337.com%2F
http://www.americanstylefridgefreezer.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.newzealandgirls.co.nz/auckland/escorts/clicks.php?click_id=NavBar_AF_AdultDirectory&target=http%3A%2F%2Fspo337.com%2F
https://wdesk.ru/go?http%3A%2F%2Fspo337.com%2F
http://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://page.yicha.cn/tp/j?url=http%3A%2F%2Fspo337.com%2F
http://www.internettrafficreport.com/cgi-bin/cgirdir.exe?http%3A%2F%2Fspo337.com%2F
http://www.interfacelift.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://good-surf.ru/r.php?g=http%3A%2F%2Fspo337.com%2F
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=http%3A%2F%2Fspo337.com%2F
https://gektor-nsk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://lekoufa.ru/banner/go?banner_id=4&link=http%3A%2F%2Fspo337.com%2F
https://forsto.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.gigatran.ru/go?url=http%3A%2F%2Fspo337.com%2F
http://www.gigaalert.com/view.php?h=&s=http%3A%2F%2Fspo337.com%2F
http://www.jkes.tyc.edu.tw/dyna/webs/gotourl.php?id=357&url=http%3A%2F%2Fspo337.com%2F
http://www.laopinpai.com/gourl.asp?url=/gourl.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.humanbrainmapping.org/i4a/etrack/track.cfm?rType=2&campaignID=3572&contactID=4524&origURL=http%3A%2F%2Fspo337.com%2F
https://gcup.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.gearguide.ru/phpbb/go.php?http%3A%2F%2Fspo337.com%2F
https://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://passport.sfacg.com/LoginOut.aspx?Returnurl=http%3A%2F%2Fspo337.com%2F
https://offers.sidex.ru/stat_ym_new.php?redir=http%3A%2F%2Fspo337.com%2F&hash=1577762
https://globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://ramset.com.au/document/url/?url=http%3A%2F%2Fspo337.com%2F
https://www.vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
http://www.cyprus-net.com/banner_click.php?banid=4&link=http%3A%2F%2Fspo337.com%2F
http://a.gongkong.com/db/adredir.asp?id=16757&url=http%3A%2F%2Fspo337.com%2F
http://gfaq.ru/go?http%3A%2F%2Fspo337.com%2F
http://gbi-12.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.global-flat.com/mobile/mobile_switch.php?dir=tofull&url=http%3A%2F%2Fspo337.com%2F
https://golden-resort.ru/out.php?out=http%3A%2F%2Fspo337.com%2F
http://avalon.gondor.ru/away.php?link=http%3A%2F%2Fspo337.com%2F
http://www.laosubenben.com/home/link.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lifeshow.com.tw/show.php?ty5_id=1599&url=http%3A%2F%2Fspo337.com%2F
http://games.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.netwalkerstore.com/redirect.asp?accid=18244&adcampaignid=2991&adcampaigntype=2&affduration=30&url=http%3A%2F%2Fspo337.com%2F
http://tsugarubrand.jp/blog/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://blackhistorydaily.com/black_historylinks/link.asp?linkid=5&URL=http%3A%2F%2Fspo337.com%2F
http://www.kitchencabinetsdirectory.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://cityprague.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://www.hachimantaishi.com/click3/click3.cgi?cnt=c5&url=http%3A%2F%2Fspo337.com%2F
http://earnupdates.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://www.careerbuilder.com.cn/cn/tallyredirector.aspx?task=Ushi&objName=SnsShare&redirectUrl=http%3A%2F%2Fspo337.com%2F&methodName=SetSnsShareLink
http://banners.knollenstein.com/adnetwork/servlet/advertBeans.TrackingServlet?seid=27709655&t=100&redirectTo=http%3A%2F%2Fspo337.com%2F&cid=DECONL&vid=986809&externalsource=jobbsquareppc
https://meyeucon.org/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://www.cnmhe.fr/spip.php?action=cookie&url=http%3A%2F%2Fspo337.com%2F
https://perezvoni.com/blog/away?url=http%3A%2F%2Fspo337.com%2F
https://www.ciymca.org/af/register-redirect/71104?url=http%3A%2F%2Fspo337.com%2F
https://whizpr.nl/tracker.php?u=http%3A%2F%2Fspo337.com%2F
http://bw.irr.by/knock.php?bid=252583&link=http%3A%2F%2Fspo337.com%2F
http://www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/hak/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://www.actuaries.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMVFIELD]EMAIL[EMV/FIELD]&cat=Techniquesculturales&url=http%3A%2F%2Fspo337.com%2F
https://www.jukujo.gs/bin/out.cgi?id=kimiomof&url=http%3A%2F%2Fspo337.com%2F
http://hansolav.net/blog/ct.ashx?id=0af6301b-e71f-44be-838f-905709eee792&url=http%3A%2F%2Fspo337.com%2F
http://bitrix.adlr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://m.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.officeservice.com.br/trackMKT/trackerFolha.aspx?DESCRIPTION=HotSite_EcrmContabil&FROM=Workshop&DESTINATION=http%3A%2F%2Fspo337.com%2F
https://www.arbsport.ru/gotourl.php?url=http%3A%2F%2Fspo337.com%2F
http://www.strana.co.il/finance/redir.aspx?site=http%3A%2F%2Fspo337.com%2F
https://www.tourplanisrael.com/redir/?url=http%3A%2F%2Fspo337.com%2F
http://www.mutualgravity.com/archangel/webcontact/d_signinsimple.php?action=signup&CID=240&EID=&S=default.css&return=http%3A%2F%2Fspo337.com%2F
http://buildingreputation.com/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
http://gals.graphis.ne.jp/mkr/out.cgi?id=04489&go=http%3A%2F%2Fspo337.com%2F
http://dir.tetsumania.net/search/rank.cgi?mode=link&id=3267&url=http%3A%2F%2Fspo337.com%2F
http://www.project24.info/mmview.php?dest=http%3A%2F%2Fspo337.com%2F
http://tfads.testfunda.com/TFServeAds.aspx?strTFAdVars=4a086196-2c64-4dd1-bff7-aa0c7823a393,TFvar,00319d4f-d81c-4818-81b1-a8413dc614e6,TFvar,GYDH-Y363-YCFJ-DFGH-5R6H,TFvar,http%3A%2F%2Fspo337.com%2F
http://www.request-response.com/blog/ct.ashx?id=d827b163-39dd-48f3-b767-002147c94e05&url=http%3A%2F%2Fspo337.com%2F
http://www.cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
http://sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=785&redirect=http%3A%2F%2Fspo337.com%2F
http://www.kyoto-osaka.com/search/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://metalist.co.il/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://i-marine.eu/pages/goto.aspx?link=http%3A%2F%2Fspo337.com%2F
http://www.teenlove.biz/cgibin/atc/out.cgi?id=24&u=http%3A%2F%2Fspo337.com%2F
http://www.altoona.com/newsletter/click.php?volume=52&url=http%3A%2F%2Fspo337.com%2F
http://www.katjushik.ru/link.php?to=http%3A%2F%2Fspo337.com%2F
http://gondor.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://proekt-gaz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.tricitiesapartmentguide.com/MobileDefault.aspx?reff=http%3A%2F%2Fspo337.com%2F
http://www.vietnamsingle.com/vn/banners/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.voxlocalis.net/enlazar/?url=http%3A%2F%2Fspo337.com%2F
http://no-smok.net/nsmk/InterWiki?action=goto&oe=EUC-KR&url=http%3A%2F%2Fspo337.com%2F
http://www.stalker-modi.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.p1-uranai.com/rank.cgi?mode=link&id=538&url=http%3A%2F%2Fspo337.com%2F
http://earthsciencescanada.com/modules/babel/redirect.php?newlang=en_us&newurl=http%3A%2F%2Fspo337.com%2F
http://psykodynamiskt.nu/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
http://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
http://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://lirasport.com.ar/bloques/bannerclick.php?id=7&url=http%3A%2F%2Fspo337.com%2F
http://www.knabstrupper.se/guestbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.pba.ph/redirect?url=http%3A%2F%2Fspo337.com%2F&id=3&type=tab
https://bondage-guru.net/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.westbloomfieldlibrary.org/includes/statistics.php?StatType=Link&StatID=Facebook&weblink=http%3A%2F%2Fspo337.com%2F
https://tenisnews.com.br/clickTracker/clickTracker.php?u=http%3A%2F%2Fspo337.com%2F
http://www.stevestechspot.com/ct.ashx?id=9fdcf4a4-6e09-4807-bc31-ac1adf836f6c&url=http%3A%2F%2Fspo337.com%2F
http://rcoi71.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.laselection.net/redir.php3?cat=int&url=http%3A%2F%2Fspo337.com%2F
http://www.masai-mara.com/cgi-bin/link2.pl?grp=mm&link=http%3A%2F%2Fspo337.com%2F
http://evenemangskalender.se/redirect/?id=15723&lank=http%3A%2F%2Fspo337.com%2F
http://anifre.com/out.html?go=http%3A%2F%2Fspo337.com%2F
http://www.restavracije-gostilne.si/banner.php?id=44&url=http%3A%2F%2Fspo337.com%2F
http://hobbyplastic.co.uk/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://ads.pukpik.com/myads/click.php?banner_id=316&banner_url=http%3A%2F%2Fspo337.com%2F
https://www.adminer.org/redirect/?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackmamas.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://primorye.ru/go.php?id=19&url=http%3A%2F%2Fspo337.com%2F
https://www.123gomme.it/it/ViewSwitcher/SwitchView?mobile=True&returnUrl=http%3A%2F%2Fspo337.com%2F
http://youmydaddy.com/cgi-bin/at3/out.cgi?id=88&trade=http%3A%2F%2Fspo337.com%2F
https://naviking.localking.com.tw/about/redirect.aspx?mid=7&url=http%3A%2F%2Fspo337.com%2F
http://sms-muzeji.si/Home/ChangeCulture?lang=sl&returnUrl=http%3A%2F%2Fspo337.com%2F
http://r.emeraldexpoinfo.com/s.ashx?ms=EXI2:125462_115115&e=krubin723@aol.com&eId=440186886&c=h&url=http%3A%2F%2Fspo337.com%2F
https://apps.firstrain.com/service/openurl.jsp?action=titleclick&src=rss&u=http%3A%2F%2Fspo337.com%2F
http://cdp.thegoldwater.com/click.php?id=101&url=http%3A%2F%2Fspo337.com%2F
http://www.appenninobianco.it/ads/adclick.php?bannerid=159&zoneid=8&source=&dest=http%3A%2F%2Fspo337.com%2F
https://www.eduplus.hk/special/emailalert/goURL.jsp?clickURL=http%3A%2F%2Fspo337.com%2F
http://www.japan.road.jp/navi/navi.cgi?jump=226&url=http%3A%2F%2Fspo337.com%2F
http://www.arch.iped.pl/artykuly.php?id=1&cookie=1&url=http%3A%2F%2Fspo337.com%2F
http://hirlevelcenter.eu/click.php?hirlevel_id=13145441085508&url=http%3A%2F%2Fspo337.com%2F
https://birds.cz/avif/redirect.php?from=avif.obs.php&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackbootywatchers.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
http://barykin.com/go.php?http%3A%2F%2Fspo337.com%2F
https://forum.everleap.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://www.mosig-online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hccincorporated.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fatnews.com/?URL=http%3A%2F%2Fspo337.com%2F
https://csirealty.com/?URL=http%3A%2F%2Fspo337.com%2F
http://asadi.de/url?q=http%3A%2F%2Fspo337.com%2F
http://treblin.de/url?q=http%3A%2F%2Fspo337.com%2F
https://kentbroom.com/?URL=http%3A%2F%2Fspo337.com%2F
http://0845.boo.jp/cgi/mt3/mt4i.cgi?id=24&mode=redirect&no=15&ref_eid=3387&url=http%3A%2F%2Fspo337.com%2F
http://110.164.66.211/ULIB6//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://110.164.92.12/ULIB//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://198.54.125.86.myopenlink.net/describe/?url=http%3A%2F%2Fspo337.com%2F
http://202.144.225.38/jmp?url=http%3A%2F%2Fspo337.com%2F
http://2cool2.be/url?q=http%3A%2F%2Fspo337.com%2F
http://4coma.net/cgi/mt4/mt4i.cgi?cat=12&mode=redirect&ref_eid=3231&url=http%3A%2F%2Fspo337.com%2F
http://4travel.jp/dynamic/redirect.php?mode=dm_tour&url=http%3A%2F%2Fspo337.com%2F
http://imaginingourselves.globalfundforwomen.org/pb/External.aspx?url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
http://search.pointcom.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.cercasostituto.it/index.php?name=GestBanner&file=counter&idbanner=40&dir_link=http%3A%2F%2Fspo337.com%2F
http://vsvejr.dk/mt/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.meteo-leran.fr/meteotemplate/template/plugins/deviations/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.eurobichons.com/fda%20alerts.php?url=http%3A%2F%2Fspo337.com%2F
https://mydojo.at/de_AT/karate/weiterleitung?redirect=http%3A%2F%2Fspo337.com%2F
http://click.phosphodiesterase4.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.mariahownersclub.com/forum/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://www.wildromance.com/buy.php?url=http%3A%2F%2Fspo337.com%2F&store=iBooks&book=omk-ibooks-us
http://mapleriverweather.com/mobile/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.archijob.co.il/index/comp_website.asp?companyId=1469&website=http%3A%2F%2Fspo337.com%2F
https://student-helpr.rminds.dev/redirect?redirectTo=http%3A%2F%2Fspo337.com%2F
http://www.kalinna.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hartmanngmbh.de/url?q=http%3A%2F%2Fspo337.com%2F
https://www.the-mainboard.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://www.betamachinery.com/?URL=http%3A%2F%2Fspo337.com%2F
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=http%3A%2F%2Fspo337.com%2F
http://www.sprang.net/url?q=http%3A%2F%2Fspo337.com%2F
https://img.2chan.net/bin/jump.php?http%3A%2F%2Fspo337.com%2F
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=http%3A%2F%2Fspo337.com%2F
http://local.rongbachkim.com/rdr.php?url=http%3A%2F%2Fspo337.com%2F
http://bachecauniversitaria.it/link/frm_top.php?url=http%3A%2F%2Fspo337.com%2F
https://www.stcwdirect.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://forum.vcoderz.com/externalredirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.momentumstudio.com/?URL=http%3A%2F%2Fspo337.com%2F
https://s-p.me/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://ww.thesteelbrothers.com/buy.php?store=iBooks&url=http%3A%2F%2Fspo337.com%2F
http://thdt.vn/convert/convert.php?link=http%3A%2F%2Fspo337.com%2F
http://www.doitweb365.de/scripts/doitweb.exe/rasklickzaehler2?http%3A%2F%2Fspo337.com%2F
https://www.guadamur.eu/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://weather.cube.com.gr/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.fimmgviterbo.org/mobfimmgviterbo/index.php?nametm=counter&idbanner=4&dir_link=http%3A%2F%2Fspo337.com%2F
http://www.mckinneyfarm.com/template/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://utmagazine.ru/r?url=http%3A%2F%2Fspo337.com%2F
http://www.evrika41.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
http://expomodel.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://facto.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://fc-zenit.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://forum-region.ru/forum/away.php?s=http%3A%2F%2Fspo337.com%2F
http://forumdate.ru/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://shckp.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://shinglas.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sibran.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://sintez-oka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://app.espace.cool/clientapi/subscribetocalendar/974?url=http%3A%2F%2Fspo337.com%2F
http://111056.net/yomisearch/rank.cgi?mode=link&id=6205&url=http%3A%2F%2Fspo337.com%2F
http://stanko.tw1.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sozialemoderne.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.showb.com/search/ranking.cgi?mode=link&id=7083&url=http%3A%2F%2Fspo337.com%2F
http://imagelibrary.asprey.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://ime.nu/http%3A%2F%2Fspo337.com%2F
http://interflex.biz/url?q=http%3A%2F%2Fspo337.com%2F
http://ivvb.de/url?q=http%3A%2F%2Fspo337.com%2F
http://j.lix7.net/?http%3A%2F%2Fspo337.com%2F
http://jacobberger.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jahn.eu/url?q=http%3A%2F%2Fspo337.com%2F
http://jamesvelvet.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jla.drmuller.net/r.php?url=http%3A%2F%2Fspo337.com%2F
http://jump.pagecs.net/http%3A%2F%2Fspo337.com%2F
http://kagarin.net/cgi/mt/mt4i.cgi?id=2&mode=redirect&no=330&ref_eid=103&url=http%3A%2F%2Fspo337.com%2F
http://karkom.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kens.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinderundjugendpsychotherapie.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinhtexaydung.net/redirect/?url=http%3A%2F%2Fspo337.com%2F
http://neoromance.info/link/rank.cgi?mode=link&id=26&url=http%3A%2F%2Fspo337.com%2F
http://www.hainberg-gymnasium.com/url?q=http%3A%2F%2Fspo337.com%2F
https://befonts.com/checkout/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.usap.gov/externalsite.cfm?http%3A%2F%2Fspo337.com%2F
https://maps.google.com.ua/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.shinobi.jp/etc/goto.html?http%3A%2F%2Fspo337.com%2F
http://images.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://supplier-portal-uat.daimler.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://strelmag.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://spb90.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://spartak.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sovaisova.ru/bitrix/redirect.php?event1=2009_mainpage&event2=go_www&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
https://seoandme.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://s-online.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://rssfeeds.wtsp.com/~/t/0/0/wtsp/home/~http%3A%2F%2Fspo337.com%2F
https://rostovmama.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://rev1.reversion.jp/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.star174.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://staten.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://stopcran.ru/go?http%3A%2F%2Fspo337.com%2F
http://studioad.ru/go?http%3A%2F%2Fspo337.com%2F
http://swepub.kb.se/setattribute?language=en&redirect=http%3A%2F%2Fspo337.com%2F
https://www.licnioglasi.org/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
http://www2.smartmail.com.ar/tl.php?p=hqf/f94/rs/1fp/4c0/rs//http%3A%2F%2Fspo337.com%2F
http://old.roofnet.org/external.php?link=http%3A%2F%2Fspo337.com%2F
http://www.bucatareasa.ro/link.php?url=http%3A%2F%2Fspo337.com%2F
http://mosprogulka.ru/go?http%3A%2F%2Fspo337.com%2F
https://uniline.co.nz/Document/Url/?url=http%3A%2F%2Fspo337.com%2F
https://tracker.onrecruit.net/api/v1/redirect/?redirect_to=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.samovar-forum.ru/go?http%3A%2F%2Fspo337.com%2F
http://staldver.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://posts.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://naruto.su/link.ext.php?url=http%3A%2F%2Fspo337.com%2F
https://justpaste.it/redirect/172fy/http%3A%2F%2Fspo337.com%2F
https://jt-pr-dot-yamm-track.appspot.com/Redirect?ukey=1iXi3dF8AuzUIsgifbcVnqqx-anF4B8R-9PC3UGhHO3E-1131306248&key=YAMMID-79725512&link=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://de.flavii.de/index.php?flavii=linker&link=http%3A%2F%2Fspo337.com%2F
https://dakke.co/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_attribute_to_url=http%3A%2F%2Fspo337.com%2F
https://contacts.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.nxp.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.esri.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2Fspo337.com%2F
https://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
https://bukkit.org/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://branch.app.link/?$deeplink_path=article%2Fjan%2F123&$fallback_url=http%3A%2F%2Fspo337.com%2F&channel=facebook&feature=affiliate
https://boowiki.info/go.php?go=http%3A%2F%2Fspo337.com%2F
https://blaze.su/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://bbs.pku.edu.cn/v2/jump-to.php?url=http%3A%2F%2Fspo337.com%2F
https://bares.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.flyingsamaritans.net/Startup/SetupSite.asp?RestartPage=http%3A%2F%2Fspo337.com%2F
https://www.ewind.cz/index.php?page=home/redirect&url=http%3A%2F%2Fspo337.com%2F
https://www.eas-racing.se/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.direkt-einkauf.de/includes/refer.php?id=170&url=http%3A%2F%2Fspo337.com%2F
https://www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.curseforge.com/linkout?remoteUrl=http%3A%2F%2Fspo337.com%2F
https://www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=http%3A%2F%2Fspo337.com%2F
https://www.autopartskart.com/buyfromamzon.php?url=http%3A%2F%2Fspo337.com%2F
https://www.autoandrv.com/linkout.aspx?websiteurl=http%3A%2F%2Fspo337.com%2F
https://www.art-prizes.com/AdRedirector.aspx?ad=MelbPrizeSculpture_2017&target=http%3A%2F%2Fspo337.com%2F
https://wirelessestimator.com/advertise/newsletter_redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://wasitviewed.com/index.php?href=http%3A%2F%2Fspo337.com%2F
https://tvtropes.org/pmwiki/no_outbounds.php?o=http%3A%2F%2Fspo337.com%2F
https://trello.com/add-card?source=mode=popup&name=click+here&desc=http%3A%2F%2Fspo337.com%2F
https://sutd.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://abiznes.com.ua/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.sv-mama.ru/shared/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sofion.ru/banner.php?r1=41&r2=2234&goto=http%3A%2F%2Fspo337.com%2F
http://www.rss.geodles.com/fwd.php?url=http%3A%2F%2Fspo337.com%2F
http://www.nuttenzone.at/jump.php?url=http%3A%2F%2Fspo337.com%2F
http://www.myhottiewife.com/cgi-bin/arpro/out.cgi?id=Jojo&url=http%3A%2F%2Fspo337.com%2F
http://www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
http://www.johnvorhees.com/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.imsnet.at/LangChange.aspx?uri=http%3A%2F%2Fspo337.com%2F
http://www.hon-cafe.net/cgi-bin/re.cgi?lid=hmw&url=http%3A%2F%2Fspo337.com%2F
http://www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
http://www.etis.ford.com/externalURL.do?url=http%3A%2F%2Fspo337.com%2F
http://www.chungshingelectronic.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.bdsmandfetish.com/cgi-bin/sites/out.cgi?id=mandymon&url=http%3A%2F%2Fspo337.com%2F
http://visits.seogaa.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
http://tharp.me/?url_to_shorten=http%3A%2F%2Fspo337.com%2F
http://speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://spbstroy.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://solo-center.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://sennheiserstore.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://school364.spb.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://sc.sie.gov.hk/TuniS/http%3A%2F%2Fspo337.com%2F
http://rzngmu.ru/go?http%3A%2F%2Fspo337.com%2F
http://rostovklad.ru/go.php?http%3A%2F%2Fspo337.com%2F
http://portalnp.snauka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://markiza.me/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://jump.5ch.net/?http%3A%2F%2Fspo337.com%2F
http://imperialoptical.com/news-redirect.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hellothai.com/wwwlink/wwwredirect.asp?hp_id=1242&url=http%3A%2F%2Fspo337.com%2F
http://guru.sanook.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fr.knubic.com/redirect_to?url=http%3A%2F%2Fspo337.com%2F
http://branch.app.link/?$deeplink_path=article/jan/123&$fallback_url=http%3A%2F%2Fspo337.com%2F
http://biz-tech.org/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://2010.russianinternetweek.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://xat.com/web_gear/chat/linkvalidator.php?link=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://www.woodlist.us/delete-company?nid=13964&element=http%3A%2F%2Fspo337.com%2F
https://www.viecngay.vn/go?to=http%3A%2F%2Fspo337.com%2F
https://www.uts.edu.co/portal/externo.php?id=http%3A%2F%2Fspo337.com%2F
https://www.talgov.com/Main/exit.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.russianrobotics.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.ruchnoi.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://www.rprofi.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.roccotube.com/cgi-bin/at3/out.cgi?id=27&tag=toplist&trade=http%3A%2F%2Fspo337.com%2F
https://www.nyl0ns.com/cgi-bin/a2/out.cgi?id=43&l=btop&u=http%3A%2F%2Fspo337.com%2F
https://www.meetme.com/apps/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=http%3A%2F%2Fspo337.com%2F
https://www.interpals.net/url_redirect.php?href=http%3A%2F%2Fspo337.com%2F
https://www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
https://www.hottystop.com/cgi-bin/at3/out.cgi?id=12&trade=http%3A%2F%2Fspo337.com%2F
https://www.hobowars.com/game/linker.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hentainiches.com/index.php?id=derris&tour=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.greencom.ru/catalog/irrigation_systems.html?jumpsite=2008&url=http%3A%2F%2Fspo337.com%2F
https://www.girlznation.com/cgi-bin/atc/out.cgi?id=50&l=side&u=http%3A%2F%2Fspo337.com%2F
https://www.funeralunion.org/delete-company?nid=39&element=http%3A%2F%2Fspo337.com%2F
https://www.fudbal91.com/tz.php?zone=America/Iqaluit&r=http%3A%2F%2Fspo337.com%2F
https://www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
https://kryvbas.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://joomluck.com/go/?http%3A%2F%2Fspo337.com%2F
https://www.anonymz.com/?http%3A%2F%2Fspo337.com%2F
https://weburg.net/redirect?url=http%3A%2F%2Fspo337.com%2F
https://tw6.jp/jump/?url=http%3A%2F%2Fspo337.com%2F
https://www.spainexpat.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.fotka.pl/link.php?u=http%3A%2F%2Fspo337.com%2F
https://www.lolinez.com/?http%3A%2F%2Fspo337.com%2F
https://nanos.jp/jmp?url=http%3A%2F%2Fspo337.com%2F
https://www.fca.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://savvylion.com/?bmDomain=http%3A%2F%2Fspo337.com%2F
https://www.soyyooestacaido.com/http%3A%2F%2Fspo337.com%2F
https://www.gta.ru/redirect/www.http%3A%2F%2Fspo337.com%2F
https://directx10.org/go?http%3A%2F%2Fspo337.com%2F
https://mejeriet.dk/link.php?id=http%3A%2F%2Fspo337.com%2F
https://ezdihan.do.am/go?http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://hiddenrefer.com/?http%3A%2F%2Fspo337.com%2F
https://romhacking.ru/go?http%3A%2F%2Fspo337.com%2F
https://turion.my1.ru/go?http%3A%2F%2Fspo337.com%2F
https://kassirs.ru/sweb.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.allods.net/redirect/http%3A%2F%2Fspo337.com%2F
https://icook.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.pasco.k12.fl.us/?URL=http%3A%2F%2Fspo337.com%2F
https://anolink.com/?link=http%3A%2F%2Fspo337.com%2F
https://www.questsociety.ca/?URL=http%3A%2F%2Fspo337.com%2F
https://www.disl.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://holidaykitchens.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.mbcarolinas.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.anibox.org/go?http%3A%2F%2Fspo337.com%2F
https://google.info/url?q=http%3A%2F%2Fspo337.com%2F
https://atlantis-tv.ru/go?http%3A%2F%2Fspo337.com%2F
https://otziv.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.sgvavia.ru/go?http%3A%2F%2Fspo337.com%2F
https://element.lv/go?url=http%3A%2F%2Fspo337.com%2F
https://karanova.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://789.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
https://krasnoeselo.su/go?http%3A%2F%2Fspo337.com%2F
https://game-era.do.am/go?http%3A%2F%2Fspo337.com%2F
https://kudago.com/go/?to=http%3A%2F%2Fspo337.com%2F
https://after.ucoz.net/go?http%3A%2F%2Fspo337.com%2F
https://kinteatr.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://nervetumours.org.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://kopyten.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://www.taker.im/go/?u=http%3A%2F%2Fspo337.com%2F
https://usehelp.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://sepoa.fr/wp/go.php?http%3A%2F%2Fspo337.com%2F
https://world-source.ru/go?http%3A%2F%2Fspo337.com%2F
https://mail2.mclink.it/SRedirect/http%3A%2F%2Fspo337.com%2F
https://www.swleague.ru/go?http%3A%2F%2Fspo337.com%2F
https://nazgull.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.rosbooks.ru/go?http%3A%2F%2Fspo337.com%2F
https://beskuda.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://richmonkey.biz/go/?http%3A%2F%2Fspo337.com%2F
https://vlpacific.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://eric.ed.gov/?redir=http%3A%2F%2Fspo337.com%2F
https://www.usich.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://sec.pn.to/jump.php?http%3A%2F%2Fspo337.com%2F
https://www.earth-policy.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.silverdart.co.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://pr-cy.ru/jump/?url=http%3A%2F%2Fspo337.com%2F
https://google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://pikmlm.ru/out.php?p=http%3A%2F%2Fspo337.com%2F
https://regnopol.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://mss.in.ua/go.php?to=http%3A%2F%2Fspo337.com%2F
https://owohho.com/away?url=http%3A%2F%2Fspo337.com%2F
https://www.youa.eu/r.php?u=http%3A%2F%2Fspo337.com%2F
https://cool4you.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://rg4u.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.de-online.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.allpn.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://click.start.me/?url=http%3A%2F%2Fspo337.com%2F
https://prizraks.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://flyd.ru/away.php?to=http%3A%2F%2Fspo337.com%2F
https://risunok.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.sr/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://www.semanticjuice.com/site/http%3A%2F%2Fspo337.com%2F
https://cse.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://s79457.gridserver.com/?URL=http%3A%2F%2Fspo337.com%2F
https://cdl.su/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.fondbtvrtkovic.hr/?URL=http%3A%2F%2Fspo337.com%2F
https://lostnationarchery.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.booktrix.com/live/?URL=http%3A%2F%2Fspo337.com%2F
https://www.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://www.marcellusmatters.psu.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://onlinesportsbettingstory.blogspot.com/2024/01/asian-handicap-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-creates-absurd-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/01/fantasy-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/connecticut-considering-new-advertisement-club-and-sports-wagering-bill
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-profit.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-official-talks-about-state-s-games-wagering-bill-and-future-of
https://bestsportsbettingarticles.blogspot.com/2024/01/consideration-sports-betting-best-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/why-sports-expectation-markets-are-like-onions-and-film-industry-film-receipts
https://bettingstratigicarticle.blogspot.com/2024/01/technology-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/iowa-bill-to-authorize-sports-wagering-advances-subtleties-need-pressing
https://sportswageringinfo.blogspot.com/2024/01/future-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://gameswageringarticles.blogspot.com/2024/01/blog-post_31.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-lawful-games-wagering-regulation-trend
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-pro-level.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-bankroll-the-executives-benefits-victors-and-discipline-1623a8ef-e6da-4dd6-bedb-5596e78ec1af
https://sportsbettingindustry.blogspot.com/2024/01/game-changing-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/sports-wagering-nfl-lines-chances-point-spreads-and-then-some
https://articleinsportsbetting.blogspot.com/2024/01/optimal-wins-sports-betting-.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-jersey-representative-pallone-submits-amicus-brief-in-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/best-odds-earnings-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/american-gaming-affiliation-records-brief-in-sports-wagering-case-in-high-court
https://bettingstratigicarticle.blogspot.com/2024/02/essential-tips-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/n-j-representative-leonard-spear-on-sports-wagering-jersey-merits
https://sportswageringinfo.blogspot.com/2024/02/sports-wagering-triumph.html
https://lucky-razi-777.mystrikingly.com/blog/7-of-the-greatest-possible-advantages-of-lawful-managed-sports-wagering
https://gameswageringarticles.blogspot.com/2024/02/games-wagering-victory-tips.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettor-at-focus-of-alabama-baseball-embarrassment-has-to-deal-with-upwards-of
https://onlinesportsbettingstory.blogspot.com/2024/02/mastering-online-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-sports-wagering-bill-starts-development-through-senate
https://sportsbettingindustry.blogspot.com/2024/02/successful-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/looking-for-bettors-and-administrators-for-wide-arriving-at-industry-reviews
https://articleinsportsbetting.blogspot.com/2024/02/savvy-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bestsportsbettingarticles.blogspot.com/2024/02/winning-streak-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/something-like-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups-f8de9e02-c6a6-495d-9ec9-14a31daf9d51
https://onlinesportsbettingstory.blogspot.com/2024/02/changing-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportsbettingindustry.blogspot.com/2024/02/mastering-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/rocha-california-drive-will-assist-harm-the-brand-of-portable-games-wagering
https://articleinsportsbetting.blogspot.com/2024/02/mastering-revolutionary-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/is-america-crummy-with-awful-games-bettors
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tip-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-s-three-portable-sportsbooks-all-set-with-send-off-advancements
https://bettingstratigicarticle.blogspot.com/2024/02/expert-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/media-note-pad-where-might-the-telecom-companies-be-without-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/successful-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/yearlings-texans-same-game-parlay-expects-pass-cheerful-undertaking
https://gameswageringarticles.blogspot.com/2024/02/secret-success-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maturing-superteams-among-wagering-top-choices-to-bring-home-nba-championship
https://onlinesportsbettingstory.blogspot.com/2024/02/proven-winnings-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/climate-abbreviated-pga-visit-occasion-sparkles-sports-wagering-discussion
https://sportsbettingindustry.blogspot.com/2024/02/mastering%20strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/an-hour-endeavors-to-handle-sports-wagering-thrashes-about-all-things-being
https://articleinsportsbetting.blogspot.com/2024/02/art-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ncaa-solicitations-restriction-on-school-player-prop-wagers-in-ohio
https://bestsportsbettingarticles.blogspot.com/2024/02/unbeatable-sports%20betting-tips.html
https://site-6861241-1631-6047.mystrikingly.com/blog/liv-golf-wagering-markets-presented-in-additional-states-for-2024-season
https://bettingstratigicarticle.blogspot.com/2024/02/profit-expert-betting-stratigic.html
https://daddys-lucky000.mystrikingly.com/blog/mississippi-online-games-wagering-bill-clears-house-missouri-bill-escapes-panel
https://sportswageringinfo.blogspot.com/2024/02/Winning-Sports-Wagering.html
https://lucky-razi-777.mystrikingly.com/blog/swift-at-the-super-bowl-can-t-wager-on-it
https://gameswageringarticles.blogspot.com/2024/02/strategies-excel-ports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-betr-getting-ready-to-leave-massachusetts-market
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/record-promotion-offers-aided-lift-pennsylvanians-wagering-the-month-before
https://sportsbettingindustry.blogspot.com/2024/02/guide-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/lexi-thompson-thought-about-a-major-wagering-longshot-in-vegas-pga-visit
https://articleinsportsbetting.blogspot.com/2024/02/successful-guide-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-controllers-acclimate-to-new-difficulties-in-betting-business
https://bestsportsbettingarticles.blogspot.com/2024/02/psychology-winning-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/greatest-administrators-amazed-to-catch-wind-of-potential-california-sports
https://bettingstratigicarticle.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-remains-school-football-wagering-number-one-after-flimsy-september
https://sportswageringinfo.blogspot.com/2024/02/proven-tactics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/fanduel-keeps-on-ruling-virginia-sports-betting-scene
https://gameswageringarticles.blogspot.com/2024/02/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/travis-kelce-prop-wagering-detonates-as-taylor-swift-gives-a-shout-out-to-him
https://onlinesportsbettingstory.blogspot.com/2024/02/tips-win-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-dark-horse-dream-asset-will-support-dependable-betting-advancement
https://sportsbettingindustry.blogspot.com/2024/02/strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-bet-is-a-lock-to-send-off-by-this-date
https://articleinsportsbetting.blogspot.com/2024/02/mastering-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-week-5-mahomes-slides-bettors-cry-coolio-rides-wan-dale-skims
https://bestsportsbettingarticles.blogspot.com/2024/02/best-sports-wagering-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ncaa-hopes-to-return-to-sports-betting-infringement-discipline-framework
https://bettingstratigicarticle.blogspot.com/2024/02/sports-wagering-skills.html
https://daddys-lucky000.mystrikingly.com/blog/finally-around-is-prepared-to-play-in-illinois
https://onlinesportsbettingstory.blogspot.com/2024/02/%20strategies-sports-wagering-triumph.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-controllers-look-for-proactive-reaction-to-digital-hacks-at
https://sportsbettingindustry.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://casino999.mystrikingly.com/blog/caesars-enters-developing-microbetting-business-sector-with-simplebet
https://bestsportsbettingarticles.blogspot.com/2024/02/crushing-online-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/bally-bet-wynnbet-granted-maryland-versatile-wagering-licenses
https://bettingstratigicarticle.blogspot.com/2024/02/online-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/wynnbet-gets-massachusetts-computerized-sports-wagering-permit
https://sportswageringinfo.blogspot.com/2024/02/dominate-online-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-once-more-sets-month-to-month-sports-betting-income-record
https://gameswageringarticles.blogspot.com/2024/02/sports-betting-revealed.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettors-bet-more-than-200-million-in-maryland-in-november
https://onlinesportsbettingstory.blogspot.com/2024/02/online-sports-betting-hacks.html
https://site-6828921-2394-9309.mystrikingly.com/blog/fan-commitment-media-organizations-will-drive-sports-wagering-dealmaking
https://sportsbettingindustry.blogspot.com/2024/02/thrills-online-sports-betting.html
https://casino999.mystrikingly.com/blog/return-of-enormous-ten-football-gives-knock-to-sportsbooks
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-strategies-pay-off.html
https://site-4763676-9520-5980.mystrikingly.com/blog/development-in-us-helps-pad-william-slope-s-q3-decrease-in-incomes
https://bestsportsbettingarticles.blogspot.com/2024/02/best-online-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/d-c-s-first-free-sportsbook-preparing-for-opening-as-neighbors-stand-up-to
https://bettingstratigicarticle.blogspot.com/2024/02/successful-online-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/star-card-sharks-blame-nv-bookmaker-for-undermining-in-game-wagers
https://sportswageringinfo.blogspot.com/2024/02/sports-betting-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/the-week-in-sports-wagering-record-breaking-month-to-month-750m-new-jersey
https://gameswageringarticles.blogspot.com/2024/02/profits-online-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/turner-sports-makes-fanduel-official-games-wagering-accomplice-for-nba
https://onlinesportsbettingstory.blogspot.com/2024/02/skill-meets-fortune-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-gambling-clubs-planned-to-return-july-1
https://sportsbettingindustry.blogspot.com/2024/02/online-sports-betting-pros.html
https://casino999.mystrikingly.com/blog/gold-silver-and-betting-how-colorado-s-gaming-towns-became
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-hobby.html
https://site-4763676-9520-5980.mystrikingly.com/blog/arlington-park-itha-at-long-last-have-arrangement-for-horse-racing-in-illinois
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tips-every-sports-bettor%20.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-moving-again-in-georgia
https://bettingstratigicarticle.blogspot.com/2024/02/guide-safe-responsible-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://sportswageringinfo.blogspot.com/2024/02/safety-sports-betting-online.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-survey-expansive-help-for-club-gaming-not-such-a-huge-amount-for
https://gameswageringarticles.blogspot.com/2024/02/secure-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sporttrade-is-a-genuine-games-wagering-roller-coaster
https://onlinesportsbettingstory.blogspot.com/2024/02/%20online-sports-betting-enthusiasts.html
https://site-6828921-2394-9309.mystrikingly.com/blog/everything-that-bet365-s-colorado-send-off-says-to-us-about-the
https://sportsbettingindustry.blogspot.com/2024/02/tips-secure-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration
https://articleinsportsbetting.blogspot.com/2024/02/priority-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-unquestionable
https://bestsportsbettingarticles.blogspot.com/2024/02/safely-world-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/regardless-of-udoka-suspension-celtics-remain-title-top-choices-all-things
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-wins.html
https://daddys-lucky000.mystrikingly.com/blog/maryland-posts-sports-wagering-handle-of-32-5m-in-january
https://onlinesportsbettingstory.blogspot.com/2024/02/enjoying-online-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nevada-controllers-caution-sportsbooks-that-detailing-postpones-won-t-go-on
https://sportsbettingindustry.blogspot.com/2024/02/safety-measures-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration-a8c987a8-5921-4dd4-8953-8ddc50b1486a
https://articleinsportsbetting.blogspot.com/2024/02/world-sports-wagering-confidence.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-absolute
https://bestsportsbettingarticles.blogspot.com/2024/02/secrets-secure-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/alberta-s-controllers-actually-haggling-with-potential-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/world-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-bettor-hits-3-million-parlay-thanks-to-late-energize-by-horses
https://sportswageringinfo.blogspot.com/2024/02/finances-well-being-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/betfair-applies-for-sports-betting-permit-at-joined-center
https://gameswageringarticles.blogspot.com/2024/02/security-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/top-sportsbooks-turn-on-prop-27-promotion-system-in-california
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-dominate-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/wynnbet-draws-nearer-to-new-york-send-off
https://sportsbettingindustry.blogspot.com/2024/02/sports-betting-strategies-revealed.html
https://casino999.mystrikingly.com/blog/ohio-gambling-club-control-commission-sets-sports-wagering-application-charges
https://articleinsportsbetting.blogspot.com/2024/02/tips-live-betting-triumphs.html
https://site-4763676-9520-5980.mystrikingly.com/blog/a-bettor-s-manual-for-the-wild-and-weird-at-the-colder-time-of-year-olympics
https://bestsportsbettingarticles.blogspot.com/2024/02/strategies-work-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/month-to-month-stock-watch-m-a-reports-whirl-as-sports-wagering-stocks-stay
https://bettingstratigicarticle.blogspot.com/2024/02/live-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/will-arkansas-versatile-betting-get-february-endorsement
https://sportswageringinfo.blogspot.com/2024/02/live-betting-tactics-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-in-louisiana-gets-off-major-areas-of-strength-for-to
https://gameswageringarticles.blogspot.com/2024/02/strategies-guaranteed-wins-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-plans-to-open-sportsbook-parlor-at-joined-center-in-chicago
https://onlinesportsbettingstory.blogspot.com/2024/02/online-betting-strategies-unleashed.html
https://site-6828921-2394-9309.mystrikingly.com/blog/april-brings-newbie-ohio-its-most-minimal-wagering-action-so-far
https://sportsbettingindustry.blogspot.com/2024/02/live-betting-strategies-exposed.html
https://casino999.mystrikingly.com/blog/issue-betting-subsidizing-authoritatively-cut-from-d-c-spending-plan
https://articleinsportsbetting.blogspot.com/2024/02/strategies-real-time-live-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://bestsportsbettingarticles.blogspot.com/2024/02/sports-betting-strategies-victory.html
https://site-6861241-1631-6047.mystrikingly.com/blog/report-senate-decision-on-north-carolina-portable-wagering-bill-just-around
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/neglecting-to-report-betting-infringement-a-no-ncaa-says
https://sportswageringinfo.blogspot.com/2024/02/live-sports-betting-techniques.html
https://lucky-razi-777.mystrikingly.com/blog/scientists-go-after-making-sense-of-card-sharks-conduct-at-vegas-social-event
https://gameswageringarticles.blogspot.com/2024/02/Live%20Betting%20Strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/zensports-to-offer-money-back-remunerations-to-tennessee-games-bettors
https://onlinesportsbettingstory.blogspot.com/2024/02/successful-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-keeps-on-kicking-the-tires-on-the-streaming-conflicts
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting-5-simple-steps.html
https://casino999.mystrikingly.com/blog/charge-documented-to-give-each-arizona-clan-an-occasion-betting-permit
https://articleinsportsbetting.blogspot.com/2024/02/blog-post.html
https://site-4763676-9520-5980.mystrikingly.com/blog/perceptions-from-a-visit-to-betmgm-sportsbook-at-nats-park
https://bestsportsbettingarticles.blogspot.com/2024/02/world-best-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/georgia-partners-hopeful-2022-is-the-year-for-lawful-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/complex-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/locationsmart-an-option-in-contrast-to-geocomply-for-ontario-igaming
https://sportswageringinfo.blogspot.com/2024/02/conquering-world-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/illinois-sportsbooks-see-wagering-income-plunge-in-december
https://gameswageringarticles.blogspot.com/2024/02/tactics-sports-betting-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/proline-retail-sports-bettors-baffled-with-chances-disparities
https://onlinesportsbettingstory.blogspot.com/2024/02/succeed-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-s-betting-bonus-at-last-endorses-5-club-however-go-live-date-muddled
https://sportsbettingindustry.blogspot.com/2024/02/exciting-world-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-club-baffled-by-absence-of-sports-wagering-endorsement
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-smarter.html
https://site-4763676-9520-5980.mystrikingly.com/blog/bclc-reports-single-game-games-wagering-income-is-watching-ontario-market
https://bestsportsbettingarticles.blogspot.com/2024/02/mastering-sports-betting-worldwide.html
https://site-6861241-1631-6047.mystrikingly.com/blog/wash-st-sports-wagering-scientist-talks-bootleg-market-relocation-portable
https://bettingstratigicarticle.blogspot.com/2024/02/sports-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/a-story-of-three-new-games-wagering-states
https://sportswageringinfo.blogspot.com/2024/02/success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/entain-promotes-strong-tech-stack-with-draftkings-romance-having-blurred
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting_01573407901.html
https://site-6828921-2394-9309.mystrikingly.com/blog/florida-high-court-expresses-no-to-taking-seminoles-hard-rock-stage-3e9c4744-c515-4536-a0c8-7bb56cf957b7
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting.html
https://casino999.mystrikingly.com/blog/california-ancestral-chief-board-officially-goes-against-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/wisconsin-adolescent-confesses-to-scheme-in-sports-wagering-hacking-case
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/record-u-s-wagering-handle-anticipated-for-las-vegas-excellent-prix
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/draftkings-reports-moderate-parlay-element-coming-soon-to-online-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/proven-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/ontario-controller-partners-start-conversations-around-new-promotion-rules
https://gameswageringarticles.blogspot.com/2024/02/foolproof-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncpg-s-new-board-president-a-hero-with-an-arrangement
https://onlinesportsbettingstory.blogspot.com/2024/02/ultimate-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-york-turns-out-to-be-first-state-to-top-2-billion-in-month-to-month-handle
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-professional.html
https://casino999.mystrikingly.com/blog/california-s-clans-open-to-novel-thoughts-however-don-t-have-any-desire-to
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-guaranteed-success.html
https://site-4763676-9520-5980.mystrikingly.com/blog/florida-parimutuels-look-for-court-request-driving-seminoles-to-bring-down
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-greatest-pr-challenge-espn-faces-when-espn-bet-goes-live
https://bettingstratigicarticle.blogspot.com/2024/02/innovative-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/north-carolina-sports-wagering-board-of-trustees-offers-second-bunch-of-rules
https://sportswageringinfo.blogspot.com/2024/02/transformative-strategies-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/expert-association-organization-wagering-on-carrom-to-take-u-s-by-tempest
https://gameswageringarticles.blogspot.com/2024/02/effective-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/illinois-the-state-with-the-20-million-games-betting-permit-and-no-takers-dbbfc4dc-8a1d-4f1d-96bc-1334337be2c0
https://onlinesportsbettingstory.blogspot.com/2024/02/advanced-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/genius-associations-informing-on-sports-wagering-respectability-may-blow-up
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-success.html
https://casino999.mystrikingly.com/blog/a-few-gubernatorial-up-and-comers-take-solid-position-on-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies-dominate.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-officials-gradual-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/professional-betting-strategies.html
https://site-6861241-1631-6047.mystrikingly.com/blog/paddy-power-betfair-declares-procurement-of-fanduel
https://bettingstratigicarticle.blogspot.com/2024/02/inside-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/new-york-state-sports-wagering-plans-picture-it-s-a-piece-confounded
https://sportswageringinfo.blogspot.com/2024/02/mastering-betting-strategies.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-and-liquor-a-story-of-two-restrictions
https://onlinesportsbettingstory.blogspot.com/2024/03/betting-strategies-consistent-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-dependable-betting-bill-language-rejected-in-everyday-get-together
https://sportsbettingindustry.blogspot.com/2024/03/revolutionize-betting-game.html
https://casino999.mystrikingly.com/blog/virginia-tops-10-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/03/elevate-betting-game.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-senate-supports-revised-sports-wagering-bill
https://bestsportsbettingarticles.blogspot.com/2024/03/proven-sports-betting-strategies-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maryland-lottery-issues-versatile-betting-permit-to-aficionados-sportsbook
https://bettingstratigicarticle.blogspot.com/2024/03/elevate-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/april-brings-novice-ohio-its-most-minimal-wagering-action-hitherto
https://sportswageringinfo.blogspot.com/2024/03/ultimate-guide-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/issue-betting-financing-formally-cut-from-d-c-spending-plan
https://gameswageringarticles.blogspot.com/2024/03/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://onlinesportsbettingstory.blogspot.com/2024/03/transform-betting-experience.html
https://site-6828921-2394-9309.mystrikingly.com/blog/media-note-pad-kelce-siblings-sound-off-on-individual-nfl-players
https://sportsbettingindustry.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/best-wagering-scenes-getting-stoned-in-the-music-of-possibility
https://articleinsportsbetting.blogspot.com/2024/03/mastering-betting-pro-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ohio-has-turned-into-the-most-intriguing-state-with-regards-to-the-sportsbook
https://bestsportsbettingarticles.blogspot.com/2024/03/betting-strategies-serious-players.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-regulator-approves-latest-sports-wagering-procedures
https://bettingstratigicarticle.blogspot.com/2024/03/Betting%20Strategies%20for%20Victory.html
https://daddys-lucky000.mystrikingly.com/blog/three-new-administrators-could-be-live-in-arizona-by-mid-2024
https://sportswageringinfo.blogspot.com/2024/03/unveiling-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://gameswageringarticles.blogspot.com/2024/03/journey-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/administrators-push-back-on-proposed-betting-methods-in-vermont
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crab-sports-dispatches-wagering-stage-in-maryland-application-not-yet
https://sportsbettingindustry.blogspot.com/2024/03/deep-dive-world-wagering.html
https://casino999.mystrikingly.com/blog/pieces-prosperity-assists-colorado-bettors-with-avoiding-may-pattern-of
https://articleinsportsbetting.blogspot.com/2024/03/journey-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://bestsportsbettingarticles.blogspot.com/2024/03/dollars-world-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maine-betting-control-boss-back-working-after-neglected-suspension
https://bettingstratigicarticle.blogspot.com/2024/03/wagering-journey-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/is-most-recent-florida-choice-actually-an-ancestral-gaming-major-advantage
https://sportswageringinfo.blogspot.com/2024/03/art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-virtuoso-games-expand-sports-wagering-information-association-through
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-first-to-attempt-to-boycott-bettors-who-undermine-competitors
https://sportsbettingindustry.blogspot.com/2024/03/unconventional-wagering-journey.html
https://casino999.mystrikingly.com/blog/sportradar-inks-sports-wagering-information-concurrence-with-south-america-s
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-wizardry-mastering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-virtuoso-games-broaden-sports-wagering-information-organization-through
https://bestsportsbettingarticles.blogspot.com/2024/03/unveiling-wagering-journey.html
https://site-6861241-1631-6047.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://bettingstratigicarticle.blogspot.com/2024/03/navigating-world-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/how-truly-do-cultivate-children-need-to-manage-the-fate-of-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/lessons-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/how-in-all-actuality-do-encourage-children-need-to-manage-the-eventual-fate
https://gameswageringarticles.blogspot.com/2024/03/journey-fortunes-made-lost.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ohio-officials-twofold-games-betting-expense
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-betting-strategies-winning-big.html
https://site-6828921-2394-9309.mystrikingly.com/blog/five-nfl-groups-with-ideal-prospects-chances
https://sportsbettingindustry.blogspot.com/2024/03/blog-post.html
https://casino999.mystrikingly.com/blog/football-challenges-of-old-made-the-present-huge-games-wagering-prominence
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-like-champion.html
https://site-4763676-9520-5980.mystrikingly.com/blog/one-more-influx-of-gambling-clubs-apply-to-join-new-jersey-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/tips-excel-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/fanduel-sportsbook-send-off-imprints-another-lawful-games-wagering-achievement
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-controllers-authorities-urge-persistence-to-try-not-to-bumble
https://sportswageringinfo.blogspot.com/2024/03/inner-champion-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/cheerful-commemoration-delaware-sports-wagering-a-champ-one-month-in
https://gameswageringarticles.blogspot.com/2024/03/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/excitement-is-high-as-mississippi-sports-wagering-arrangements-in-progress
https://onlinesportsbettingstory.blogspot.com/2024/03/Navigate-art-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-wagering-lines-problem-what-happens-when-everybody-risks-everything-group
https://sportsbettingindustry.blogspot.com/2024/03/decoding-art-sports-wagering.html
https://casino999.mystrikingly.com/blog/report-ny-will-have-greatest-games-wagering-business-sector-in-u-s
https://articleinsportsbetting.blogspot.com/2024/03/maximizing-odds-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pga-visit-concurs-it-d-be-stupid-to-restrict-portable-wagering-to-on-premises
https://bestsportsbettingarticles.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/kentucky-putting-money-on-sports-wagering-to-assist-with-fixing-annuity-issue
https://sportswageringinfo.blogspot.com/2024/03/dominate-art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/legitimate-games-wagering-now-regulation-in-rhode-island-and-state-gets-51
https://gameswageringarticles.blogspot.com/2024/03/comprehensive-guide-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-nearby-according-to-an-understudy-s-point-of-view
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-wagering-guaranteed-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pittsburgh-privateers-take-sports-wagering-trustworthiness-expense-to
https://sportsbettingindustry.blogspot.com/2024/03/game-changing-strategy-sports-betting.html
https://casino999.mystrikingly.com/blog/greatest-chances-movers-after-first-rounds-of-world-cup
https://articleinsportsbetting.blogspot.com/2024/03/Winwinning-strategies-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/monitoring-west-virginia-s-games-wagering-plans-and-send-off
https://bestsportsbettingarticles.blogspot.com/2024/03/art-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/oregon-lottery-preparing-portable-application-with-versatile-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/level-up-betting-game.html
https://daddys-lucky000.mystrikingly.com/blog/rhode-island-makes-stride-nearer-to-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/03/excel-sports-wagering-like-pro.html
https://lucky-razi-777.mystrikingly.com/blog/seriously-fighting-yet-no-democratic-in-new-york-state
https://gameswageringarticles.blogspot.com/2024/03/psychological-tricks-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/choctaw-intend-to-be-first-in-quite-a-while-to-offer-games-wagering
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/murphy-makes-sports-wagering-lawful-in-new-jersey
https://sportsbettingindustry.blogspot.com/2024/03/key-success-sports-betting.html
https://casino999.mystrikingly.com/blog/stage-set-for-decision-on-legitimate-new-york-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/why-is-murphy-delayed-to-make-nj-sports-wagering-regulation
https://bestsportsbettingarticles.blogspot.com/2024/03/mental-aspect-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/at-any-rate-so-what-in-blazes-is-uprightness-checking
https://bettingstratigicarticle.blogspot.com/2024/03/power-mind-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/lead-representative-wagers-on-phillies-to-start-up-lawful-delaware-sports
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-mental-mastery.html
https://lucky-razi-777.mystrikingly.com/blog/mailbag-mythbusters-uigea-and-sports-wagering-made-sense
https://gameswageringarticles.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/massachusetts-preparing-to-make-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-divisional-round-weekend-sportsbooks-say-victories-are-coming
https://sportsbettingindustry.blogspot.com/2024/03/successful-sports-betting.html
https://casino999.mystrikingly.com/blog/pennsylvania-closures-notable-year-with-record-month-to-month-sports-betting
https://articleinsportsbetting.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/somewhere-around-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bestsportsbettingarticles.blogspot.com/2024/03/Comprehensiveuide-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting-success.html
https://daddys-lucky000.mystrikingly.com/blog/versatile-games-betting-authoritatively-dispatches-in-vermont
https://sportswageringinfo.blogspot.com/2024/03/strategy-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-administrator-once-again-introduces-sports-wagering-bill
https://gameswageringarticles.blogspot.com/2024/03/mastering-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-sharps-activity-giving-prime-games-looked-for-in-ohio
https://onlinesportsbettingstory.blogspot.com/2024/03/world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-legislators-might-put-destiny-of-sports-wagering-in-citizens-grasp
https://sportsbettingindustry.blogspot.com/2024/03/blog-post_18.html
https://casino999.mystrikingly.com/blog/draftkings-perspectives-sports-wagering-as-tremendous-learning-experience
https://articleinsportsbetting.blogspot.com/2024/03/mental-side-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/kansas-legislators-get-educated-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-mind-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-seeking-after-different-roads-to-sanction-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-mindset-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/could-face-sports-book-become-a-thing
https://sportswageringinfo.blogspot.com/2024/03/mastering-mind-game-betting.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-lead-representative-expresses-associations-a-desire-for-peace
https://gameswageringarticles.blogspot.com/2024/03/mastering-psychology-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-bill-russell-denounces-any-kind-of-authority-on-miami
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting_01715168387.html
https://site-6828921-2394-9309.mystrikingly.com/blog/high-court-strikes-down-government-sports-wagering-boycott-opening-entryway
https://sportsbettingindustry.blogspot.com/2024/03/strategies-betting-success.html
https://casino999.mystrikingly.com/blog/ancestral-worries-supposedly-easing-back-michigan-on-sports-wagering-regulation
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mental-game-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-fiend-examines-strife-recuperation
https://bestsportsbettingarticles.blogspot.com/2024/03/secrets-strategic-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-tangle
https://bettingstratigicarticle.blogspot.com/2024/03/expert-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/the-most-effective-method-to-explore-the-games-wagering-data-and-promote
https://sportswageringinfo.blogspot.com/2024/03/key-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/connecticut-bill-escapes-council-yet-clans-in-conflict-with-state
https://gameswageringarticles.blogspot.com/2024/03/winning-mindset-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/a-terrible-bet-slighting-sanctioned-betting-s-expected-new-clients
https://onlinesportsbettingstory.blogspot.com/2024/03/mastering-mind-game-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/monetary-examination-the-amount-will-connecticut-advantage-from-sports
https://sportsbettingindustry.blogspot.com/2024/03/mastery-sports-betting-success.html
https://casino999.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://articleinsportsbetting.blogspot.com/2024/03/sports-bet-wins-redefine-strategy.html
https://site-4763676-9520-5980.mystrikingly.com/blog/what-precisely-is-a-games-wagering-promote
https://bestsportsbettingarticles.blogspot.com/2024/03/ultimate-guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/possibilities-obscure-for-entry-of-connecticut-sports-wagering-bill
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/how-a-sex-embarrassment-is-easing-back-missouri-s-capacity-to-legitimize
https://sportswageringinfo.blogspot.com/2024/03/dark-side-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/players-associations-stake-guarantee-for-cut-of-sports-wagering-pie-now-as-well
https://onlinesportsbettingstory.blogspot.com/2024/03/winning-sports-bets-every-time.html
https://site-6828921-2394-9309.mystrikingly.com/blog/massachusetts-deliveries-extensive-survey-of-state-s-games-wagering-an
https://sportsbettingindustry.blogspot.com/2024/03/psychology-behind-successful-sports-betting.html
https://casino999.mystrikingly.com/blog/the-wire-demonstration-of-1961-when-the-web-met-the-bedrock-government
https://articleinsportsbetting.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-not-keen-on-paying-associations-a-games-wagering-sovereignty
https://bestsportsbettingarticles.blogspot.com/2024/03/beginners-guide-making-money-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/laissez-les-bons-temps-rouler-louisiana-opens-sports-wagering-discussion
https://bettingstratigicarticle.blogspot.com/2024/03/revolutionizing-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/connecticut-dems-squeezing-for-legitimized-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-strategies-work.html
https://lucky-razi-777.mystrikingly.com/blog/oklahoma-ancestral-games-wagering-bill-clears-board-heads-to-full-house
https://gameswageringarticles.blogspot.com/2024/03/sports-outcomes-beat-odds.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-jersey-tolerating-sports-wagering-applications-chief-affirms
https://onlinesportsbettingstory.blogspot.com/2024/03/tips-tricks-insider-knowledge-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-prepares-crazy-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/03/art-sports-betting-like-champ.html
https://casino999.mystrikingly.com/blog/west-virginia-official-examines-state-s-games-wagering-bill-and-future-of
https://articleinsportsbetting.blogspot.com/2024/03/biggest-sports-bet-wins.html
https://site-4763676-9520-5980.mystrikingly.com/blog/iowa-bill-to-legitimize-sports-wagering-advances-subtleties-need-pressing
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-art-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/charge-presented-in-missouri-pointed-toward-sanctioning-games-wagering-in-state
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post_25.html
https://daddys-lucky000.mystrikingly.com/blog/ball-gets-going-on-iowa-authorization-of-sports-wagering-with-hsb-592
https://sportswageringinfo.blogspot.com/2024/03/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-sports-wagering-bill-would-allow-betting-at-five-club-and-on
https://gameswageringarticles.blogspot.com/2024/03/enhance-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-legitimate-games-wagering-regulation-temporary-fad
https://onlinesportsbettingstory.blogspot.com/2024/03/sharpening-your-skills-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-junkie-talks-about-disturbance-recuperation
https://sportsbettingindustry.blogspot.com/2024/03/level-up-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/instructions-to-explore-the-games-wagering-data-and-promote-industry
https://bestsportsbettingarticles.blogspot.com/2024/04/advanced-sports-betting-triumph.html
https://site-6861241-1631-6047.mystrikingly.com/blog/in-the-event-that-sports-wagering-made-lawful-mississippi-intends-to
https://bettingstratigicarticle.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/a-terrible-bet-disregarding-legitimized-betting-s-expected-new-clients
https://sportswageringinfo.blogspot.com/2024/04/improving-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/monetary-investigation-the-amount-will-connecticut-advantage-from-sports
https://gameswageringarticles.blogspot.com/2024/04/refining-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://onlinesportsbettingstory.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maine-senate-supersedes-sports-wagering-blackball-sets-state-on-way-for
https://sportsbettingindustry.blogspot.com/2024/04/supercharge-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/wading-into-controversy-dialing-back-kentucky-games-wagering-vote
https://articleinsportsbetting.blogspot.com/2024/04/improving-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nonattendances-defer-maine-blackball-supersede-decision-on-legitimate-games
https://bestsportsbettingarticles.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/colorado-inches-nearer-to-may-sports-wagering-send-off-notwithstanding
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/in-the-midst-of-epic-u-s-sports-wagering-turf-war-penn-public-plants
https://sportswageringinfo.blogspot.com/2024/04/secrets-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-principles-among-plan-things-for-illinois-gaming-executive
https://gameswageringarticles.blogspot.com/2024/04/elevate-skills-rookie-pro.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maine-lead-representative-denials-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/04/essential-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/report-ncaa-has-found-175-games-betting-infringement-beginning-around-2018
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-skills-today.html
https://casino999.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/04/elevate-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/mlb-rule-changes-lead-to-additional-wagering-on-taken-bases
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sportsbetting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/concentrate-on-shows-huge-flood-in-wagering-among-ladies-on-ladies-games
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-skills-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/crisis-kentucky-games-wagering-guidelines-preclude-deluding-publicizing
https://sportswageringinfo.blogspot.com/2024/04/sharpen-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/three-occasion-betting-licenses-destined-to-be-available-for-anyone-in-arizona
https://gameswageringarticles.blogspot.com/2024/04/strategies-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/seminoles-will-not-have-the-option-to-send-off-sports-betting-in-florida
https://onlinesportsbettingstory.blogspot.com/2024/04/improve-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/hold-rate-for-new-york-sports-betting-remaining-parts-stuck-on-high
https://sportsbettingindustry.blogspot.com/2024/04/essential-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/most-recent-surveying-recommends-backing-for-california-betting-drives
https://articleinsportsbetting.blogspot.com/2024/04/enhance-sports-betting-skills-now.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-gaming-commission-sitting-tight-for-public-remarks
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/elys-game-innovation-uses-d-c-as-retail-sports-betting-model
https://bettingstratigicarticle.blogspot.com/2024/04/polishing-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/capitalizing-on-changing-out-bookmaker-dishes-on-money-out-apparatus
https://sportswageringinfo.blogspot.com/2024/04/mastering-sports-betting-pro.html
https://lucky-razi-777.mystrikingly.com/blog/maryland-lottery-chief-offers-versatile-send-off-course-of-events-subtleties
https://gameswageringarticles.blogspot.com/2024/04/master-sports-betting-5-steps.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-in-possessing-mahowny-the-activity-is-the-juice
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-insider-tips.html
https://site-6828921-2394-9309.mystrikingly.com/blog/oregon-lottery-carries-out-application-sports-wagering-coming-the-following
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/sports-wagering-and-wounds-in-school-football-isolating-great-data-from-clamor
https://articleinsportsbetting.blogspot.com/2024/04/mastering-art-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/elite-athletics-associations-proceed-with-full-court-push-on-west-virginia
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-wins.html
https://site-6861241-1631-6047.mystrikingly.com/blog/where-the-midwest-is-at-on-sports-wagering-regulation
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/aga-to-schumer-sports-wagering-doesn-t-need-government-oversight
https://sportswageringinfo.blogspot.com/2024/04/mastering-science-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/arkansas-gaming-sports-wagering-polling-form-drive-enduring-an-onslaught
https://gameswageringarticles.blogspot.com/2024/04/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-expert-will-congress-engage-with-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/mind-master-sports-bettor.html
https://site-6828921-2394-9309.mystrikingly.com/blog/intelligent-bettors-ought-to-pay-attention-to-the-buzz-prior-to-wagering
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-management-techniques.html
https://casino999.mystrikingly.com/blog/maine-officials-move-toward-legitimate-games-wagering
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-analytics.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-s-conflict-over-betting-gets-more-sizzling
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/tennessee-posts-370-million-games-wagering-handle-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://daddys-lucky000.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://sportswageringinfo.blogspot.com/2024/04/master-sports-betting-intelligence.html
https://lucky-razi-777.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-bill-bites-the-dust-in-panel-on-conclusive-day-of-kentucky
https://onlinesportsbettingstory.blogspot.com/2024/04/master-sports-betting-like-pro.html
https://site-6828921-2394-9309.mystrikingly.com/blog/kentucky-crisis-sports-wagering-regs-could-make-worry-among-administrators
https://sportsbettingindustry.blogspot.com/2024/04/mastering-art-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-report-records-conceivable-dependable-betting-upgrades
https://articleinsportsbetting.blogspot.com/2024/04/journey-sports-betting-succes.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-draws-nearer-to-inking-manage-new-games-betting-accomplice
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-techniques.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-controller-endorses-most-recent-games-betting-methods
https://bettingstratigicarticle.blogspot.com/2024/04/become-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://sportswageringinfo.blogspot.com/2024/04/tips-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/concentrate-on-shows-critical-flood-in-wagering-among-ladies-on-ladies-games
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-maximum-returns.html
https://site-6861405-2997-4499.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://onlinesportsbettingstory.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://site-6828921-2394-9309.mystrikingly.com/blog/is-vermont-getting-ready-to-consider-sanctioning-games-wagering
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://casino999.mystrikingly.com/blog/espn-refreshing-growing-focusing-on-sports-betting-substance
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies_01879442689.html
https://site-4763676-9520-5980.mystrikingly.com/blog/survey-says-most-ohioans-don-t-want-to-bet-on-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pointsbet-plans-to-handle-wagers-in-less-than-a-second-as-shift-to-in-game
https://bettingstratigicarticle.blogspot.com/2024/04/strategies-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/online-sportsbooks-are-playing-get-up-to-speed-with-regards-to-ada-consistence
https://sportswageringinfo.blogspot.com/2024/04/becoming-master-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/bally-s-applies-to-offer-versatile-games-betting-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesotans-aren-t-in-with-no-reservations-on-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/future-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/yasiel-puig-pulls-out-blameworthy-request-in-unlawful-games-wagering-case
https://sportsbettingindustry.blogspot.com/2024/04/revolutionizing-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-affirms-postpone-in-sports-wagering-send-off-as-iger-behaviors-key-audit
https://articleinsportsbetting.blogspot.com/2024/04/top-sports-betting-trends-2024.html
https://site-4763676-9520-5980.mystrikingly.com/blog/will-sports-wagering-twitter-endure-musk-s-fart-mode-second
https://bestsportsbettingarticles.blogspot.com/2024/04/inside-world-in-play-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/massachusetts-gaming-commission-consents-to-acknowledge-mgm-s-late-application
https://bettingstratigicarticle.blogspot.com/2024/04/rise-mobile-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-computerized-betting-authorizing-contest-not-really-solid
https://sportswageringinfo.blogspot.com/2024/04/data-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-times-fiercely-comes-up-short-on-sports-wagering-story
https://gameswageringarticles.blogspot.com/2024/04/blog-post_12.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-might-ftx-s-crash-affect-crypto-in-sports-wagering-5cbefb65-9344-4f22-bb25-1dcf520d525a
https://onlinesportsbettingstory.blogspot.com/2024/04/AI-transforming-sports-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/versatile-sportsbooks-will-go-live-in-maryland-on-wednesday-lottery-reports
https://sportsbettingindustry.blogspot.com/2024/04/traditional-sports-betting.html
https://casino999.mystrikingly.com/blog/california-betting-drives-made-the-most-awful-sort-of-history
https://articleinsportsbetting.blogspot.com/2024/04/deep-dive-prop-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://bestsportsbettingarticles.blogspot.com/2024/04/popularity-virtual-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-wrongfully-on-sports
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-beginners.html
https://daddys-lucky000.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://sportswageringinfo.blogspot.com/2024/04/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/california-betting-drives-made-the-most-obviously-awful-sort-of-history
https://gameswageringarticles.blogspot.com/2024/04/blockchain-sports-betting-security.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-responsible-gaming.html
https://site-6828921-2394-9309.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-unlawfully-on-sports
https://sportsbettingindustry.blogspot.com/2024/04/trends-live-betting.html
https://casino999.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://articleinsportsbetting.blogspot.com/2024/04/dynamic-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/maryland-portable-send-off-unavoidable-as-betting-commission-grants-10-licenses
https://bestsportsbettingarticles.blogspot.com/2024/04/cross-section-sports-betting-social-media.html
https://site-6861241-1631-6047.mystrikingly.com/blog/caesars-offering-100-in-credits-in-front-of-maryland-sports-wagering-send-off
https://bettingstratigicarticle.blogspot.com/2024/04/latest-mobile-betting-apps.html
https://daddys-lucky000.mystrikingly.com/blog/texas-representative-acquaints-goal-with-permit-sports-betting-club-betting
https://sportswageringinfo.blogspot.com/2024/04/predictive-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/holey-strokes-little-golf-betting-endorsed-in-colorado-wyoming
https://gameswageringarticles.blogspot.com/2024/04/sports-betting-regulations.html
https://site-6861405-2997-4499.mystrikingly.com/blog/one-day-on-one-free-day-plan-for-maryland-send-off-certain-to-befuddle-bettors
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-markets-rise.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sportsbook-celebrity-projects-ain-t-what-they-used-to-be-bettors-say
https://sportsbettingindustry.blogspot.com/2024/04/leverage-data-smart-betting-decisions.html
https://casino999.mystrikingly.com/blog/month-to-month-stock-watch-sports-wagering-stocks-keep-afloat-in-front-of
https://articleinsportsbetting.blogspot.com/2024/04/popularity-niche-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/not-dead-yet-sports-wagering-restored-in-missouri
https://bestsportsbettingarticles.blogspot.com/2024/04/influence-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ohio-sports-betting-application-window-opens-june-15
https://bettingstratigicarticle.blogspot.com/2024/04/live-streaming-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/the-usfl-needs-to-embrace-sports-bettors-and-dfs-players-right-away
https://sportswageringinfo.blogspot.com/2024/04/sports-betting-trends-social-media.html
https://lucky-razi-777.mystrikingly.com/blog/best-games-wagering-scenes-riding-the-borail-with-mine-that-bird-in-50-to-1
https://gameswageringarticles.blogspot.com/2024/04/tips-sports-betting-beginners.html
https://site-6861405-2997-4499.mystrikingly.com/blog/arizona-sports-wagering-handle-falls-barely-short-of-500m-for-december
https://onlinesportsbettingstory.blogspot.com/2024/04/evolution-parlay-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pennsylvania-posts-800-million-games-wagering-handle-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/mind-betting-rofessional.html
https://casino999.mystrikingly.com/blog/nba-hands-lifetime-boycott-to-jontay-watchman-for-conspicuous-betting
https://articleinsportsbetting.blogspot.com/2024/04/think-like-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/draftkings-and-enthusiasts-go-head-to-head-in-court-over-leader-abandonment
https://bestsportsbettingarticles.blogspot.com/2024/04/journey-becoming-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-changing-game.html
https://daddys-lucky000.mystrikingly.com/blog/espn-unlv-declare-beneficiaries-of-first-dependable-betting-partnerships
https://sportswageringinfo.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://gameswageringarticles.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-decrees-bettors-need-to-follow-for-capable-parlay-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/secrets-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-sports-wagering-handle-aggregates-1-07-billion-for-february
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-playbook.html
https://casino999.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-sanctioning-endeavors-fizzled
https://articleinsportsbetting.blogspot.com/2024/04/insights-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-bettors-bound-to-knock-back-the-firewater-than-different-players
https://bestsportsbettingarticles.blogspot.com/2024/04/evolution-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/iowa-south-carolina-establishes-ladies-games-wagering-standards
https://bettingstratigicarticle.blogspot.com/2024/04/true-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/caitlin-clark-previously-carrying-gigantic-wagering-interest-to-the-wnba
https://sportswageringinfo.blogspot.com/2024/04/world-inside-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-bill-spotlights-on-issue-betting-third-minnesota
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-vs-casual-bettors.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesota-sports-wagering-sanctioning-discussion-warms-up-in-house-advisory
https://onlinesportsbettingstory.blogspot.com/2024/04/psychology-behind-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-recruits-lori-kalani-as-first-boss-dependable-gaming-official
https://sportsbettingindustry.blogspot.com/2024/04/winning-like-betting-professional.html
https://casino999.mystrikingly.com/blog/more-secure-betting-conversation-addresses-late-embarrassments
https://articleinsportsbetting.blogspot.com/2024/04/consistent-success-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ontario-sportsbooks-not-permitted-to-take-wagers-on-wba-boxing
https://bestsportsbettingarticles.blogspot.com/2024/04/truth-behind-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-sports-wagering-polling-form-drive-outperforms-300-000-marks
https://bettingstratigicarticle.blogspot.com/2024/04/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/washington-d-c-sports-wagering-handle-under-16-million-for-spring
https://sportswageringinfo.blogspot.com/2024/04/life-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://onlinesportsbettingstory.blogspot.com/2024/04/tools-resources-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-jersey-sports-wagering-handle-tops-1-3-billion-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/transitioning-betting-professional.html
https://casino999.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://articleinsportsbetting.blogspot.com/2024/04/financial-realities-professional-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/the-precepts-bettors-need-to-follow-for-dependable-parlay-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/betting-professionals-trends-make-decisions.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-guide.html
https://daddys-lucky000.mystrikingly.com/blog/2024-nba-season-finisher-wagering-review
https://sportswageringinfo.blogspot.com/2024/04/betting-professionals-stay-top.html
https://lucky-razi-777.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-future.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-becomes-essential-games-wagering-application-in-washington-d-c
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crusade-for-more-pleasant-betting-billions-bet-illicitly-on-ncaa-competitions
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-balancing-luck-skill.html
https://casino999.mystrikingly.com/blog/bally-bet-plans-for-june-sports-wagering-send-off-in-massachusetts
https://articleinsportsbetting.blogspot.com/2024/04/takes-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/tennessee-games-wagering-handle-hits-472-million-for-spring
https://bestsportsbettingarticles.blogspot.com/2024/04/ethical-dilemmas-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/here-s-to-you-mr-robinson-nate-robinson-dishes-on-the-knicks-sports
https://bettingstratigicarticle.blogspot.com/2024/04/betting-like-pro.html
https://daddys-lucky000.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-authorization-endeavors-fizzled
https://sportswageringinfo.blogspot.com/2024/04/secrets-behind-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/sports-bettors-bound-to-hit-the-bottle-hard-than-different-speculators
https://gameswageringarticles.blogspot.com/2024/04/psychology-betting-professionals.html
https://site-6861405-2997-4499.mystrikingly.com/blog/caitlin-clark-previously-carrying-monstrous-wagering-interest-to-the-wnba
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post_25.html
https://site-6828921-2394-9309.mystrikingly.com/blog/more-modest-games-associations-hustling-for-legitimate-games-wagering-open-doors
https://sportsbettingindustry.blogspot.com/2024/05/betting-change-perspective.html
https://casino999.mystrikingly.com/blog/football-is-top-dog-ms-september-sports-wagering-handle-ranges-31-77-million
https://articleinsportsbetting.blogspot.com/2024/04/betting-blunders-brilliance.html
https://site-4763676-9520-5980.mystrikingly.com/blog/after-lively-hearing-indiana-legislators-will-keep-on-investigating-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/lessons-learned-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://bettingstratigicarticle.blogspot.com/2024/04/lessons-from-world-betting.html
https://daddys-lucky000.mystrikingly.com/blog/study-mlb-nba-to-yield-joined-1-7-billion-from-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/04/essential-life-lessons-learned-betting.html
https://lucky-razi-777.mystrikingly.com/blog/hearing-uncovers-illinois-legislators-advancing-toward-legitimizing-sports
https://onlinesportsbettingstory.blogspot.com/2024/04/Career-betting-professional.html
https://site-6828921-2394-9309.mystrikingly.com/blog/the-arrangements-keep-comin-new-york-planes-mgm-figure-out-advertising
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-top-predictions.html
https://casino999.mystrikingly.com/blog/looking-at-replies-to-enter-sports-wagering-inquiries-in-illinois
https://articleinsportsbetting.blogspot.com/2024/04/mysteries-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pgcb-to-hold-hearings-decision-on-three-additional-games-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/highly-effective-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/minnesota-administrator-hopeful-but-still-guarded-about-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-exposed.html
https://daddys-lucky000.mystrikingly.com/blog/mgm-resorts-turns-into-nhl-s-true-games-wagering-accomplice-in-memorable-new
https://sportswageringinfo.blogspot.com/2024/04/lessons-learned-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/with-significant-declaration-impending-nhl-supposedly-betting-everything-on
https://gameswageringarticles.blogspot.com/2024/04/betting-will-blow-your-mind.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncaa-declares-foundation-of-new-advisory-group-to-look-at-sports-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/unconventional-lessons-learned-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/louisiana-officials-looking-at-sports-wagering-legitimization
https://sportsbettingindustry.blogspot.com/2024/04/lessons-learned-betting-strategies.html
https://casino999.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://articleinsportsbetting.blogspot.com/2024/04/unveiling-betting-wisdom.html
https://site-4763676-9520-5980.mystrikingly.com/blog/more-modest-games-associations-dashing-for-legitimate-games-wagering-open-doors
https://bestsportsbettingarticles.blogspot.com/2024/04/essential-lessons-every-risk-taker.html
https://site-6861241-1631-6047.mystrikingly.com/blog/football-is-above-all-else-ms-september-sports-wagering-handle-ranges-31-77
https://bettingstratigicarticle.blogspot.com/2024/04/betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/after-energetic-hearing-indiana-officials-will-keep-on-investigating-sports
https://sportswageringinfo.blogspot.com/2024/04/blog-post_29.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/blog-post_26.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://onlinesportsbettingstory.blogspot.com/2024/05/practical-lessons-smarter-decision-making.html
https://site-6828921-2394-9309.mystrikingly.com/blog/legislators-pushing-for-legitimate-games-wagering-in-washington-d-c
https://sportsbettingindustry.blogspot.com/2024/05/betting-lessons-kickstart-journey.html
https://casino999.mystrikingly.com/blog/sports-wagering-dispatches-in-new-mexico-we-anticipate-a-major-weekend
https://articleinsportsbetting.blogspot.com/2024/05/betting-lessons-learned-strategic-living.html
https://site-4763676-9520-5980.mystrikingly.com/blog/where-do-gubernatorial-competitors-stand-west-version
https://bestsportsbettingarticles.blogspot.com/2024/05/essential-lessons-every-gambler.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-examiner-in-game-betting-one-of-smartest-choices-to-make
https://bettingstratigicarticle.blogspot.com/2024/05/lessons-learned-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-on-plan-this-week-in-indiana-illinois-and-washington-d-c
https://sportswageringinfo.blogspot.com/2024/05/betting-secrets-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/september-new-jersey-sports-wagering-handle-leaps-to-184m-income-24m
https://gameswageringarticles.blogspot.com/2024/05/lessons-learned-high-stakes-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/where-do-gubernatorial-up-and-comers-stand-on-sports-wagering-east-version
해외배팅사이트 추천 https://spo337.com/
해외스포츠배팅사이트 추천 https://spo337.com/
해외배팅사이트에이전시 추천 https://spo337.com/
머니라인247 https://spo337.com/ml247/
황룡카지노 https://spo337.com/gdcasino/
아시안커넥트 https://spo337.com/asianconnect/
스보벳 https://spo337.com/sbobet/
피나클 https://spo337.com/pinbet88/
맥스벳 https://spo337.com/maxbet/
파워볼 https://spo337.com/category/powerball/
BTI Sports https://spo337.com/btisports/
https://rb.gy/4y0o7
https://sites.google.com/view/spo337/
https://bit.ly/45dAsE6
https://cutt.ly/dwbyj5Dq
https://ln.run/Cl2oj
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/iv97s
https://cutt.ly/2wbyki06
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/
https://ln.run/VribE
https://sites.google.com/view/hdcasino/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/3vh3j
https://cutt.ly/Cwbykk2t
https://sites.google.com/view/hdcasino/
https://ln.run/6Bw_0
https://sites.google.com/view/moneyline247/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/03f4u
https://sites.google.com/view/moneyline247/
https://duckduckgo.com/?q=https%3A%2F%2Fspo337.com&t=h_&ia=web
mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
materinstvo.ru/forward?link=http%3A%2F%2Fspo337.com%2F
http://computer-chess.org/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
https://enchantedcottageshop.com/shop/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
https://buist-keatch.org/sphider/include/click_counter.php?url=http%3A%2F%2Fspo337.com%2F
www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
rel.chubu-gu.ac.jp/soumokuji/cgi-bin/go.cgi?http%3A%2F%2Fspo337.com%2F
fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
www.veloxbox.us/link/?h=http%3A%2F%2Fspo337.com%2F
www.adult-plus.com/ys/rank.php?mode=link&id=592&url=http%3A%2F%2Fspo337.com%2F
mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
sparktime.justclick.ru/lms/api-login/?hash=MO18szcRUQdzpT%2FrstSCW5K8Gz6ts1NvTJLVa34vf1A%3D&authBhvr=1&email=videotrend24%40mail.ru&expire=1585462818&lms%5BrememberMe%5D=1&targetPath=http%3A%2F%2Fspo337.com%2F
http://vcteens.com/cgi-bin/at3/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.bquest.org/Links/Redirect.aspx?ID=164&url=http%3A%2F%2Fspo337.com%2F
www.stipendije.info/phpAdsNew/adclick.php?bannerid=129&zoneid=1&source=&dest=http%3A%2F%2Fspo337.com%2F
today.od.ua/redirect.php?url=http%3A%2F%2Fspo337.com%2F
search.kcm.co.kr/jump.php?url=http%3A%2F%2Fspo337.com%2F
laskma.megastart-slot.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
www.mir-stalkera.ru/go?http%3A%2F%2Fspo337.com%2F
duhocphap.edu.vn/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.millerovo161.ru/go?http%3A%2F%2Fspo337.com%2F
www.naturaltranssexuals.com/cgi-bin/a2/out.cgi?id=97&l=toplist&u=http%3A%2F%2Fspo337.com%2F
https://amanaimages.com/lsgate/?lstid=pM6b0jdQgVM-Y9ibFgTe6Zv1N0oD2nYuMA&lsurl=http%3A%2F%2Fspo337.com%2F
planszowkiap.pl/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://covenantpeoplesministry.org/cpm/wp/sermons/?show&url=http%3A%2F%2Fspo337.com%2F
https://mightypeople.asia/link.php?id=M0ZGNHFISkd2bFh0RmlwSFU4bDN4QT09&destination=http%3A%2F%2Fspo337.com%2F
www.chinaleatheroid.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
i.s0580.cn/module/adsview/content/?action=click&bid=5&aid=163&url=http%3A%2F%2Fspo337.com%2F&variable=&source=https%3A%2F%2Fcutepix.info%2Fsex%2Friley-reyes.php
jsv3.recruitics.com/redirect?rx_cid=506&rx_jobId=39569207&rx_url=http%3A%2F%2Fspo337.com%2F
www.supermoto8.com/sidebanner/62?href=http%3A%2F%2Fspo337.com%2F
http://www.youtube.de/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.es/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ca/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.nl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ch/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.be/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.se/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.dk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.no/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.gr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.at/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.bg/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.sk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.rs/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.si/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.hr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ee/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lu/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.tn/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.ke/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.cr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.kz/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cat/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ge/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F&gl=ml
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=DE
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=IT
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AR
https://www.youtube.at/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ch/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.fr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.es/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.jp/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.co.uk/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ru/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.pl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.gr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.nl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ca/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.cz/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AU
https://www.youtube.com.tw/redirect?q=http%3A%2F%2Fspo337.com%2F
www.mexicolore.co.uk/click.php?url=http%3A%2F%2Fspo337.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=http%3A%2F%2Fspo337.com%2F
www.invisalign-doctor.in/api/redirect?url=http%3A%2F%2Fspo337.com%2F
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=http%3A%2F%2Fspo337.com%2F
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=http%3A%2F%2Fspo337.com%2F
trackingapp4.embluejet.com/Mod_Campaigns/tracking.asp?idem=31069343&em=larauz@untref.edu.ar&ca=73143&ci=0&me=72706&of=581028&adirecta=0&url=http%3A%2F%2Fspo337.com%2F
smils.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=http%3A%2F%2Fspo337.com/
cipresso.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.vacacionartravel.com/DTCSpot/public/banner_redirect.aspx?idca=286&ids=75665176&cp=167&idcli=0&ci=2&p=http%3A%2F%2Fspo337.com%2F
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=http%3A%2F%2Fspo337.com%2F
www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=http%3A%2F%2Fspo337.com%2F
www.cccowe.org/lang.php?lang=en&url=http%3A%2F%2Fspo337.com%2F
www.joeshouse.org/booking?link=http%3A%2F%2Fspo337.com%2F&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://okane-antena.com/redirect/index/fid100269/?u=http%3A%2F%2Fspo337.com%2F
www.gmwebsite.com/web/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
swra.backagent.net/ext/rdr/?http%3A%2F%2Fspo337.com%2F
www.mytokachi.jp/index.php?type=click&mode=sbm&code=2981&url=http%3A%2F%2Fspo337.com%2F
www.wqketang.com/logout?goto=http%3A%2F%2Fspo337.com%2F
access.bridges.com/externalRedirector.do?url=http%3A%2F%2Fspo337.com%2F
www.dolgin.net/zen_dolgin/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
bot.buymeapie.com/recipe?url=http%3A%2F%2Fspo337.com%2F
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=http%3A%2F%2Fspo337.com%2F
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
http://blackwhitepleasure.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.knet-web.net/m/pRedirect.php?uID=2&iID=259&iURL=http%3A%2F%2Fspo337.com%2F
azlan.techdata.com/InTouch/GUIBnrT3/BnrTrackerPublic.aspx?CountryCode=18&BannerLangCulture=nl-nl&URL=http%3A%2F%2Fspo337.com%2F&Target=2&BannerId=41919&Zoneid=281&Parameters=&cos=Azlan
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44__zoneid=1__cb=7743e8d201__oadest=http%3A%2F%2Fspo337.com%2F
www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=http%3A%2F%2Fspo337.com%2F
www.lzmfjj.com/Go.asp?URL=http%3A%2F%2Fspo337.com%2F
http://alexmovs.com/cgi-bin/atx/out.cgi?id=148&tag=topatx&trade=http%3A%2F%2Fspo337.com%2F
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2__bannerid=40__zoneid=16__cb=fc1d72225c__oadest=http%3A%2F%2Fspo337.com%2F
psylive.ru/success.aspx?id=0&goto=spo337.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=http%3A%2F%2Fspo337.com%2F
www.mybunnies.net/te3/out.php?u=http%3A%2F%2Fspo337.com%2F
lubaczowskie.pl/rdir/?l=http%3A%2F%2Fspo337.com%2F&lid=1315
www.kowaisite.com/bin/out.cgi?id=kyouhuna&url=http%3A%2F%2Fspo337.com%2F
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=http%3A%2F%2Fspo337.com%2F
www.wave24.net/cgi-bin/linkrank/out.cgi?id=106248&cg=1&url=spo337.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
zh-hk.guitarians.com/home/redirect/ubid/1015?r=http%3A%2F%2Fspo337.com%2F
www.cumshoter.com/cgi-bin/at3/out.cgi?id=98&tag=top&trade=http%3A%2F%2Fspo337.com%2F
shp.hu/hpc_uj/click.php?ml=5&url=http%3A%2F%2Fspo337.com%2F
lrnews.ru/xgo.php?url=http%3A%2F%2Fspo337.com%2F
www.dive-international.net/places/redirect.php?b=797&web=www.http%3A%2F%2Fspo337.com%2F
www.themza.com/redirect.php?r=http%3A%2F%2Fspo337.com%2F
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://http%3A%2F%2Fspo337.com%2F
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=http%3A%2F%2Fspo337.com%2F
spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
forest.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
reefcentral.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=http%3A%2F%2Fspo337.com%2F
https://trackdaytoday.com/redirect-out?url=http%3A%2F%2Fspo337.com%2F
https://bigjobslittlejobs.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=4__zoneid=4__cb=68dbdae1d1__oadest=http%3A%2F%2Fspo337.com%2F
www.obertaeva.com/include/get.php?go=http%3A%2F%2Fspo337.com%2F
www.quanmama.com/t/goto.aspx?url=http%3A%2F%2Fspo337.com%2F
quartiernetz-friesenberg.ch/links-go.php?to=http%3A%2F%2Fspo337.com%2F
http://anorexicpornmovies.com/cgi-bin/atc/out.cgi?id=20&u=http%3A%2F%2Fspo337.com%2F
www.maultalk.com/url.php?to=http%3A%2F%2Fspo337.com%2F
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=http%3A%2F%2Fspo337.com%2F
www.e-expo.net/category/click_url.html?url=http%3A%2F%2Fspo337.com%2F
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=http%3A%2F%2Fspo337.com%2F
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=http%3A%2F%2Fspo337.com%2F
http://getdatasheet.com/url.php?url=http%3A%2F%2Fspo337.com%2F
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=http%3A%2F%2Fspo337.com%2F
www.aldolarcher.com/tools/esstat/esdown.asp?File=http%3A%2F%2Fspo337.com%2F
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&no_chat=1&domain=http%3A%2F%2Fspo337.com%2F
cs.payeasy.com.tw/click?url=http%3A%2F%2Fspo337.com%2F
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=http%3A%2F%2Fspo337.com%2F
http://tswzjs.com/go.asp?url=http%3A%2F%2Fspo337.com%2F
asstomouth.guru/out.php?url=http%3A%2F%2Fspo337.com%2F
advtest.exibart.com/adv/adv.php?id_banner=7201&link=http%3A%2F%2Fspo337.com%2F
https://thairesidents.com/l.php?b=85&p=2,5&l=http%3A%2F%2Fspo337.com%2F
www.latestnigeriannews.com/link_channel.php?channel=http%3A%2F%2Fspo337.com%2F
www.haogaoyao.com/proad/default.aspx?url=http%3A%2F%2Fspo337.com%2F
globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
citysafari.nl/Home/setCulture?language=en&returnUrl=http%3A%2F%2Fspo337.com%2F
es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
lorena-kuhni.kz/redirect?link=http%3A%2F%2Fspo337.com%2F
www.webshoptrustmark.fr/Change/en?returnUrl=http%3A%2F%2Fspo337.com%2F
https://processon.com/setting/locale?language=zh&back=http%3A%2F%2Fspo337.com%2F
c.yam.com/srh/wsh/r.c?http%3A%2F%2Fspo337.com%2F
www.jolletorget.no/J/l.php?l=http%3A%2F%2Fspo337.com%2F
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=http%3A%2F%2Fspo337.com%2F
www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
www.sparetimeteaching.dk/forward.php?link=http%3A%2F%2Fspo337.com%2F
https://paspn.net/default.asp?p=90&gmaction=40&linkid=52&linkurl=http%3A%2F%2Fspo337.com%2F
www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
www.poddebiczak.pl/?action=set-desktop&url=http%3A%2F%2Fspo337.com%2F
ant53.ru/file/link.php?url=http%3A%2F%2Fspo337.com%2F
www.docin.com/jsp_cn/mobile/tip/android_v1.jsp?forward=http%3A%2F%2Fspo337.com%2F
old.magictower.ru/cgi-bin/redir/redir.pl?http%3A%2F%2Fspo337.com%2F
go.flx1.com/click?id=1&m=11&pl=113&dmcm=16782&euid=16603484876&out=http%3A%2F%2Fspo337.com%2F
moba-hgh.de/link2http.php?href=http%3A%2F%2Fspo337.com%2F
https://gazetablic.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=34__zoneid=26__cb=0e0dfef92b__oadest=http%3A%2F%2Fspo337.com%2F
watchvideo.co/go.php?url=http%3A%2F%2Fspo337.com%2F
www.todoku.info/gpt/rank.cgi?mode=link&id=29649&url=http%3A%2F%2Fspo337.com%2F
www.fotochki.com/redirect.php?go=http%3A%2F%2Fspo337.com%2F
bayerwald.tips/plugins/bannerverwaltung/bannerredirect.php?bannerid=1&url=http%3A%2F%2Fspo337.com%2F
ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=http%3A%2F%2Fspo337.com%2F
www.widgetinfo.net/read.php?sym=FRA_LM&url=http%3A%2F%2Fspo337.com%2F
arctic.nyheter24.se/rdb/nyheter24_eed6ad4b451f2fb8193922f832bc91ed/5?url=http%3A%2F%2Fspo337.com%2F
www.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
school.mosreg.ru/soc/moderation/abuse.aspx?link=http%3A%2F%2Fspo337.com%2F
affiliates.kanojotoys.com/affiliate/scripts/click.php?a_aid=widdi77&desturl=http%3A%2F%2Fspo337.com%2F
www.gotomypctech.com/affiliates/scripts/click.php?a_aid=ed983915&a_bid=&desturl=http%3A%2F%2Fspo337.com%2F
www.my-sms.ru/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F&rel=external
www.genderpsychology.com/http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
www.360wichita.com/amp-banner-tracking?adid=192059&url=http%3A%2F%2Fspo337.com%2F
www.tsv-bad-blankenburg.de/cms/page/mod/url/url.php?eid=16&urlpf=http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://zerlong.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
catalog.flexcom.ru/go?z=36047&i=55&u=http%3A%2F%2Fspo337.com%2F
www.wellvit.nl/response/forward/c1e41491e30c5af3c20f80a2af44e440.php?link=0&target=http%3A%2F%2Fspo337.com%2F
www.ident.de/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=76__zoneid=2__cb=8a18c95a9e__oadest=http%3A%2F%2Fspo337.com%2F
www.fertilab.net/background_manager.aspx?ajxName=link_banner&id_banner=50&url=http%3A%2F%2Fspo337.com%2F
shop-uk.fmworld.com/Queue/Index?url=http%3A%2F%2Fspo337.com%2F
www.weddinginlove.com/redirect/?url=http%3A%2F%2Fspo337.com%2F
nieuws.rvent.nl/bitmailer/statistics/mailstatclick/42261?link=http%3A%2F%2Fspo337.com%2F
https://jobanticipation.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobanticipation.com
www.xsbaseball.com/tracker/index.html?t=ad&pool_id=3&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
eos.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
b.sm.su/click.php?bannerid=56&zoneid=10&source=&dest=http%3A%2F%2Fspo337.com%2F
https://bethlehem-alive.com/abnrs/countguideclicks.cfm?targeturl=http%3A%2F%2Fspo337.com%2F&businessid=29579
www.bookmark-favoriten.com/?goto=http%3A%2F%2Fspo337.com%2F
shop.yuliyababich.eu/RU/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
www.depmode.com/go.php?http%3A%2F%2Fspo337.com%2F
https://urgankardesler.com/anasayfa/yonlen?link=http%3A%2F%2Fspo337.com%2F
www.metalindex.ru/netcat/modules/redir/?&site=http%3A%2F%2Fspo337.com%2F
www.rprofi.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://darudar.org/external/?link=http%3A%2F%2Fspo337.com%2F
www.postsabuy.com/autopost4/page/generate/?link=http%3A%2F%2Fspo337.com%2F&list=PL9d7lAncfCDSkF4UPyhzO59Uh8cOoD-8q&fb_node=942812362464093&picture&name=%E0%B9%82%E0%B8%9B%E0%B8%A3%E0%B9%81%E0%B8%81%E0%B8%A3%E0%B8%A1%E0%B9%82%E0%B8%9E%E0%B8%AA%E0%B8%82%E0%B8%B2%E0%B8%A2%E0%B8%AA%E0%B8%B4%E0%B8%99%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%AD%E0%B8%AD%E0%B8%99%E0%B9%84%E0%B8%A5%E0%B8%99%E0%B9%8C+&caption=%E0%B9%80%E0%B8%A5%E0%B8%82%E0%B8%B2%E0%B8%AA%E0%B9%88%E0%B8%A7%E0%B8%99%E0%B8%95%E0%B8%B1%E0%B8%A7+%E0%B8%97%E0%B8%B5%E0%B8%84%E0%B8%B8%E0%B8%93%E0%B8%A5%E0%B8%B7%E0%B8%A1%E0%B9%84%E0%B8%A1%E0%B9%88%E0%B8%A5%E0%B8%87+Line+%40postsabuy&description=%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%B2%E0%B8%96%E0%B8%B9%E0%B8%81%E0%B8%97%E0%B8%B5%E0%B9%88%E0%B8%AA%E0%B8%B8%E0%B8%94%E0%B9%83%E0%B8%99+3+%E0%B9%82%E0%B8%A5%E0%B8%81+%E0%B8%AD%E0%B8%B4%E0%B8%AD%E0%B8%B4
www.perimeter.org/track.pdf?url=http%3A%2F%2Fspo337.com%2F
forum.darievna.ru/go.php?http%3A%2F%2Fspo337.com%2F
techlab.rarus.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.spiritualforums.com/vb/redir.php?link=http%3A%2F%2Fspo337.com%2F
www.review-mag.com/cdn/www/delivery/view.php?ct=1&oaparams=2__bannerid=268__zoneid=1__cb=8c1317f219__oadest=http%3A%2F%2Fspo337.com%2F
belantara.or.id/lang/s/ID?url=http%3A%2F%2Fspo337.com%2F
ele-market.ru/consumer.php?url=http%3A%2F%2Fspo337.com%2F
https://www.хорошие-сайты.рф/r.php?r=http%3A%2F%2Fspo337.com%2F
https://jobsflagger.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://gpoltava.com/away/?go=http%3A%2F%2Fspo337.com%2F
www.cardexchange.com/index.php/tools/packages/tony_mailing_list/services/?mode=link&mlm=62&mlu=0&u=2&url=http%3A%2F%2Fspo337.com%2F
services.nfpa.org/Authentication/GetSSOSession.aspx?return=http%3A%2F%2Fspo337.com%2F
http://spaceup.org/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
yarko-zhivi.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
365sekretov.ru/redirect.php?action=url&goto=http%3A%2F%2Fspo337.com%2F%20
www.sgdrivingtest.com/redirect.php?page=http%3A%2F%2Fspo337.com%2F
www.jxren.com/news/link/link.asp?id=7&url=http%3A%2F%2Fspo337.com%2F
particularcareers.co.uk/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://bsaonline.com/MunicipalDirectory/SelectUnit?unitId=411&returnUrl=http%3A%2F%2Fspo337.com%2F&sitetransition=true
www.elit-apartament.ru/go?http%3A%2F%2Fspo337.com%2F
sendai.japansf.net/rank.cgi?mode=link&id=1216&url=http%3A%2F%2Fspo337.com%2F
www.bmwfanatics.ru/goto.php?l=http%3A%2F%2Fspo337.com%2F
www.saabsportugal.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
cms.sive.it/Jump.aspx?gotourl=http%3A%2F%2Fspo337.com%2F
largusladaclub.ru/go/url=https:/http%3A%2F%2Fspo337.com%2F
https://kekeeimpex.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=GBP&cRate=77.86247
mycounter.com.ua/go.php?http%3A%2F%2Fspo337.com%2F
l2base.su/go?http%3A%2F%2Fspo337.com%2F
https://freeseotool.org/url/?q=http%3A%2F%2Fspo337.com%2F
www.duomodicagliari.it/reg_link.php?link_ext=http%3A%2F%2Fspo337.com%2F&prov=1
assine.hostnet.com.br/cadastro/?rep=17&url=http%3A%2F%2Fspo337.com%2F
www.dvnlp.de/profile/gruppe/redirect/5?url=http%3A%2F%2Fspo337.com%2F
http://hotmaturegirlfriends.com/cl.php?porno=MzB4MzB4NjEw&url=http%3A%2F%2Fspo337.com%2F
www.smkn5pontianak.sch.id/redirect/?alamat=http%3A%2F%2Fspo337.com%2F
www.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
bilometro.brksedu.com.br/tracking?url=http%3A%2F%2Fspo337.com%2F&zorigem=hotsite-blackfriday
www.omschweiz.ch/select-your-country?publicUrl=http%3A%2F%2Fspo337.com%2F
https://anacolle.net/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.cubamusic.com/Home/ChangeLanguage?lang=es-ES&returnUrl=http%3A%2F%2Fspo337.com%2F
expoclub.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.deypenburgschecourant.nl/reklame/www/delivery/ck.php?oaparams=2__bannerid=44__zoneid=11__cb=078c2a52ea__oadest=http%3A%2F%2Fspo337.com%2F
tramplintk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
cnc.extranet.gencat.cat/treball_cnc/AppJava/FileDownload.do?pdf=http%3A%2F%2Fspo337.com%2F&codi_cnv=9998045
www.gamecollections.co.uk/search/redirect.php?retailer=127&deeplink=http%3A%2F%2Fspo337.com%2F
bearcong.no1.sexy/hobby-delicious/rank.cgi?mode=link&id=19&url=http%3A%2F%2Fspo337.com%2F
dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
www.surinenglish.com/backend/conectar.php?url=http%3A%2F%2Fspo337.com%2F
www.reference-cannabis.com/interface/sortie.php?adresse=http%3A%2F%2Fspo337.com%2F
login.0×69416d.co.uk/sso/logout?tenantId=tnl&gotoUrl=http%3A%2F%2Fspo337.com%2F&domain=0×69416d.co.uk
blog.link-usa.jp/emi?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://damki.net/go/?http%3A%2F%2Fspo337.com%2F
opensesame.wellymulia.zaxaa.com/b/66851136?s=1&redir=http%3A%2F%2Fspo337.com%2F
ism3.infinityprosports.com/ismdata/2009100601/std-sitebuilder/sites/200901/www/en/tracker/index.html?t=ad&pool_id=1&ad_id=112&url=http%3A%2F%2Fspo337.com%2F
apps.cancaonova.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=149__zoneid=20__cb=87d2c6208d__oadest=http%3A%2F%2Fspo337.com%2F
www.lespritjardin.be/?advp_click_bimage_id=19&url=http%3A%2F%2Fspo337.com%2F&shortcode_id=10
www.dresscircle-net.com/psr/rank.cgi?mode=link&id=14&url=http%3A%2F%2Fspo337.com%2F
www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
fms.csonlineschool.com.au/changecurrency/1?returnurl=http%3A%2F%2Fspo337.com%2F
www.v-archive.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.jagat.co.jp/analysis/analysis.php?url=http%3A%2F%2Fspo337.com%2F
https://vse-doski.com/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.karatetournaments.net/link7.asp?LRURL=http%3A%2F%2Fspo337.com%2F&LRTYP=O
simracing.su/go/?http%3A%2F%2Fspo337.com%2F
click.cheshi.com/go.php?proid=218&clickid=1393306648&url=http%3A%2F%2Fspo337.com%2F
fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
flypoet.toptenticketing.com/index.php?url=http%3A%2F%2Fspo337.com%2F
www.horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
d-click.fiemg.com.br/u/18081/131/75411/137_0/82cb7/?url=http%3A%2F%2Fspo337.com%2F
www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
www.packmage.net/uc/goto/?url=http%3A%2F%2Fspo337.com%2F
my.9991.com/login_for_index_0327.php?action=logout&forward=http%3A%2F%2Fspo337.com%2F
www.sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=986&redirect=http%3A%2F%2Fspo337.com%2F
www.naturum.co.jp/ad/linkshare/?siteID=p_L785d6UQY-V4Fh4Rxs7wNzOPgtzv95Tg&lsurl=http%3A%2F%2Fspo337.com%2F
www.d-e-a.eu/newsletter/redirect.php?link=http%3A%2F%2Fspo337.com%2F
www.bom.ai/goweburl?go=http%3A%2F%2Fspo337.com%2F
enews2.sfera.net/newsletter/redirect.php?id=sabricattani@gmail.com_0000006566_144&link=http%3A%2F%2Fspo337.com%2F
underwater.com.au/redirect_url/id/7509/?redirect=http%3A%2F%2Fspo337.com%2F
https://eqsoftwares.com/languages/setlanguage?languagesign=en&redirect=http%3A%2F%2Fspo337.com%2F
www.jagdambasarees.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=MYR&cRate=14.554
www.priegeltje.nl/gastenboek/go.php?url=http%3A%2F%2Fspo337.com%2F
www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.rz114.cn/url.html?url=http%3A%2F%2Fspo337.com%2F
www.greatdealsindia.com/redirects/infibeam.aspx?url=http%3A%2F%2Fspo337.com%2F
rs.345kei.net/rank.php?id=37&mode=link&url=http%3A%2F%2Fspo337.com%2F
webapp.jgz.la/?c=scene&a=link&id=8665466&url=http%3A%2F%2Fspo337.com%2F
www.erotiikkalelut.com/url.php?link=http%3A%2F%2Fspo337.com%2F
vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
https://fachowiec.com/zliczanie-bannera?id=24&url=http%3A%2F%2Fspo337.com%2F
ieea.ir/includes/change_lang.php?lang=en&goto=http%3A%2F%2Fspo337.com%2F
scribe.mmonline.io/click?evt_nm=Clicked+Registration+Completion&evt_typ=clickEmail&app_id=m4marry&eml_sub=Registration+Successful&usr_did=4348702&cpg_sc=NA&cpg_md=email&cpg_nm=&cpg_cnt=&cpg_tm=NA&link_txt=Live+Chat&em_type=Notification&url=http%3A%2F%2Fspo337.com%2F
polo-v1.feathr.co/v1/analytics/crumb?flvr=email_link_click&rdr=http%3A%2F%2Fspo337.com%2F
sintesi.provincia.mantova.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F
https://careerchivy.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
shinsekai.type.org/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
https://maned.com/scripts/lm/lm.php?tk=CQkJZWNuZXdzQGluZm90b2RheS5jb20JW05ld3NdIE1FSSBBbm5vdW5jZXMgUGFydG5lcnNoaXAgV2l0aCBUd2l4bCBNZWRpYQkxNjcyCVBSIE1lZGlhIENvbnRhY3RzCTI1OQljbGljawl5ZXMJbm8=&url=http%3A%2F%2Fspo337.com%2F
www.etaigou.com/turn2.php?ad_id=276&link=http%3A%2F%2Fspo337.com%2F
crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
www.tagirov.org/out.php?url=http%3A%2F%2Fspo337.com%2F
https://startlist.club/MSF/Language/Set?languageIsoCode=en&returnUrl=http%3A%2F%2Fspo337.com%2F
www.tetsumania.net/search/rank.cgi?mode=link&id=947&url=http%3A%2F%2Fspo337.com%2F
https://imperial-info.net/link?idp=125&url=http%3A%2F%2Fspo337.com%2F
www.brainlanguage-sa.com/setcookie.php?lang=en&file=http%3A%2F%2Fspo337.com%2F
www.asensetranslations.com/modules/babel/redirect.php?newlang=en_US&newurl=http%3A%2F%2Fspo337.com%2F
gameshock.jeez.jp/rank.cgi?mode=link&id=307&url=http%3A%2F%2Fspo337.com%2F
https://tripyar.com/go.php?http%3A%2F%2Fspo337.com%2F
www.floridafilmofficeinc.com/?goto=http%3A%2F%2Fspo337.com%2F
tsvc1.teachiworld.com/bin/checker?mode=4&module=11&mailidx=19130&dmidx=0&emidx=0&service=0&cidx=&etime=20120328060000&seqidx=3&objidx=22&encoding=0&url=http%3A%2F%2Fspo337.com%2F
rechner.atikon.at/lbg.at/newsletter/linktracking?subscriber=&delivery=38116&url=http%3A%2F%2Fspo337.com%2F
www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
go.eniro.dk/lg/ni/cat-2611/http:/http%3A%2F%2Fspo337.com%2F
www.fisherly.com/redirect?type=website&ref=listing_detail&url=http%3A%2F%2Fspo337.com%2F
bio-pack.ru/bitrix/redirect.php?goto=http://http%3A%2F%2Fspo337.com%2F
fid.com.ua/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.modernipanelak.cz/?b=618282165&redirect=http%3A%2F%2Fspo337.com%2F
h5.hbifeng.com/index.php?c=scene&a=link&id=14240604&url=http%3A%2F%2Fspo337.com%2F
www.bkdc.ru/bitrix/redirect.php?event1=news_out&event2=32reg.roszdravnadzor.ru/&event3=A0A0B5A09180D0%A09582A0BBA1A085%D0E2A084D0D1C2D0%A085+A0A0B5A182B0A0%C2D0D0D096+A1A0BBA0B180D0%A09795+A0A0B0A09582A1%D1D0D0D0A182B5+A0A091A08695A0%D1D0A6A185A0A085%D0D1D0D082A1A085%D0D0D1D0A095B1A0%C2D0D0D091&goto=http%3A%2F%2Fspo337.com%2F
record.affiliatelounge.com/WS-jvV39_rv4IdwksK4s0mNd7ZgqdRLk/7/?deeplink=http%3A%2F%2Fspo337.com%2F
d-click.artenaescola.org.br/u/3806/290/32826/1416_0/53052/?url=http%3A%2F%2Fspo337.com%2F
www.morroccoaffiliate.com/aff.php?id=883&url=http%3A%2F%2Fspo337.com%2F
www.flooble.com/cgi-bin/clicker.pl?id=grabbadl&url=http%3A%2F%2Fspo337.com%2F
www.sportsbook.ag/ctr/acctmgt/pl/openLink.ctr?ctrPage=http%3A%2F%2Fspo337.com%2F
www.ra2d.com/directory/redirect.asp?id=596&url=http%3A%2F%2Fspo337.com%2F
www.anorexicnudes.net/cgi-bin/atc/out.cgi?u=http%3A%2F%2Fspo337.com%2F
www.zjjiajiao.com.cn/ad/adredir.asp?url=http%3A%2F%2Fspo337.com%2F
https://hakobo.com/wp/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
t.agrantsem.com/tt.aspx?cus=216&eid=1&p=216-2-71016b553a1fa2c9.3b14d1d7ea8d5f86&d=http%3A%2F%2Fspo337.com%2F
moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.cheek.co.jp/location/location.php?id=keibaseminar&url=http%3A%2F%2Fspo337.com%2F
www.quantixtickets3.com/php-bin-8/kill_session_and_redirect.php?redirect=http%3A%2F%2Fspo337.com%2F
www.photokonkurs.com/cgi-bin/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.nbda.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.thislife.net/cgi-bin/webcams/out.cgi?id=playgirl&url=http%3A%2F%2Fspo337.com%2F
https://www.dans-web.nu/klick.php?url=http%3A%2F%2Fspo337.com%2F
https://voobrajulya.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.nafta-him.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.kyoto-osaka.com/search/rank.cgi?mode=link&id=9143&url=http%3A%2F%2Fspo337.com%2F
www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
red.ribbon.to/~zkcsearch/zkc-search/rank.cgi?mode=link&id=156&url=http%3A%2F%2Fspo337.com%2F
akademik.tkyd.org/Home/SetCulture?culture=en-US&returnUrl=http%3A%2F%2Fspo337.com%2F
www.rencai8.com/web/jump_to_ad_url.php?id=642&url=http%3A%2F%2Fspo337.com%2F
https://www.baby22.com.tw/Web/turn.php?ad_id=160&link=http%3A%2F%2Fspo337.com%2F
https://www.kichink.com/home/issafari?uri=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=1032500&url=http%3A%2F%2Fspo337.com%2F
5cfxm.hxrs6.servertrust.com/v/affiliate/setCookie.asp?catId=1180&return=http%3A%2F%2Fspo337.com%2F
http://3dcreature.com/cgi-bin/at3/out.cgi?id=187&trade=http%3A%2F%2Fspo337.com%2F
https://nowlifestyle.com/redir.php?k=9a4e080456dabe5eebc8863cde7b1b48&url=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
www.acutenet.co.jp/cgi-bin/lcount/lcounter.cgi?link=http%3A%2F%2Fspo337.com%2F
http://news-matome.com/method.php?method=1&url=http%3A%2F%2Fspo337.com%2F
https://lifecollection.top/site/gourl?url=http%3A%2F%2Fspo337.com%2F
https://www.toscanapiu.com/web/lang.php?lang=DEU&oldlang=ENG&url=http%3A%2F%2Fspo337.com%2F
speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.sainttropeztourisme.com/en/bannieres/redirection/index.html?id=649&lien=http%3A%2F%2Fspo337.com%2F
www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
www.interracialhall.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
https://www.tumimusic.com/link.php?url=http%3A%2F%2Fspo337.com%2F
www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
mail.resen.gov.mk/redir.hsp?url=http%3A%2F%2Fspo337.com%2F
https://www.pompengids.net/followlink.php?id=495&link=http%3A%2F%2Fspo337.com%2F
https://www.hirforras.net/scripts/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://lens-club.ru/link?go=http%3A%2F%2Fspo337.com%2F
https://t.raptorsmartadvisor.com/.lty?url=http%3A%2F%2Fspo337.com%2F&loyalty_id=14481&member_id=b01bbee6-4592-4345-a0ee-5d71ed6f1929
https://evoautoshop.com/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://beautycottageshop.com/change.php?lang=cn&url=http%3A%2F%2Fspo337.com%2F
http://1000love.net/lovelove/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.ffw-ellar.de/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://www.contactlenshouse.com/currency.asp?c=CAD&r=http%3A%2F%2Fspo337.com%2F
https://sogo.i2i.jp/link_go.php?url=http%3A%2F%2Fspo337.com%2F
https://ceb.bg/catalog/statistic/?id=61&location=http%3A%2F%2Fspo337.com%2F
https://malehealth.ie/redirect/?age=40&part=waist&illness=obesity&refer=http%3A%2F%2Fspo337.com%2F
https://magicode.me/affiliate/go?url=http%3A%2F%2Fspo337.com%2F
https://jobregistry.net/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobregistry.net&rgp_m=title13&et=4495
https://www.goatzz.com/adredirect.aspx?adType=SiteAd&ItemID=9595&ReturnURL=http%3A%2F%2Fspo337.com%2F
https://miyagi.lawyer-search.tv/details/linkchk.aspx?type=o&url=http%3A%2F%2Fspo337.com%2F
https://www.snwebcastcenter.com/event/page/count_download_time.php?url=http%3A%2F%2Fspo337.com%2F
https://go.onelink.me/v1xd?pid=Patch&c=Mobile%20Footer&af_web_dp=http%3A%2F%2Fspo337.com%2F
https://www.weather.net/cgi-bin/redir?http%3A%2F%2Fspo337.com%2F
https://gaggedtop.com/cgi-bin/top/out.cgi?ses=sBZFnVYGjF&id=206&url=http%3A%2F%2Fspo337.com%2F
https://e-bike-test.net/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://www.luckyplants.com/cgi-bin/toplist/out.cgi?id=rmontero&url=http%3A%2F%2Fspo337.com%2F
https://webankety.cz/dalsi.aspx?site=http%3A%2F%2Fspo337.com%2F
https://runkeeper.com/apps/authorize?redirect_uri=http%3A%2F%2Fspo337.com%2F
https://www.emiratesvoice.com/footer/comment_like_dislike_ajax/?code=like&commentid=127&redirect=http%3A%2F%2Fspo337.com%2F
https://envios.uces.edu.ar/control/click.mod.php?id_envio=8147&email=gramariani@gmail.com&url=http%3A%2F%2Fspo337.com%2F
https://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://www.ab-search.com/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.studyrama.be/tracking.php?origine=ficheform5683&lien=http://http%3A%2F%2Fspo337.com%2F
https://shop.merchtable.com/users/authorize?return_url=http%3A%2F%2Fspo337.com%2F
https://nudewwedivas.forumcommunity.net/m/ext.php?url=http%3A%2F%2Fspo337.com%2F
https://cztt.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://mientaynet.com/advclick.php?o=textlink&u=15&l=http%3A%2F%2Fspo337.com%2F
https://atlanticleague.com/tracker/index.html?t=ad&pool_id=11&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
https://www.podcastone.com/site/rd?satype=40&said=4&aaid=email&camid=-4999600036534929178&url=http%3A%2F%2Fspo337.com%2F
https://www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://monarchbeachmembers.play18.com/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
https://wayi.com.tw/wayi_center.aspx?flag=banner&url=http%3A%2F%2Fspo337.com%2F&idno=443
https://d.agkn.com/pixel/2389/?che=2979434297&col=22204979,1565515,238211572,435508400,111277757&l1=http%3A%2F%2Fspo337.com%2F
https://mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
https://bemidji.bigdealsmedia.net/include/sort.php?return_url=http%3A%2F%2Fspo337.com%2F&sort=a:3:{s:9:%E2%80%9Ddirection%E2%80%9D;s:3:%E2%80%9DASC%E2%80%9D;s:5:%E2%80%9Dfield%E2%80%9D;s:15:%E2%80%9DItems.PriceList%E2%80%9D;s:5:%E2%80%9Dlabel%E2%80%9D;s:9:%E2%80%9Dvalue_asc%E2%80%9D;}
https://hslda.org/content/a/LinkTracker.aspx?id=4015475&appeal=385&package=36&uri=http%3A%2F%2Fspo337.com%2F
https://panarmenian.net/eng/tofv?tourl=http%3A%2F%2Fspo337.com%2F
https://lavoro.provincia.como.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=935
https://www.dodeley.com/?action=show_ad&url=http%3A%2F%2Fspo337.com%2F
https://www.ignicaodigital.com.br/affiliate/?idev_id=270&u=http%3A%2F%2Fspo337.com%2F
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://m.wedkuje.pl/mobile/redirect.php?redir=http%3A%2F%2Fspo337.com%2F
https://area51.to/go/out.php?s=100&l=site&u=http%3A%2F%2Fspo337.com%2F
https://games4ever.3dn.ru/go?http%3A%2F%2Fspo337.com%2F
https://de.reasonable.shop/SetCurrency.aspx?currency=CNY&returnurl=http%3A%2F%2Fspo337.com%2F
https://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
https://mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.shop-bell.com/out.php?id=kibocase&category=ladies&url=http%3A%2F%2Fspo337.com%2F
https://m.addthis.com/live/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
https://horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
https://soom.cz/projects/get2mail/redir.php?id=c2e52da9ad&url=http%3A%2F%2Fspo337.com%2F
https://www.iaai.com/VehicleInspection/InspectionProvidersUrl?name=AA%20Transit%20Pros%20Inspection%20Service&url=http%3A%2F%2Fspo337.com%2F
https://cingjing.fun-taiwan.com/AdRedirector.aspx?padid=303&target=http%3A%2F%2Fspo337.com%2F
https://rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
https://campaign.unitwise.com/click?emid=31452&emsid=ee720b9f-a315-47ce-9552-fd5ee4c1c5fa&url=http%3A%2F%2Fspo337.com%2F
https://www.swipeclock.com/sc/cookie.asp?sitealias=79419397&redirect=http%3A%2F%2Fspo337.com%2F
https://www.gutscheinaffe.de/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://home.uceusa.com/Redirect.aspx?r=http%3A%2F%2Fspo337.com%2F
https://www.seankenney.com/include/jump.php?num=http%3A%2F%2Fspo337.com%2F
https://runningcheese.com/go?url=http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
https://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
https://crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
http://dstats.net/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://union.591.com.tw/stats/event/redirect?e=eyJpdiI6IjdUd1B5Z2FPTmNWQzBmZk1LblR2R0E9PSIsInZhbHVlIjoiQTI4TnVKMzdjMkxrUjcrSWlkcXdzbjRQeGRtZ0ZGbXdNSWxkSkVieENwNjQ1cHF5aDZmWmFobU92ZGVyUk5jRTlxVnI2TG5pb0dJVHZSUUlHcXFTbGo3UDliYWU5UE5MSjlMY0xOQnFmbVRQSFNoZDRGd2dqVDZXZEU4WFoyajJ0S0JITlQ2XC9SXC9jRklPekdmcnFGb09vRllqNHVtTHlYT284ZmN3d0ozOHFkclRYYnU5UlY2NTFXSGRheW5SbGxJb3BmYjQ2Mm9TWUFCTEJuXC9iT25nYkg4QXpOd2pHVlBWTWxWXC91aWRQMVhKQmVJXC9qMW9IdlZaVVlBdWlCYW4rS0JualhSMElFeVZYN3NnUW1qcUdxcWUrSlFROFhKbWttdkdvMUJ3aWVRa2I3MVV5TXpER3doa2ZuekFWNWd3OGpuQ1VSczFDREVKaklaUks0TTRIY2pUeXYrQmdZYUFTK1F4RWpTY0RRaW5Nc0krdVJ2N2VUT1wvSUxVVWVKN3hnQU92QmlCbjQyMUpRdTZKVWJcL0RCSVFOcWl0azl4V2pBazBHWmVhdWptZGREVXh0VkRNWWxkQmFSYXhBRmZtMHA5dTlxMzIzQzBVaWRKMEFqSG0wbGkxME01RDBaaElTaU5QKzIxbSswaUltS0FYSzViZlFmZjZ1XC9Yclg0U2VKdHFCc0pTNndcL09FWklUdjlQM2dcL2RuN0szQ3plWmcyYWdpQzJDQ2NIcWROczVua3dIM1Q3OXpJY3Z0XC93MVk3SHUyODZHU3Z5aHFVbWEwRFU1ZFdyMGt0YWpsb3BkQitsZER5aWk4YWMrZWYzSFNHNERhOGdDeUJWeEtoSm9wQ0hQb2EzOHZ3eHFGVTQ2Mk1QSEZERzlXZWxRRTJldjJkdnZUM0ZwaW1KcEVVc3ZXWjRHaTZWRDJOK0YxR3d4bXhMR3BhWmZBNkJ6eUYxQjR4ODVxc0d0YkFpYU8yZ2tuWGdzelBpU3dFUjJVYUVtYUlpZllSUTVpaHZMbjhySFp4VEpQR3EyYnRLTmdcLzRvKzQwRmtGNUdWWnQ0VjFpcTNPc0JubEdWenFiajRLRFg5a2dRZFJOZ1wvaUEwVHR3ZnYzakpYVmVtT294aFk1TXBUZ3FmVnF2dnNSVWJ5VEE0WGZpV3o3Y0k2SjJhM2RDK2hoQ0FvV2YrSW9QWnhuZG5QN1hlOEFaTVZrcFZ3c0pXVHhtNkRTUkpmenpibG8zdTM0cGF6Q3oxTEJsdDdiOUgwWXFOUkNHWjlEbTBFYzdIRUcyalYrcW4wYklFbnlGYlZJUG00R1VDQTZLZEVJRklIbFVNZFdpS3RkeCt5bVpTNUkrOXE3dDlxWmZ2bitjSGlSeE9wZTg5Yk9wS0V6N1wvd1EzUTNVenNtbjlvQUJhdGsxMzNkZTdjTU1LNkd4THJMYTBGUEJ4elEycFNTNGZabEJnalhJc0pYZit1c1wvWDBzSm1JMzRad3F3PT0iLCJtYWMiOiI4MjNhNDJlYTMwOTlmY2VlYzgxNmU1N2JiM2QzODk5YjI5MDFhYThhMDBkYzNhODljOTRmMTMzMzk0YTM5OGIzIn0=&source=BANNER.2913&url=http%3A%2F%2Fspo337.com%2F
https://fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
https://pdcn.co/e/http%3A%2F%2Fspo337.com%2F
https://api.webconnex.com/v1/postmaster/track/click/4f8036d14ee545798599c8921fbfcd22/db005310dba511e89fb606f49a4ee876?url=http%3A%2F%2Fspo337.com%2F
https://www.lecake.com/stat/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://www.im-harz.com/counter/counter.php?url=http%3A%2F%2Fspo337.com%2F
https://pzz.to/click?uid=8571&target_url=http%3A%2F%2Fspo337.com%2F
https://www.ricacorp.com/Ricapih09/redirect.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.tremblant.ca/Shared/LanguageSwitcher/ChangeCulture?culture=en&url=http%3A%2F%2Fspo337.com%2F
https://www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
https://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://igert2011.videohall.com/to_client?target=http%3A%2F%2Fspo337.com%2F
https://sete.gr/app_plugins/newsletterstudio/pages/tracking/trackclick.aspx?nid=218221206039243162226109144018149003034132019130&e=000220142174231130224127060133189018075115154134&url=http%3A%2F%2Fspo337.com%2F
https://ovatu.com/e/c?url=http%3A%2F%2Fspo337.com%2F
https://primepartners.globalprime.com/afs/wcome.php?c=427|0|1&e=GP204519&url=http%3A%2F%2Fspo337.com%2F
https://ltp.org/home/setlocale?locale=en&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.morhipo.com/shared/partnercookie?k=gort&url=http%3A%2F%2Fspo337.com%2F
https://www.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bg/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://maps.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://za.zalo.me/v3/verifyv2/pc?token=OcNsmjfpL0XY2F3BtHzNRs4A-hhQ5q5sPXtbk3O&continue=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://scanmail.trustwave.com/?c=8510&d=4qa02KqxZJadHuhFUvy7ZCUfI_2L10yeH0EeBz7FGQ&u=http%3A%2F%2Fspo337.com%2F
https://images.google.co.kr/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://animal.doctorsfile.jp/redirect/?ct=doctor&id=178583&url=http%3A%2F%2Fspo337.com%2F
https://hr.pecom.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://cse.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://jump2.bdimg.com/mo/q/checkurl?url=http%3A%2F%2Fspo337.com%2F
https://severeweather.wmo.int/cgi-bin/goto?where=http%3A%2F%2Fspo337.com%2F
https://beam.jpn.org/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=414788&url=http%3A%2F%2Fspo337.com%2F
https://wtk.db.com/777554543598768/optout?redirect=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.lv/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://m.caijing.com.cn/member/logout?referer=http%3A%2F%2Fspo337.com%2F
https://jamesattorney.agilecrm.com/click?u=http%3A%2F%2Fspo337.com%2F
https://wikimapia.org/external_link?url=http%3A%2F%2Fspo337.com%2F
https://rsv.nta.co.jp/affiliate/set/af100101.aspx?site_id=66108024&redi_url=http%3A%2F%2Fspo337.com%2F
https://adengine.old.rt.ru/go.jsp?to=http%3A%2F%2Fspo337.com%2F
https://thediplomat.com/ads/books/ad.php?i=4&r=http%3A%2F%2Fspo337.com%2F
https://mitsui-shopping-park.com/lalaport/iwata/redirect.html?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.es/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.it/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.br/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.au/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.be/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.at/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.se/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ch/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.my/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.co/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ie/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ph/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.no/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pe/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ae/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ee/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.eg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.si/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ec/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.qa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.li/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.uy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ba/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.is/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.az/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ng/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.np/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.by/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.as/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.do/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.jo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ai/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ni/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.la/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.jm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ps/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.af/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.fj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.lb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ms/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ci/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.py/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.am/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ad/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ly/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.na/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.me/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.je/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ge/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ht/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.im/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ml/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ac/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.st/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.td/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.nf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ne/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mn/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://www.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://asia.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://www.macro.ua/out.php?link=http%3A%2F%2Fspo337.com%2F
http://www.aurki.com/jarioa/redirect?id_feed=510&url=http%3A%2F%2Fspo337.com%2F
http://www.cnainterpreta.it/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.site-navi.net/sponavi/rank.cgi?mode=link&id=890&url=http%3A%2F%2Fspo337.com%2F
https://www.mattias.nu/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
http://teenstgp.us/cgi-bin/out.cgi?u=http%3A%2F%2Fspo337.com%2F
http://congovibes.com/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.humaniplex.com/jscs.html?hj=y&ru=http%3A%2F%2Fspo337.com%2F
http://www.qlt-online.de/cgi-bin/click/clicknlog.pl?link=http%3A%2F%2Fspo337.com%2F
http://ww.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
http://fosteringsuccessmichigan.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fotos24.org/url?q=http%3A%2F%2Fspo337.com%2F
http://fouillez-tout.com/cgi-bin/redirurl.cgi?http%3A%2F%2Fspo337.com%2F
http://frag-den-doc.de/index.php?s=verlassen&url=http%3A%2F%2Fspo337.com%2F
http://freethailand.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://freshcannedfrozen.ca/?URL=http%3A%2F%2Fspo337.com%2F
http://funkhouse.de/url?q=http%3A%2F%2Fspo337.com%2F
http://ga.naaar.nl/link/?url=http%3A%2F%2Fspo337.com%2F
http://gb.poetzelsberger.org/show.php?c453c4=http%3A%2F%2Fspo337.com%2F
http://getmethecd.com/?URL=http%3A%2F%2Fspo337.com%2F
http://goldankauf-oberberg.de/out.php?link=http%3A%2F%2Fspo337.com%2F
http://distributors.hrsprings.com/?URL=http%3A%2F%2Fspo337.com%2F
http://dorf-v8.de/url?q=http%3A%2F%2Fspo337.com%2F
http://doverwx.com/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://dr-guitar.de/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://drdrum.biz/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://ds-media.info/url?q=http%3A%2F%2Fspo337.com%2F
http://excitingperformances.com/?URL=http%3A%2F%2Fspo337.com%2F
http://familie-huettler.de/link.php?link=http%3A%2F%2Fspo337.com%2F
http://forum.vizslancs.hu/lnks.php?uid=net&url=http%3A%2F%2Fspo337.com%2F
http://forum.wonaruto.com/redirection.php?redirection=http%3A%2F%2Fspo337.com%2F
https://www.fairsandfestivals.net/?URL=http%3A%2F%2Fspo337.com%2F
http://chatx2.whocares.jp/redir.jsp?u=http%3A%2F%2Fspo337.com%2F
http://chuanroi.com/Ajax/dl.aspx?u=http%3A%2F%2Fspo337.com%2F
http://club.dcrjs.com/link.php?url=http%3A%2F%2Fspo337.com%2F
http://conny-grote.de/url?q=http%3A%2F%2Fspo337.com%2F
http://crazyfrag91.free.fr/?URL=http%3A%2F%2Fspo337.com%2F
http://crewe.de/url?q=http%3A%2F%2Fspo337.com%2F
http://cwa4100.org/uebimiau/redir.php?http%3A%2F%2Fspo337.com%2F
http://d-quintet.com/i/index.cgi?id=1&mode=redirect&no=494&ref_eid=33&url=http%3A%2F%2Fspo337.com%2F
http://data.allie.dbcls.jp/fct/rdfdesc/usage.vsp?g=http%3A%2F%2Fspo337.com%2F
http://data.linkedevents.org/describe/?url=http%3A%2F%2Fspo337.com%2F
http://dayviews.com/externalLinkRedirect.php?url=http%3A%2F%2Fspo337.com%2F
http://db.cbservices.org/cbs.nsf/forward?openform&http%3A%2F%2Fspo337.com%2F
http://simvol-veri.ru/xp/?goto=http%3A%2F%2Fspo337.com%2F
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=http%3A%2F%2Fspo337.com%2F
https://stroim100.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://kakaku-navi.net/items/detail.php?url=http%3A%2F%2Fspo337.com%2F
http://www.paladiny.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://tido.al/vazhdo.php?url=http%3A%2F%2Fspo337.com%2F
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=http%3A%2F%2Fspo337.com%2F
https://www.usjournal.com/go.php?campusID=190&url=http%3A%2F%2Fspo337.com%2F
https://temptationsaga.com/buy.php?url=http%3A%2F%2Fspo337.com%2F
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=http%3A%2F%2Fspo337.com%2F
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=http%3A%2F%2Fspo337.com%2F
https://spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
https://www.info-realty.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.sermemole.com/public/serbook/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hradycz.cz/redir.php?b=445&t=http%3A%2F%2Fspo337.com%2F
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=http%3A%2F%2Fspo337.com%2F
https://uk.kindofbook.com/redirect.php/?red=http%3A%2F%2Fspo337.com%2F
http://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
http://auyttrv.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bartos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://cllfather.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littlejohnny.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://semesmemos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://faultypirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mmurugesamnfo.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://katihfmaxtron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ikkemandar.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://googlejfgdlenewstoday.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrecadominicana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://anupam-bestprice89.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrepublica.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allinspirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://weallfreieds.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://shanmegurad.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://manualmfuctional.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://verybeayurifull.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prasat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://meri.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prabu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://easehipranaam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://nidatyi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://krodyit.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://naine.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://breajwasi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://beithe.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allthingnbeyondblog.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://usafunworldt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://financialallorner.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mjghouthernmatron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://chandancomputers.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingshopping.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptritam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tech-universes.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littleboy.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://correctinspiration.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://esujkamien.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ujkaeltnx.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ausnangck2809.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://supermu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://buyfcyclingteam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tumari.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hariomm.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://lejano.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://jotumko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bestandroid.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://discoverable.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://puriagatratt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://azizlemon.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptrfsiitan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://boardgame-breking.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://flowwergulab.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingcreater-uk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://skinnyskin.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://amil.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mohan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://dilip.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://avinasg.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://virat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hsadyttk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ashu-quality.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tinko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://konkaruna.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://udavhav.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://matura.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://patana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bopal.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gwl.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://delhi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gopi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kripa.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://charanraj.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pream.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://sang.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pasas.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kanchan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://assadaaka.nl/?URL=http%3A%2F%2Fspo337.com%2F
http://atchs.jp/jump?u=http%3A%2F%2Fspo337.com%2F
http://away3d.com/?URL=http%3A%2F%2Fspo337.com%2F
http://blackberryvietnam.net/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://boogiewoogie.com/?URL=http%3A%2F%2Fspo337.com%2F
http://bsumzug.de/url?q=http%3A%2F%2Fspo337.com%2F
http://business.eatonton.com/list?globaltemplate=http%3A%2F%2Fspo337.com%2F
http://archives.midweek.com/?URL=http%3A%2F%2Fspo337.com%2F
http://armdrag.com/mb/get.php?url=http%3A%2F%2Fspo337.com%2F
http://asai-kota.com/acc/acc.cgi?REDIRECT=http%3A%2F%2Fspo337.com%2F
http://ass-media.de/wbb2/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://cdiabetes.com/redirects/offer.php?URL=http%3A%2F%2Fspo337.com%2F
https://padletcdn.com/cgi/fetch?disposition=attachment&url=http%3A%2F%2Fspo337.com%2F
http://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
http://centuryofaction.org/?URL=http%3A%2F%2Fspo337.com%2F
http://Somewh.a.T.dfqw@www.newsdiffs.org/article-history/www.findabeautyschool.com/map.aspx?url=http%3A%2F%2Fspo337.com%2F
http://actontv.org/?URL=http%3A%2F%2Fspo337.com%2F
http://bigbarganz.com/g/?http%3A%2F%2Fspo337.com%2F
http://andreasgraef.de/url?q=http%3A%2F%2Fspo337.com%2F
http://app.espace.cool/ClientApi/SubscribeToCalendar/1039?url=http%3A%2F%2Fspo337.com%2F
http://archive.paulrucker.com/?URL=http%3A%2F%2Fspo337.com%2F
http://baseballpodcasts.net/Feed2JS/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
http://bbs.mottoki.com/index?bbs=hpsenden&act=link&page=94&linkk=http%3A%2F%2Fspo337.com%2F
http://bernhardbabel.com/url?q=http%3A%2F%2Fspo337.com%2F
http://albertaaerialapplicators.com/?URL=http%3A%2F%2Fspo337.com%2F
http://alexanderroth.de/url?q=http%3A%2F%2Fspo337.com%2F
http://alt.baunetzwissen.de/rd/nl-track/?obj=bnw&cg1=redirect&cg2=wissen-newsletter:beton&cg3=partner&datum=2017-01&titel=partnerklick-beton&firma=informationszentrumbeton&link=http%3A%2F%2Fspo337.com%2F
https://duckduckgo.com/?q=https%3A%2F%2Fspo1top.com&t=h_&ia=web
https://www.google.com/search?q=https%3A%2F%2Fspo1top.com&sca_esv=567933587&sxsrf=AM9HkKkLNk4hFHvgRG-dTHcW476TZakh3Q%3A1695527512326&ei=WLIPZZPIE66t4-EPwYOS4AE&ved=0ahUKEwiT1NCYrMKBAxWu1jgGHcGBBBwQ4dUDCBA&uact=5&oq=https%3A%2F%2Fspo1top.com&gs_lp=Egxnd3Mtd2l6LXNlcnAiE2h0dHBzOi8vc3BvMXRvcC5jb20yBBAjGCdI2x9QkAtY2xpwAXgBkAEAmAGHAaABygeqAQMwLji4AQPIAQD4AQHCAgoQABhHGNYEGLAD4gMEGAAgQYgGAZAGBg&sclient=gws-wiz-serp
http://client.paltalk.com/client/webapp/client/External.wmt?url=http%3A%2F%2Fspo337.com%2F
http://community.nxp.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
http://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://ceskapozice.lidovky.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mitsui-shopping-park.com/lalaport/ebina/redirect.html?url=http%3A%2F%2Fspo337.com%2F
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=http%3A%2F%2Fspo337.com%2F
http://fishbiz.seagrant.uaf.edu/click-thru.html?url=http%3A%2F%2Fspo337.com%2F
http://kupiauto.zr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://ipx.bcove.me/?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.td/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
https://home.guanzhuang.org/link.php?url=http%3A%2F%2Fspo337.com%2F
http://dvd24online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://twindish-electronics.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.beigebraunapartment.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.mix-choice.com/yomi/rank.cgi?mode=link&id=391&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
http://www.1soft-tennis.com/search/rank.cgi?mode=link&id=17&url=http%3A%2F%2Fspo337.com%2F
http://big-data-fr.com/linkedin.php?lien=http%3A%2F%2Fspo337.com%2F
http://reg.kost.ru/cgi-bin/go?http%3A%2F%2Fspo337.com%2F
http://www.kirstenulrich.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.inkwell.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.co.je/url?q=http%3A%2F%2Fspo337.com%2F
https://n1653.funny.ge/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://clients1.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
http://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
http://www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.nickl-architects.com/url?q=http%3A%2F%2Fspo337.com%2F
https://www.ocbin.com/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lobenhausen.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
http://redirect.me/?http%3A%2F%2Fspo337.com%2F
http://kank.o.oo7.jp/cgi-bin/ys4/rank.cgi?mode=link&id=569&url=http%3A%2F%2Fspo337.com%2F
http://ruslog.com/forum/noreg.php?http%3A%2F%2Fspo337.com%2F
http://forum.ahigh.ru/away.htm?link=http%3A%2F%2Fspo337.com%2F
http://www.wildner-medien.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.tifosy.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.co.ck/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://ads.icorp.ro/others/STS/?t=CeNortjKxUjK0NDFVsgZcMBADAm4-&g=http%3A%2F%2Fspo337.com%2F
http://deai-ranking.org/search/rank.cgi?mode=link&id=28&url=http%3A%2F%2Fspo337.com%2F
https://izispicy.com/go.php?url=http%3A%2F%2Fspo337.com%2F
http://demo.vieclamcantho.vn/baohiemthatnghiep/Redirect.aspx?sms=90bb20bb20tbb20thc3%B4ng&link=http%3A%2F%2Fspo337.com%2F
http://www.city-fs.de/url?q=http%3A%2F%2Fspo337.com%2F
http://p.profmagic.com/urllink.php?url=http%3A%2F%2Fspo337.com%2F
https://www.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ee/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://www.arndt-am-abend.de/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
http://maxnetworks.org/searchlink/rank.cgi?mode=link&id=321&url=http%3A%2F%2Fspo337.com%2F
https://image.google.com.ng/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
https://www.kenzai-navi.com/location/location_topbanner.php?bs=238&m=899&href=http%3A%2F%2Fspo337.com%2F
https://www.anybeats.jp/jump/?http%3A%2F%2Fspo337.com%2F
http://url-collector.appspot.com/positiveVotes?topic=Hate%20speech&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.be/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://top.hange.jp/linkdispatch/dispatch?targetUrl=http%3A%2F%2Fspo337.com%2F
http://www.link.gokinjyo-eikaiwa.com/rank.cgi?mode=link&id=5&url=http%3A%2F%2Fspo337.com%2F
http://www.air-dive.com/au/mt4i.cgi?mode=redirect&ref_eid=697&url=http%3A%2F%2Fspo337.com%2F
http://okozukai.j-web.jp/j-web/okozukai/ys4/rank.cgi?mode=link&id=6817&url=http%3A%2F%2Fspo337.com%2F
https://sfmission.com/rss/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
https://www.watersportstaff.co.uk/extern.aspx?src=http%3A%2F%2Fspo337.com%2F&cu=60096&page=1&t=1&s=42"
http://siamcafe.net/board/go/go.php?http%3A%2F%2Fspo337.com%2F
http://www.whitening-navi.info/cgi/search-smartphone/rank.cgi?mode=link&id=1431&url=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channeldescription&q=http%3A%2F%2Fspo337.com%2F
http://www.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://go.e-frontier.co.jp/rd2.php?uri=http%3A%2F%2Fspo337.com%2F
http://adchem.net/Click.aspx?url=http%3A%2F%2Fspo337.com%2F
http://www.reddotmedia.de/url?q=http%3A%2F%2Fspo337.com%2F
https://10ways.com/fbredir.php?orig=http%3A%2F%2Fspo337.com%2F
https://emailtrackerapi.leadforensics.com/api/URLOpen?EmailSentRecordID=17006&URL=http%3A%2F%2Fspo337.com%2F
http://search.haga-f.net/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://7ba.ru/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.51queqiao.net/link.php?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://rsyosetsu.bookmarks.jp/ys4/rank.cgi?mode=link&id=3519&url=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://underwood.ru/away.html?url=http%3A%2F%2Fspo337.com%2F
https://unicom.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.unifin.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://uogorod.ru/feed/520?redirect=http%3A%2F%2Fspo337.com%2F
http://uvbnb.ru/go?http%3A%2F%2Fspo337.com%2F
https://skibaza.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://smartservices.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.topkam.ru/gtu/?url=http%3A%2F%2Fspo337.com%2F
https://torggrad.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://torgi-rybinsk.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://tpprt.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.al/url?q=http%3A%2F%2Fspo337.com%2F
http://toolbarqueries.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.capitalbikepark.se/bok/go.php?url=http%3A%2F%2Fspo337.com%2F
http://cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
https://joomlinks.org/?url=http%3A%2F%2Fspo337.com%2F
https://managementportal.de/modules/mod_jw_srfr/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://www.green-cross.pro/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://m.adlf.jp/jump.php?l=http%3A%2F%2Fspo337.com%2F
https://www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
http://www.mac52ipod.cn/urlredirect.php?go=http%3A%2F%2Fspo337.com%2F
http://chrison.net/ct.ashx?id=dad2849a-8862-4a6d-b842-ef628cbfd3c3&url=http%3A%2F%2Fspo337.com%2F
http://datasheetcatalog.biz/url.php?url=http%3A%2F%2Fspo337.com%2F
http://minlove.biz/out.html?id=nhmode&go=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/win/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://request-response.com/blog/ct.ashx?url=http%3A%2F%2Fspo337.com%2F
https://turbazar.ru/url/index?url=http%3A%2F%2Fspo337.com%2F
http://members.asoa.org/sso/logout.aspx?returnurl=http%3A%2F%2Fspo337.com%2F
http://login.mediafort.ru/autologin/mail/?code=14844×02ef859015x290299&url=http%3A%2F%2Fspo337.com%2F
https://www.sports-central.org/cgi-bin/axs/ax.pl?http%3A%2F%2Fspo337.com%2F
http://dot.boss.sk/o/rs.php?ssid=1&szid=4&dsid=12&dzid=32&url=http%3A%2F%2Fspo337.com%2F
http://www.americanstylefridgefreezer.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.newzealandgirls.co.nz/auckland/escorts/clicks.php?click_id=NavBar_AF_AdultDirectory&target=http%3A%2F%2Fspo337.com%2F
https://wdesk.ru/go?http%3A%2F%2Fspo337.com%2F
http://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://page.yicha.cn/tp/j?url=http%3A%2F%2Fspo337.com%2F
http://www.internettrafficreport.com/cgi-bin/cgirdir.exe?http%3A%2F%2Fspo337.com%2F
http://www.interfacelift.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://good-surf.ru/r.php?g=http%3A%2F%2Fspo337.com%2F
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=http%3A%2F%2Fspo337.com%2F
https://gektor-nsk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://lekoufa.ru/banner/go?banner_id=4&link=http%3A%2F%2Fspo337.com%2F
https://forsto.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.gigatran.ru/go?url=http%3A%2F%2Fspo337.com%2F
http://www.gigaalert.com/view.php?h=&s=http%3A%2F%2Fspo337.com%2F
http://www.jkes.tyc.edu.tw/dyna/webs/gotourl.php?id=357&url=http%3A%2F%2Fspo337.com%2F
http://www.laopinpai.com/gourl.asp?url=/gourl.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.humanbrainmapping.org/i4a/etrack/track.cfm?rType=2&campaignID=3572&contactID=4524&origURL=http%3A%2F%2Fspo337.com%2F
https://gcup.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.gearguide.ru/phpbb/go.php?http%3A%2F%2Fspo337.com%2F
https://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://passport.sfacg.com/LoginOut.aspx?Returnurl=http%3A%2F%2Fspo337.com%2F
https://offers.sidex.ru/stat_ym_new.php?redir=http%3A%2F%2Fspo337.com%2F&hash=1577762
https://globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://ramset.com.au/document/url/?url=http%3A%2F%2Fspo337.com%2F
https://www.vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
http://www.cyprus-net.com/banner_click.php?banid=4&link=http%3A%2F%2Fspo337.com%2F
http://a.gongkong.com/db/adredir.asp?id=16757&url=http%3A%2F%2Fspo337.com%2F
http://gfaq.ru/go?http%3A%2F%2Fspo337.com%2F
http://gbi-12.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.global-flat.com/mobile/mobile_switch.php?dir=tofull&url=http%3A%2F%2Fspo337.com%2F
https://golden-resort.ru/out.php?out=http%3A%2F%2Fspo337.com%2F
http://avalon.gondor.ru/away.php?link=http%3A%2F%2Fspo337.com%2F
http://www.laosubenben.com/home/link.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lifeshow.com.tw/show.php?ty5_id=1599&url=http%3A%2F%2Fspo337.com%2F
http://games.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.netwalkerstore.com/redirect.asp?accid=18244&adcampaignid=2991&adcampaigntype=2&affduration=30&url=http%3A%2F%2Fspo337.com%2F
http://tsugarubrand.jp/blog/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://blackhistorydaily.com/black_historylinks/link.asp?linkid=5&URL=http%3A%2F%2Fspo337.com%2F
http://www.kitchencabinetsdirectory.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://cityprague.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://www.hachimantaishi.com/click3/click3.cgi?cnt=c5&url=http%3A%2F%2Fspo337.com%2F
http://earnupdates.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://www.careerbuilder.com.cn/cn/tallyredirector.aspx?task=Ushi&objName=SnsShare&redirectUrl=http%3A%2F%2Fspo337.com%2F&methodName=SetSnsShareLink
http://banners.knollenstein.com/adnetwork/servlet/advertBeans.TrackingServlet?seid=27709655&t=100&redirectTo=http%3A%2F%2Fspo337.com%2F&cid=DECONL&vid=986809&externalsource=jobbsquareppc
https://meyeucon.org/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://www.cnmhe.fr/spip.php?action=cookie&url=http%3A%2F%2Fspo337.com%2F
https://perezvoni.com/blog/away?url=http%3A%2F%2Fspo337.com%2F
https://www.ciymca.org/af/register-redirect/71104?url=http%3A%2F%2Fspo337.com%2F
https://whizpr.nl/tracker.php?u=http%3A%2F%2Fspo337.com%2F
http://bw.irr.by/knock.php?bid=252583&link=http%3A%2F%2Fspo337.com%2F
http://www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/hak/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://www.actuaries.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMVFIELD]EMAIL[EMV/FIELD]&cat=Techniquesculturales&url=http%3A%2F%2Fspo337.com%2F
https://www.jukujo.gs/bin/out.cgi?id=kimiomof&url=http%3A%2F%2Fspo337.com%2F
http://hansolav.net/blog/ct.ashx?id=0af6301b-e71f-44be-838f-905709eee792&url=http%3A%2F%2Fspo337.com%2F
http://bitrix.adlr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://m.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.officeservice.com.br/trackMKT/trackerFolha.aspx?DESCRIPTION=HotSite_EcrmContabil&FROM=Workshop&DESTINATION=http%3A%2F%2Fspo337.com%2F
https://www.arbsport.ru/gotourl.php?url=http%3A%2F%2Fspo337.com%2F
http://www.strana.co.il/finance/redir.aspx?site=http%3A%2F%2Fspo337.com%2F
https://www.tourplanisrael.com/redir/?url=http%3A%2F%2Fspo337.com%2F
http://www.mutualgravity.com/archangel/webcontact/d_signinsimple.php?action=signup&CID=240&EID=&S=default.css&return=http%3A%2F%2Fspo337.com%2F
http://buildingreputation.com/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
http://gals.graphis.ne.jp/mkr/out.cgi?id=04489&go=http%3A%2F%2Fspo337.com%2F
http://dir.tetsumania.net/search/rank.cgi?mode=link&id=3267&url=http%3A%2F%2Fspo337.com%2F
http://www.project24.info/mmview.php?dest=http%3A%2F%2Fspo337.com%2F
http://tfads.testfunda.com/TFServeAds.aspx?strTFAdVars=4a086196-2c64-4dd1-bff7-aa0c7823a393,TFvar,00319d4f-d81c-4818-81b1-a8413dc614e6,TFvar,GYDH-Y363-YCFJ-DFGH-5R6H,TFvar,http%3A%2F%2Fspo337.com%2F
http://www.request-response.com/blog/ct.ashx?id=d827b163-39dd-48f3-b767-002147c94e05&url=http%3A%2F%2Fspo337.com%2F
http://www.cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
http://sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=785&redirect=http%3A%2F%2Fspo337.com%2F
http://www.kyoto-osaka.com/search/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://metalist.co.il/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://i-marine.eu/pages/goto.aspx?link=http%3A%2F%2Fspo337.com%2F
http://www.teenlove.biz/cgibin/atc/out.cgi?id=24&u=http%3A%2F%2Fspo337.com%2F
http://www.altoona.com/newsletter/click.php?volume=52&url=http%3A%2F%2Fspo337.com%2F
http://www.katjushik.ru/link.php?to=http%3A%2F%2Fspo337.com%2F
http://gondor.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://proekt-gaz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.tricitiesapartmentguide.com/MobileDefault.aspx?reff=http%3A%2F%2Fspo337.com%2F
http://www.vietnamsingle.com/vn/banners/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.voxlocalis.net/enlazar/?url=http%3A%2F%2Fspo337.com%2F
http://no-smok.net/nsmk/InterWiki?action=goto&oe=EUC-KR&url=http%3A%2F%2Fspo337.com%2F
http://www.stalker-modi.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.p1-uranai.com/rank.cgi?mode=link&id=538&url=http%3A%2F%2Fspo337.com%2F
http://earthsciencescanada.com/modules/babel/redirect.php?newlang=en_us&newurl=http%3A%2F%2Fspo337.com%2F
http://psykodynamiskt.nu/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
http://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
http://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://lirasport.com.ar/bloques/bannerclick.php?id=7&url=http%3A%2F%2Fspo337.com%2F
http://www.knabstrupper.se/guestbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.pba.ph/redirect?url=http%3A%2F%2Fspo337.com%2F&id=3&type=tab
https://bondage-guru.net/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.westbloomfieldlibrary.org/includes/statistics.php?StatType=Link&StatID=Facebook&weblink=http%3A%2F%2Fspo337.com%2F
https://tenisnews.com.br/clickTracker/clickTracker.php?u=http%3A%2F%2Fspo337.com%2F
http://www.stevestechspot.com/ct.ashx?id=9fdcf4a4-6e09-4807-bc31-ac1adf836f6c&url=http%3A%2F%2Fspo337.com%2F
http://rcoi71.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.laselection.net/redir.php3?cat=int&url=http%3A%2F%2Fspo337.com%2F
http://www.masai-mara.com/cgi-bin/link2.pl?grp=mm&link=http%3A%2F%2Fspo337.com%2F
http://evenemangskalender.se/redirect/?id=15723&lank=http%3A%2F%2Fspo337.com%2F
http://anifre.com/out.html?go=http%3A%2F%2Fspo337.com%2F
http://www.restavracije-gostilne.si/banner.php?id=44&url=http%3A%2F%2Fspo337.com%2F
http://hobbyplastic.co.uk/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://ads.pukpik.com/myads/click.php?banner_id=316&banner_url=http%3A%2F%2Fspo337.com%2F
https://www.adminer.org/redirect/?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackmamas.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://primorye.ru/go.php?id=19&url=http%3A%2F%2Fspo337.com%2F
https://www.123gomme.it/it/ViewSwitcher/SwitchView?mobile=True&returnUrl=http%3A%2F%2Fspo337.com%2F
http://youmydaddy.com/cgi-bin/at3/out.cgi?id=88&trade=http%3A%2F%2Fspo337.com%2F
https://naviking.localking.com.tw/about/redirect.aspx?mid=7&url=http%3A%2F%2Fspo337.com%2F
http://sms-muzeji.si/Home/ChangeCulture?lang=sl&returnUrl=http%3A%2F%2Fspo337.com%2F
http://r.emeraldexpoinfo.com/s.ashx?ms=EXI2:125462_115115&e=krubin723@aol.com&eId=440186886&c=h&url=http%3A%2F%2Fspo337.com%2F
https://apps.firstrain.com/service/openurl.jsp?action=titleclick&src=rss&u=http%3A%2F%2Fspo337.com%2F
http://cdp.thegoldwater.com/click.php?id=101&url=http%3A%2F%2Fspo337.com%2F
http://www.appenninobianco.it/ads/adclick.php?bannerid=159&zoneid=8&source=&dest=http%3A%2F%2Fspo337.com%2F
https://www.eduplus.hk/special/emailalert/goURL.jsp?clickURL=http%3A%2F%2Fspo337.com%2F
http://www.japan.road.jp/navi/navi.cgi?jump=226&url=http%3A%2F%2Fspo337.com%2F
http://www.arch.iped.pl/artykuly.php?id=1&cookie=1&url=http%3A%2F%2Fspo337.com%2F
http://hirlevelcenter.eu/click.php?hirlevel_id=13145441085508&url=http%3A%2F%2Fspo337.com%2F
https://birds.cz/avif/redirect.php?from=avif.obs.php&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackbootywatchers.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
http://barykin.com/go.php?http%3A%2F%2Fspo337.com%2F
https://forum.everleap.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://www.mosig-online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hccincorporated.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fatnews.com/?URL=http%3A%2F%2Fspo337.com%2F
https://csirealty.com/?URL=http%3A%2F%2Fspo337.com%2F
http://asadi.de/url?q=http%3A%2F%2Fspo337.com%2F
http://treblin.de/url?q=http%3A%2F%2Fspo337.com%2F
https://kentbroom.com/?URL=http%3A%2F%2Fspo337.com%2F
http://0845.boo.jp/cgi/mt3/mt4i.cgi?id=24&mode=redirect&no=15&ref_eid=3387&url=http%3A%2F%2Fspo337.com%2F
http://110.164.66.211/ULIB6//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://110.164.92.12/ULIB//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://198.54.125.86.myopenlink.net/describe/?url=http%3A%2F%2Fspo337.com%2F
http://202.144.225.38/jmp?url=http%3A%2F%2Fspo337.com%2F
http://2cool2.be/url?q=http%3A%2F%2Fspo337.com%2F
http://4coma.net/cgi/mt4/mt4i.cgi?cat=12&mode=redirect&ref_eid=3231&url=http%3A%2F%2Fspo337.com%2F
http://4travel.jp/dynamic/redirect.php?mode=dm_tour&url=http%3A%2F%2Fspo337.com%2F
http://imaginingourselves.globalfundforwomen.org/pb/External.aspx?url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
http://search.pointcom.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.cercasostituto.it/index.php?name=GestBanner&file=counter&idbanner=40&dir_link=http%3A%2F%2Fspo337.com%2F
http://vsvejr.dk/mt/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.meteo-leran.fr/meteotemplate/template/plugins/deviations/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.eurobichons.com/fda%20alerts.php?url=http%3A%2F%2Fspo337.com%2F
https://mydojo.at/de_AT/karate/weiterleitung?redirect=http%3A%2F%2Fspo337.com%2F
http://click.phosphodiesterase4.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.mariahownersclub.com/forum/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://www.wildromance.com/buy.php?url=http%3A%2F%2Fspo337.com%2F&store=iBooks&book=omk-ibooks-us
http://mapleriverweather.com/mobile/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.archijob.co.il/index/comp_website.asp?companyId=1469&website=http%3A%2F%2Fspo337.com%2F
https://student-helpr.rminds.dev/redirect?redirectTo=http%3A%2F%2Fspo337.com%2F
http://www.kalinna.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hartmanngmbh.de/url?q=http%3A%2F%2Fspo337.com%2F
https://www.the-mainboard.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://www.betamachinery.com/?URL=http%3A%2F%2Fspo337.com%2F
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=http%3A%2F%2Fspo337.com%2F
http://www.sprang.net/url?q=http%3A%2F%2Fspo337.com%2F
https://img.2chan.net/bin/jump.php?http%3A%2F%2Fspo337.com%2F
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=http%3A%2F%2Fspo337.com%2F
http://local.rongbachkim.com/rdr.php?url=http%3A%2F%2Fspo337.com%2F
http://bachecauniversitaria.it/link/frm_top.php?url=http%3A%2F%2Fspo337.com%2F
https://www.stcwdirect.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://forum.vcoderz.com/externalredirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.momentumstudio.com/?URL=http%3A%2F%2Fspo337.com%2F
https://s-p.me/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://ww.thesteelbrothers.com/buy.php?store=iBooks&url=http%3A%2F%2Fspo337.com%2F
http://thdt.vn/convert/convert.php?link=http%3A%2F%2Fspo337.com%2F
http://www.doitweb365.de/scripts/doitweb.exe/rasklickzaehler2?http%3A%2F%2Fspo337.com%2F
https://www.guadamur.eu/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://weather.cube.com.gr/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.fimmgviterbo.org/mobfimmgviterbo/index.php?nametm=counter&idbanner=4&dir_link=http%3A%2F%2Fspo337.com%2F
http://www.mckinneyfarm.com/template/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://utmagazine.ru/r?url=http%3A%2F%2Fspo337.com%2F
http://www.evrika41.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
http://expomodel.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://facto.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://fc-zenit.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://forum-region.ru/forum/away.php?s=http%3A%2F%2Fspo337.com%2F
http://forumdate.ru/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://shckp.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://shinglas.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sibran.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://sintez-oka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://app.espace.cool/clientapi/subscribetocalendar/974?url=http%3A%2F%2Fspo337.com%2F
http://111056.net/yomisearch/rank.cgi?mode=link&id=6205&url=http%3A%2F%2Fspo337.com%2F
http://stanko.tw1.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sozialemoderne.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.showb.com/search/ranking.cgi?mode=link&id=7083&url=http%3A%2F%2Fspo337.com%2F
http://imagelibrary.asprey.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://ime.nu/http%3A%2F%2Fspo337.com%2F
http://interflex.biz/url?q=http%3A%2F%2Fspo337.com%2F
http://ivvb.de/url?q=http%3A%2F%2Fspo337.com%2F
http://j.lix7.net/?http%3A%2F%2Fspo337.com%2F
http://jacobberger.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jahn.eu/url?q=http%3A%2F%2Fspo337.com%2F
http://jamesvelvet.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jla.drmuller.net/r.php?url=http%3A%2F%2Fspo337.com%2F
http://jump.pagecs.net/http%3A%2F%2Fspo337.com%2F
http://kagarin.net/cgi/mt/mt4i.cgi?id=2&mode=redirect&no=330&ref_eid=103&url=http%3A%2F%2Fspo337.com%2F
http://karkom.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kens.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinderundjugendpsychotherapie.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinhtexaydung.net/redirect/?url=http%3A%2F%2Fspo337.com%2F
http://neoromance.info/link/rank.cgi?mode=link&id=26&url=http%3A%2F%2Fspo337.com%2F
http://www.hainberg-gymnasium.com/url?q=http%3A%2F%2Fspo337.com%2F
https://befonts.com/checkout/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.usap.gov/externalsite.cfm?http%3A%2F%2Fspo337.com%2F
https://maps.google.com.ua/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.shinobi.jp/etc/goto.html?http%3A%2F%2Fspo337.com%2F
http://images.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://supplier-portal-uat.daimler.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://strelmag.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://spb90.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://spartak.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sovaisova.ru/bitrix/redirect.php?event1=2009_mainpage&event2=go_www&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
https://seoandme.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://s-online.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://rssfeeds.wtsp.com/~/t/0/0/wtsp/home/~http%3A%2F%2Fspo337.com%2F
https://rostovmama.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://rev1.reversion.jp/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.star174.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://staten.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://stopcran.ru/go?http%3A%2F%2Fspo337.com%2F
http://studioad.ru/go?http%3A%2F%2Fspo337.com%2F
http://swepub.kb.se/setattribute?language=en&redirect=http%3A%2F%2Fspo337.com%2F
https://www.licnioglasi.org/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
http://www2.smartmail.com.ar/tl.php?p=hqf/f94/rs/1fp/4c0/rs//http%3A%2F%2Fspo337.com%2F
http://old.roofnet.org/external.php?link=http%3A%2F%2Fspo337.com%2F
http://www.bucatareasa.ro/link.php?url=http%3A%2F%2Fspo337.com%2F
http://mosprogulka.ru/go?http%3A%2F%2Fspo337.com%2F
https://uniline.co.nz/Document/Url/?url=http%3A%2F%2Fspo337.com%2F
https://tracker.onrecruit.net/api/v1/redirect/?redirect_to=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.samovar-forum.ru/go?http%3A%2F%2Fspo337.com%2F
http://staldver.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://posts.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://naruto.su/link.ext.php?url=http%3A%2F%2Fspo337.com%2F
https://justpaste.it/redirect/172fy/http%3A%2F%2Fspo337.com%2F
https://jt-pr-dot-yamm-track.appspot.com/Redirect?ukey=1iXi3dF8AuzUIsgifbcVnqqx-anF4B8R-9PC3UGhHO3E-1131306248&key=YAMMID-79725512&link=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://de.flavii.de/index.php?flavii=linker&link=http%3A%2F%2Fspo337.com%2F
https://dakke.co/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_attribute_to_url=http%3A%2F%2Fspo337.com%2F
https://contacts.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.nxp.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.esri.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2Fspo337.com%2F
https://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
https://bukkit.org/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://branch.app.link/?$deeplink_path=article%2Fjan%2F123&$fallback_url=http%3A%2F%2Fspo337.com%2F&channel=facebook&feature=affiliate
https://boowiki.info/go.php?go=http%3A%2F%2Fspo337.com%2F
https://blaze.su/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://bbs.pku.edu.cn/v2/jump-to.php?url=http%3A%2F%2Fspo337.com%2F
https://bares.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.flyingsamaritans.net/Startup/SetupSite.asp?RestartPage=http%3A%2F%2Fspo337.com%2F
https://www.ewind.cz/index.php?page=home/redirect&url=http%3A%2F%2Fspo337.com%2F
https://www.eas-racing.se/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.direkt-einkauf.de/includes/refer.php?id=170&url=http%3A%2F%2Fspo337.com%2F
https://www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.curseforge.com/linkout?remoteUrl=http%3A%2F%2Fspo337.com%2F
https://www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=http%3A%2F%2Fspo337.com%2F
https://www.autopartskart.com/buyfromamzon.php?url=http%3A%2F%2Fspo337.com%2F
https://www.autoandrv.com/linkout.aspx?websiteurl=http%3A%2F%2Fspo337.com%2F
https://www.art-prizes.com/AdRedirector.aspx?ad=MelbPrizeSculpture_2017&target=http%3A%2F%2Fspo337.com%2F
https://wirelessestimator.com/advertise/newsletter_redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://wasitviewed.com/index.php?href=http%3A%2F%2Fspo337.com%2F
https://tvtropes.org/pmwiki/no_outbounds.php?o=http%3A%2F%2Fspo337.com%2F
https://trello.com/add-card?source=mode=popup&name=click+here&desc=http%3A%2F%2Fspo337.com%2F
https://sutd.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://abiznes.com.ua/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.sv-mama.ru/shared/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sofion.ru/banner.php?r1=41&r2=2234&goto=http%3A%2F%2Fspo337.com%2F
http://www.rss.geodles.com/fwd.php?url=http%3A%2F%2Fspo337.com%2F
http://www.nuttenzone.at/jump.php?url=http%3A%2F%2Fspo337.com%2F
http://www.myhottiewife.com/cgi-bin/arpro/out.cgi?id=Jojo&url=http%3A%2F%2Fspo337.com%2F
http://www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
http://www.johnvorhees.com/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.imsnet.at/LangChange.aspx?uri=http%3A%2F%2Fspo337.com%2F
http://www.hon-cafe.net/cgi-bin/re.cgi?lid=hmw&url=http%3A%2F%2Fspo337.com%2F
http://www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
http://www.etis.ford.com/externalURL.do?url=http%3A%2F%2Fspo337.com%2F
http://www.chungshingelectronic.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.bdsmandfetish.com/cgi-bin/sites/out.cgi?id=mandymon&url=http%3A%2F%2Fspo337.com%2F
http://visits.seogaa.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
http://tharp.me/?url_to_shorten=http%3A%2F%2Fspo337.com%2F
http://speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://spbstroy.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://solo-center.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://sennheiserstore.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://school364.spb.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://sc.sie.gov.hk/TuniS/http%3A%2F%2Fspo337.com%2F
http://rzngmu.ru/go?http%3A%2F%2Fspo337.com%2F
http://rostovklad.ru/go.php?http%3A%2F%2Fspo337.com%2F
http://portalnp.snauka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://markiza.me/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://jump.5ch.net/?http%3A%2F%2Fspo337.com%2F
http://imperialoptical.com/news-redirect.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hellothai.com/wwwlink/wwwredirect.asp?hp_id=1242&url=http%3A%2F%2Fspo337.com%2F
http://guru.sanook.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fr.knubic.com/redirect_to?url=http%3A%2F%2Fspo337.com%2F
http://branch.app.link/?$deeplink_path=article/jan/123&$fallback_url=http%3A%2F%2Fspo337.com%2F
http://biz-tech.org/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://2010.russianinternetweek.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://xat.com/web_gear/chat/linkvalidator.php?link=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://www.woodlist.us/delete-company?nid=13964&element=http%3A%2F%2Fspo337.com%2F
https://www.viecngay.vn/go?to=http%3A%2F%2Fspo337.com%2F
https://www.uts.edu.co/portal/externo.php?id=http%3A%2F%2Fspo337.com%2F
https://www.talgov.com/Main/exit.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.russianrobotics.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.ruchnoi.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://www.rprofi.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.roccotube.com/cgi-bin/at3/out.cgi?id=27&tag=toplist&trade=http%3A%2F%2Fspo337.com%2F
https://www.nyl0ns.com/cgi-bin/a2/out.cgi?id=43&l=btop&u=http%3A%2F%2Fspo337.com%2F
https://www.meetme.com/apps/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=http%3A%2F%2Fspo337.com%2F
https://www.interpals.net/url_redirect.php?href=http%3A%2F%2Fspo337.com%2F
https://www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
https://www.hottystop.com/cgi-bin/at3/out.cgi?id=12&trade=http%3A%2F%2Fspo337.com%2F
https://www.hobowars.com/game/linker.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hentainiches.com/index.php?id=derris&tour=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.greencom.ru/catalog/irrigation_systems.html?jumpsite=2008&url=http%3A%2F%2Fspo337.com%2F
https://www.girlznation.com/cgi-bin/atc/out.cgi?id=50&l=side&u=http%3A%2F%2Fspo337.com%2F
https://www.funeralunion.org/delete-company?nid=39&element=http%3A%2F%2Fspo337.com%2F
https://www.fudbal91.com/tz.php?zone=America/Iqaluit&r=http%3A%2F%2Fspo337.com%2F
https://www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
https://kryvbas.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://joomluck.com/go/?http%3A%2F%2Fspo337.com%2F
https://www.anonymz.com/?http%3A%2F%2Fspo337.com%2F
https://weburg.net/redirect?url=http%3A%2F%2Fspo337.com%2F
https://tw6.jp/jump/?url=http%3A%2F%2Fspo337.com%2F
https://www.spainexpat.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.fotka.pl/link.php?u=http%3A%2F%2Fspo337.com%2F
https://www.lolinez.com/?http%3A%2F%2Fspo337.com%2F
https://nanos.jp/jmp?url=http%3A%2F%2Fspo337.com%2F
https://www.fca.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://savvylion.com/?bmDomain=http%3A%2F%2Fspo337.com%2F
https://www.soyyooestacaido.com/http%3A%2F%2Fspo337.com%2F
https://www.gta.ru/redirect/www.http%3A%2F%2Fspo337.com%2F
https://directx10.org/go?http%3A%2F%2Fspo337.com%2F
https://mejeriet.dk/link.php?id=http%3A%2F%2Fspo337.com%2F
https://ezdihan.do.am/go?http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://hiddenrefer.com/?http%3A%2F%2Fspo337.com%2F
https://romhacking.ru/go?http%3A%2F%2Fspo337.com%2F
https://turion.my1.ru/go?http%3A%2F%2Fspo337.com%2F
https://kassirs.ru/sweb.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.allods.net/redirect/http%3A%2F%2Fspo337.com%2F
https://icook.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.pasco.k12.fl.us/?URL=http%3A%2F%2Fspo337.com%2F
https://anolink.com/?link=http%3A%2F%2Fspo337.com%2F
https://www.questsociety.ca/?URL=http%3A%2F%2Fspo337.com%2F
https://www.disl.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://holidaykitchens.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.mbcarolinas.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.anibox.org/go?http%3A%2F%2Fspo337.com%2F
https://google.info/url?q=http%3A%2F%2Fspo337.com%2F
https://atlantis-tv.ru/go?http%3A%2F%2Fspo337.com%2F
https://otziv.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.sgvavia.ru/go?http%3A%2F%2Fspo337.com%2F
https://element.lv/go?url=http%3A%2F%2Fspo337.com%2F
https://karanova.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://789.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
https://krasnoeselo.su/go?http%3A%2F%2Fspo337.com%2F
https://game-era.do.am/go?http%3A%2F%2Fspo337.com%2F
https://kudago.com/go/?to=http%3A%2F%2Fspo337.com%2F
https://after.ucoz.net/go?http%3A%2F%2Fspo337.com%2F
https://kinteatr.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://nervetumours.org.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://kopyten.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://www.taker.im/go/?u=http%3A%2F%2Fspo337.com%2F
https://usehelp.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://sepoa.fr/wp/go.php?http%3A%2F%2Fspo337.com%2F
https://world-source.ru/go?http%3A%2F%2Fspo337.com%2F
https://mail2.mclink.it/SRedirect/http%3A%2F%2Fspo337.com%2F
https://www.swleague.ru/go?http%3A%2F%2Fspo337.com%2F
https://nazgull.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.rosbooks.ru/go?http%3A%2F%2Fspo337.com%2F
https://beskuda.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://richmonkey.biz/go/?http%3A%2F%2Fspo337.com%2F
https://vlpacific.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://eric.ed.gov/?redir=http%3A%2F%2Fspo337.com%2F
https://www.usich.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://sec.pn.to/jump.php?http%3A%2F%2Fspo337.com%2F
https://www.earth-policy.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.silverdart.co.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://pr-cy.ru/jump/?url=http%3A%2F%2Fspo337.com%2F
https://google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://pikmlm.ru/out.php?p=http%3A%2F%2Fspo337.com%2F
https://regnopol.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://mss.in.ua/go.php?to=http%3A%2F%2Fspo337.com%2F
https://owohho.com/away?url=http%3A%2F%2Fspo337.com%2F
https://www.youa.eu/r.php?u=http%3A%2F%2Fspo337.com%2F
https://cool4you.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://rg4u.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.de-online.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.allpn.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://click.start.me/?url=http%3A%2F%2Fspo337.com%2F
https://prizraks.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://flyd.ru/away.php?to=http%3A%2F%2Fspo337.com%2F
https://risunok.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.sr/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://www.semanticjuice.com/site/http%3A%2F%2Fspo337.com%2F
https://cse.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://s79457.gridserver.com/?URL=http%3A%2F%2Fspo337.com%2F
https://cdl.su/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.fondbtvrtkovic.hr/?URL=http%3A%2F%2Fspo337.com%2F
https://lostnationarchery.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.booktrix.com/live/?URL=http%3A%2F%2Fspo337.com%2F
https://www.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://www.marcellusmatters.psu.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://onlinesportsbettingstory.blogspot.com/2024/01/asian-handicap-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-creates-absurd-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/01/fantasy-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/connecticut-considering-new-advertisement-club-and-sports-wagering-bill
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-profit.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-official-talks-about-state-s-games-wagering-bill-and-future-of
https://bestsportsbettingarticles.blogspot.com/2024/01/consideration-sports-betting-best-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/why-sports-expectation-markets-are-like-onions-and-film-industry-film-receipts
https://bettingstratigicarticle.blogspot.com/2024/01/technology-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/iowa-bill-to-authorize-sports-wagering-advances-subtleties-need-pressing
https://sportswageringinfo.blogspot.com/2024/01/future-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://gameswageringarticles.blogspot.com/2024/01/blog-post_31.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-lawful-games-wagering-regulation-trend
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-pro-level.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-bankroll-the-executives-benefits-victors-and-discipline-1623a8ef-e6da-4dd6-bedb-5596e78ec1af
https://sportsbettingindustry.blogspot.com/2024/01/game-changing-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/sports-wagering-nfl-lines-chances-point-spreads-and-then-some
https://articleinsportsbetting.blogspot.com/2024/01/optimal-wins-sports-betting-.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-jersey-representative-pallone-submits-amicus-brief-in-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/best-odds-earnings-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/american-gaming-affiliation-records-brief-in-sports-wagering-case-in-high-court
https://bettingstratigicarticle.blogspot.com/2024/02/essential-tips-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/n-j-representative-leonard-spear-on-sports-wagering-jersey-merits
https://sportswageringinfo.blogspot.com/2024/02/sports-wagering-triumph.html
https://lucky-razi-777.mystrikingly.com/blog/7-of-the-greatest-possible-advantages-of-lawful-managed-sports-wagering
https://gameswageringarticles.blogspot.com/2024/02/games-wagering-victory-tips.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettor-at-focus-of-alabama-baseball-embarrassment-has-to-deal-with-upwards-of
https://onlinesportsbettingstory.blogspot.com/2024/02/mastering-online-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-sports-wagering-bill-starts-development-through-senate
https://sportsbettingindustry.blogspot.com/2024/02/successful-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/looking-for-bettors-and-administrators-for-wide-arriving-at-industry-reviews
https://articleinsportsbetting.blogspot.com/2024/02/savvy-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bestsportsbettingarticles.blogspot.com/2024/02/winning-streak-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/something-like-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups-f8de9e02-c6a6-495d-9ec9-14a31daf9d51
https://onlinesportsbettingstory.blogspot.com/2024/02/changing-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportsbettingindustry.blogspot.com/2024/02/mastering-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/rocha-california-drive-will-assist-harm-the-brand-of-portable-games-wagering
https://articleinsportsbetting.blogspot.com/2024/02/mastering-revolutionary-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/is-america-crummy-with-awful-games-bettors
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tip-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-s-three-portable-sportsbooks-all-set-with-send-off-advancements
https://bettingstratigicarticle.blogspot.com/2024/02/expert-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/media-note-pad-where-might-the-telecom-companies-be-without-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/successful-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/yearlings-texans-same-game-parlay-expects-pass-cheerful-undertaking
https://gameswageringarticles.blogspot.com/2024/02/secret-success-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maturing-superteams-among-wagering-top-choices-to-bring-home-nba-championship
https://onlinesportsbettingstory.blogspot.com/2024/02/proven-winnings-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/climate-abbreviated-pga-visit-occasion-sparkles-sports-wagering-discussion
https://sportsbettingindustry.blogspot.com/2024/02/mastering%20strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/an-hour-endeavors-to-handle-sports-wagering-thrashes-about-all-things-being
https://articleinsportsbetting.blogspot.com/2024/02/art-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ncaa-solicitations-restriction-on-school-player-prop-wagers-in-ohio
https://bestsportsbettingarticles.blogspot.com/2024/02/unbeatable-sports%20betting-tips.html
https://site-6861241-1631-6047.mystrikingly.com/blog/liv-golf-wagering-markets-presented-in-additional-states-for-2024-season
https://bettingstratigicarticle.blogspot.com/2024/02/profit-expert-betting-stratigic.html
https://daddys-lucky000.mystrikingly.com/blog/mississippi-online-games-wagering-bill-clears-house-missouri-bill-escapes-panel
https://sportswageringinfo.blogspot.com/2024/02/Winning-Sports-Wagering.html
https://lucky-razi-777.mystrikingly.com/blog/swift-at-the-super-bowl-can-t-wager-on-it
https://gameswageringarticles.blogspot.com/2024/02/strategies-excel-ports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-betr-getting-ready-to-leave-massachusetts-market
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/record-promotion-offers-aided-lift-pennsylvanians-wagering-the-month-before
https://sportsbettingindustry.blogspot.com/2024/02/guide-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/lexi-thompson-thought-about-a-major-wagering-longshot-in-vegas-pga-visit
https://articleinsportsbetting.blogspot.com/2024/02/successful-guide-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-controllers-acclimate-to-new-difficulties-in-betting-business
https://bestsportsbettingarticles.blogspot.com/2024/02/psychology-winning-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/greatest-administrators-amazed-to-catch-wind-of-potential-california-sports
https://bettingstratigicarticle.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-remains-school-football-wagering-number-one-after-flimsy-september
https://sportswageringinfo.blogspot.com/2024/02/proven-tactics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/fanduel-keeps-on-ruling-virginia-sports-betting-scene
https://gameswageringarticles.blogspot.com/2024/02/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/travis-kelce-prop-wagering-detonates-as-taylor-swift-gives-a-shout-out-to-him
https://onlinesportsbettingstory.blogspot.com/2024/02/tips-win-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-dark-horse-dream-asset-will-support-dependable-betting-advancement
https://sportsbettingindustry.blogspot.com/2024/02/strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-bet-is-a-lock-to-send-off-by-this-date
https://articleinsportsbetting.blogspot.com/2024/02/mastering-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-week-5-mahomes-slides-bettors-cry-coolio-rides-wan-dale-skims
https://bestsportsbettingarticles.blogspot.com/2024/02/best-sports-wagering-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ncaa-hopes-to-return-to-sports-betting-infringement-discipline-framework
https://bettingstratigicarticle.blogspot.com/2024/02/sports-wagering-skills.html
https://daddys-lucky000.mystrikingly.com/blog/finally-around-is-prepared-to-play-in-illinois
https://onlinesportsbettingstory.blogspot.com/2024/02/%20strategies-sports-wagering-triumph.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-controllers-look-for-proactive-reaction-to-digital-hacks-at
https://sportsbettingindustry.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://casino999.mystrikingly.com/blog/caesars-enters-developing-microbetting-business-sector-with-simplebet
https://bestsportsbettingarticles.blogspot.com/2024/02/crushing-online-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/bally-bet-wynnbet-granted-maryland-versatile-wagering-licenses
https://bettingstratigicarticle.blogspot.com/2024/02/online-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/wynnbet-gets-massachusetts-computerized-sports-wagering-permit
https://sportswageringinfo.blogspot.com/2024/02/dominate-online-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-once-more-sets-month-to-month-sports-betting-income-record
https://gameswageringarticles.blogspot.com/2024/02/sports-betting-revealed.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettors-bet-more-than-200-million-in-maryland-in-november
https://onlinesportsbettingstory.blogspot.com/2024/02/online-sports-betting-hacks.html
https://site-6828921-2394-9309.mystrikingly.com/blog/fan-commitment-media-organizations-will-drive-sports-wagering-dealmaking
https://sportsbettingindustry.blogspot.com/2024/02/thrills-online-sports-betting.html
https://casino999.mystrikingly.com/blog/return-of-enormous-ten-football-gives-knock-to-sportsbooks
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-strategies-pay-off.html
https://site-4763676-9520-5980.mystrikingly.com/blog/development-in-us-helps-pad-william-slope-s-q3-decrease-in-incomes
https://bestsportsbettingarticles.blogspot.com/2024/02/best-online-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/d-c-s-first-free-sportsbook-preparing-for-opening-as-neighbors-stand-up-to
https://bettingstratigicarticle.blogspot.com/2024/02/successful-online-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/star-card-sharks-blame-nv-bookmaker-for-undermining-in-game-wagers
https://sportswageringinfo.blogspot.com/2024/02/sports-betting-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/the-week-in-sports-wagering-record-breaking-month-to-month-750m-new-jersey
https://gameswageringarticles.blogspot.com/2024/02/profits-online-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/turner-sports-makes-fanduel-official-games-wagering-accomplice-for-nba
https://onlinesportsbettingstory.blogspot.com/2024/02/skill-meets-fortune-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-gambling-clubs-planned-to-return-july-1
https://sportsbettingindustry.blogspot.com/2024/02/online-sports-betting-pros.html
https://casino999.mystrikingly.com/blog/gold-silver-and-betting-how-colorado-s-gaming-towns-became
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-hobby.html
https://site-4763676-9520-5980.mystrikingly.com/blog/arlington-park-itha-at-long-last-have-arrangement-for-horse-racing-in-illinois
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tips-every-sports-bettor%20.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-moving-again-in-georgia
https://bettingstratigicarticle.blogspot.com/2024/02/guide-safe-responsible-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://sportswageringinfo.blogspot.com/2024/02/safety-sports-betting-online.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-survey-expansive-help-for-club-gaming-not-such-a-huge-amount-for
https://gameswageringarticles.blogspot.com/2024/02/secure-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sporttrade-is-a-genuine-games-wagering-roller-coaster
https://onlinesportsbettingstory.blogspot.com/2024/02/%20online-sports-betting-enthusiasts.html
https://site-6828921-2394-9309.mystrikingly.com/blog/everything-that-bet365-s-colorado-send-off-says-to-us-about-the
https://sportsbettingindustry.blogspot.com/2024/02/tips-secure-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration
https://articleinsportsbetting.blogspot.com/2024/02/priority-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-unquestionable
https://bestsportsbettingarticles.blogspot.com/2024/02/safely-world-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/regardless-of-udoka-suspension-celtics-remain-title-top-choices-all-things
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-wins.html
https://daddys-lucky000.mystrikingly.com/blog/maryland-posts-sports-wagering-handle-of-32-5m-in-january
https://onlinesportsbettingstory.blogspot.com/2024/02/enjoying-online-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nevada-controllers-caution-sportsbooks-that-detailing-postpones-won-t-go-on
https://sportsbettingindustry.blogspot.com/2024/02/safety-measures-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration-a8c987a8-5921-4dd4-8953-8ddc50b1486a
https://articleinsportsbetting.blogspot.com/2024/02/world-sports-wagering-confidence.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-absolute
https://bestsportsbettingarticles.blogspot.com/2024/02/secrets-secure-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/alberta-s-controllers-actually-haggling-with-potential-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/world-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-bettor-hits-3-million-parlay-thanks-to-late-energize-by-horses
https://sportswageringinfo.blogspot.com/2024/02/finances-well-being-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/betfair-applies-for-sports-betting-permit-at-joined-center
https://gameswageringarticles.blogspot.com/2024/02/security-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/top-sportsbooks-turn-on-prop-27-promotion-system-in-california
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-dominate-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/wynnbet-draws-nearer-to-new-york-send-off
https://sportsbettingindustry.blogspot.com/2024/02/sports-betting-strategies-revealed.html
https://casino999.mystrikingly.com/blog/ohio-gambling-club-control-commission-sets-sports-wagering-application-charges
https://articleinsportsbetting.blogspot.com/2024/02/tips-live-betting-triumphs.html
https://site-4763676-9520-5980.mystrikingly.com/blog/a-bettor-s-manual-for-the-wild-and-weird-at-the-colder-time-of-year-olympics
https://bestsportsbettingarticles.blogspot.com/2024/02/strategies-work-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/month-to-month-stock-watch-m-a-reports-whirl-as-sports-wagering-stocks-stay
https://bettingstratigicarticle.blogspot.com/2024/02/live-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/will-arkansas-versatile-betting-get-february-endorsement
https://sportswageringinfo.blogspot.com/2024/02/live-betting-tactics-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-in-louisiana-gets-off-major-areas-of-strength-for-to
https://gameswageringarticles.blogspot.com/2024/02/strategies-guaranteed-wins-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-plans-to-open-sportsbook-parlor-at-joined-center-in-chicago
https://onlinesportsbettingstory.blogspot.com/2024/02/online-betting-strategies-unleashed.html
https://site-6828921-2394-9309.mystrikingly.com/blog/april-brings-newbie-ohio-its-most-minimal-wagering-action-so-far
https://sportsbettingindustry.blogspot.com/2024/02/live-betting-strategies-exposed.html
https://casino999.mystrikingly.com/blog/issue-betting-subsidizing-authoritatively-cut-from-d-c-spending-plan
https://articleinsportsbetting.blogspot.com/2024/02/strategies-real-time-live-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://bestsportsbettingarticles.blogspot.com/2024/02/sports-betting-strategies-victory.html
https://site-6861241-1631-6047.mystrikingly.com/blog/report-senate-decision-on-north-carolina-portable-wagering-bill-just-around
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/neglecting-to-report-betting-infringement-a-no-ncaa-says
https://sportswageringinfo.blogspot.com/2024/02/live-sports-betting-techniques.html
https://lucky-razi-777.mystrikingly.com/blog/scientists-go-after-making-sense-of-card-sharks-conduct-at-vegas-social-event
https://gameswageringarticles.blogspot.com/2024/02/Live%20Betting%20Strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/zensports-to-offer-money-back-remunerations-to-tennessee-games-bettors
https://onlinesportsbettingstory.blogspot.com/2024/02/successful-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-keeps-on-kicking-the-tires-on-the-streaming-conflicts
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting-5-simple-steps.html
https://casino999.mystrikingly.com/blog/charge-documented-to-give-each-arizona-clan-an-occasion-betting-permit
https://articleinsportsbetting.blogspot.com/2024/02/blog-post.html
https://site-4763676-9520-5980.mystrikingly.com/blog/perceptions-from-a-visit-to-betmgm-sportsbook-at-nats-park
https://bestsportsbettingarticles.blogspot.com/2024/02/world-best-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/georgia-partners-hopeful-2022-is-the-year-for-lawful-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/complex-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/locationsmart-an-option-in-contrast-to-geocomply-for-ontario-igaming
https://sportswageringinfo.blogspot.com/2024/02/conquering-world-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/illinois-sportsbooks-see-wagering-income-plunge-in-december
https://gameswageringarticles.blogspot.com/2024/02/tactics-sports-betting-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/proline-retail-sports-bettors-baffled-with-chances-disparities
https://onlinesportsbettingstory.blogspot.com/2024/02/succeed-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-s-betting-bonus-at-last-endorses-5-club-however-go-live-date-muddled
https://sportsbettingindustry.blogspot.com/2024/02/exciting-world-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-club-baffled-by-absence-of-sports-wagering-endorsement
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-smarter.html
https://site-4763676-9520-5980.mystrikingly.com/blog/bclc-reports-single-game-games-wagering-income-is-watching-ontario-market
https://bestsportsbettingarticles.blogspot.com/2024/02/mastering-sports-betting-worldwide.html
https://site-6861241-1631-6047.mystrikingly.com/blog/wash-st-sports-wagering-scientist-talks-bootleg-market-relocation-portable
https://bettingstratigicarticle.blogspot.com/2024/02/sports-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/a-story-of-three-new-games-wagering-states
https://sportswageringinfo.blogspot.com/2024/02/success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/entain-promotes-strong-tech-stack-with-draftkings-romance-having-blurred
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting_01573407901.html
https://site-6828921-2394-9309.mystrikingly.com/blog/florida-high-court-expresses-no-to-taking-seminoles-hard-rock-stage-3e9c4744-c515-4536-a0c8-7bb56cf957b7
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting.html
https://casino999.mystrikingly.com/blog/california-ancestral-chief-board-officially-goes-against-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/wisconsin-adolescent-confesses-to-scheme-in-sports-wagering-hacking-case
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/record-u-s-wagering-handle-anticipated-for-las-vegas-excellent-prix
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/draftkings-reports-moderate-parlay-element-coming-soon-to-online-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/proven-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/ontario-controller-partners-start-conversations-around-new-promotion-rules
https://gameswageringarticles.blogspot.com/2024/02/foolproof-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncpg-s-new-board-president-a-hero-with-an-arrangement
https://onlinesportsbettingstory.blogspot.com/2024/02/ultimate-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-york-turns-out-to-be-first-state-to-top-2-billion-in-month-to-month-handle
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-professional.html
https://casino999.mystrikingly.com/blog/california-s-clans-open-to-novel-thoughts-however-don-t-have-any-desire-to
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-guaranteed-success.html
https://site-4763676-9520-5980.mystrikingly.com/blog/florida-parimutuels-look-for-court-request-driving-seminoles-to-bring-down
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-greatest-pr-challenge-espn-faces-when-espn-bet-goes-live
https://bettingstratigicarticle.blogspot.com/2024/02/innovative-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/north-carolina-sports-wagering-board-of-trustees-offers-second-bunch-of-rules
https://sportswageringinfo.blogspot.com/2024/02/transformative-strategies-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/expert-association-organization-wagering-on-carrom-to-take-u-s-by-tempest
https://gameswageringarticles.blogspot.com/2024/02/effective-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/illinois-the-state-with-the-20-million-games-betting-permit-and-no-takers-dbbfc4dc-8a1d-4f1d-96bc-1334337be2c0
https://onlinesportsbettingstory.blogspot.com/2024/02/advanced-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/genius-associations-informing-on-sports-wagering-respectability-may-blow-up
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-success.html
https://casino999.mystrikingly.com/blog/a-few-gubernatorial-up-and-comers-take-solid-position-on-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies-dominate.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-officials-gradual-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/professional-betting-strategies.html
https://site-6861241-1631-6047.mystrikingly.com/blog/paddy-power-betfair-declares-procurement-of-fanduel
https://bettingstratigicarticle.blogspot.com/2024/02/inside-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/new-york-state-sports-wagering-plans-picture-it-s-a-piece-confounded
https://sportswageringinfo.blogspot.com/2024/02/mastering-betting-strategies.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-and-liquor-a-story-of-two-restrictions
https://onlinesportsbettingstory.blogspot.com/2024/03/betting-strategies-consistent-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-dependable-betting-bill-language-rejected-in-everyday-get-together
https://sportsbettingindustry.blogspot.com/2024/03/revolutionize-betting-game.html
https://casino999.mystrikingly.com/blog/virginia-tops-10-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/03/elevate-betting-game.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-senate-supports-revised-sports-wagering-bill
https://bestsportsbettingarticles.blogspot.com/2024/03/proven-sports-betting-strategies-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maryland-lottery-issues-versatile-betting-permit-to-aficionados-sportsbook
https://bettingstratigicarticle.blogspot.com/2024/03/elevate-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/april-brings-novice-ohio-its-most-minimal-wagering-action-hitherto
https://sportswageringinfo.blogspot.com/2024/03/ultimate-guide-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/issue-betting-financing-formally-cut-from-d-c-spending-plan
https://gameswageringarticles.blogspot.com/2024/03/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://onlinesportsbettingstory.blogspot.com/2024/03/transform-betting-experience.html
https://site-6828921-2394-9309.mystrikingly.com/blog/media-note-pad-kelce-siblings-sound-off-on-individual-nfl-players
https://sportsbettingindustry.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/best-wagering-scenes-getting-stoned-in-the-music-of-possibility
https://articleinsportsbetting.blogspot.com/2024/03/mastering-betting-pro-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ohio-has-turned-into-the-most-intriguing-state-with-regards-to-the-sportsbook
https://bestsportsbettingarticles.blogspot.com/2024/03/betting-strategies-serious-players.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-regulator-approves-latest-sports-wagering-procedures
https://bettingstratigicarticle.blogspot.com/2024/03/Betting%20Strategies%20for%20Victory.html
https://daddys-lucky000.mystrikingly.com/blog/three-new-administrators-could-be-live-in-arizona-by-mid-2024
https://sportswageringinfo.blogspot.com/2024/03/unveiling-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://gameswageringarticles.blogspot.com/2024/03/journey-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/administrators-push-back-on-proposed-betting-methods-in-vermont
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crab-sports-dispatches-wagering-stage-in-maryland-application-not-yet
https://sportsbettingindustry.blogspot.com/2024/03/deep-dive-world-wagering.html
https://casino999.mystrikingly.com/blog/pieces-prosperity-assists-colorado-bettors-with-avoiding-may-pattern-of
https://articleinsportsbetting.blogspot.com/2024/03/journey-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://bestsportsbettingarticles.blogspot.com/2024/03/dollars-world-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maine-betting-control-boss-back-working-after-neglected-suspension
https://bettingstratigicarticle.blogspot.com/2024/03/wagering-journey-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/is-most-recent-florida-choice-actually-an-ancestral-gaming-major-advantage
https://sportswageringinfo.blogspot.com/2024/03/art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-virtuoso-games-expand-sports-wagering-information-association-through
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-first-to-attempt-to-boycott-bettors-who-undermine-competitors
https://sportsbettingindustry.blogspot.com/2024/03/unconventional-wagering-journey.html
https://casino999.mystrikingly.com/blog/sportradar-inks-sports-wagering-information-concurrence-with-south-america-s
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-wizardry-mastering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-virtuoso-games-broaden-sports-wagering-information-organization-through
https://bestsportsbettingarticles.blogspot.com/2024/03/unveiling-wagering-journey.html
https://site-6861241-1631-6047.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://bettingstratigicarticle.blogspot.com/2024/03/navigating-world-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/how-truly-do-cultivate-children-need-to-manage-the-fate-of-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/lessons-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/how-in-all-actuality-do-encourage-children-need-to-manage-the-eventual-fate
https://gameswageringarticles.blogspot.com/2024/03/journey-fortunes-made-lost.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ohio-officials-twofold-games-betting-expense
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-betting-strategies-winning-big.html
https://site-6828921-2394-9309.mystrikingly.com/blog/five-nfl-groups-with-ideal-prospects-chances
https://sportsbettingindustry.blogspot.com/2024/03/blog-post.html
https://casino999.mystrikingly.com/blog/football-challenges-of-old-made-the-present-huge-games-wagering-prominence
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-like-champion.html
https://site-4763676-9520-5980.mystrikingly.com/blog/one-more-influx-of-gambling-clubs-apply-to-join-new-jersey-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/tips-excel-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/fanduel-sportsbook-send-off-imprints-another-lawful-games-wagering-achievement
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-controllers-authorities-urge-persistence-to-try-not-to-bumble
https://sportswageringinfo.blogspot.com/2024/03/inner-champion-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/cheerful-commemoration-delaware-sports-wagering-a-champ-one-month-in
https://gameswageringarticles.blogspot.com/2024/03/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/excitement-is-high-as-mississippi-sports-wagering-arrangements-in-progress
https://onlinesportsbettingstory.blogspot.com/2024/03/Navigate-art-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-wagering-lines-problem-what-happens-when-everybody-risks-everything-group
https://sportsbettingindustry.blogspot.com/2024/03/decoding-art-sports-wagering.html
https://casino999.mystrikingly.com/blog/report-ny-will-have-greatest-games-wagering-business-sector-in-u-s
https://articleinsportsbetting.blogspot.com/2024/03/maximizing-odds-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pga-visit-concurs-it-d-be-stupid-to-restrict-portable-wagering-to-on-premises
https://bestsportsbettingarticles.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/kentucky-putting-money-on-sports-wagering-to-assist-with-fixing-annuity-issue
https://sportswageringinfo.blogspot.com/2024/03/dominate-art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/legitimate-games-wagering-now-regulation-in-rhode-island-and-state-gets-51
https://gameswageringarticles.blogspot.com/2024/03/comprehensive-guide-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-nearby-according-to-an-understudy-s-point-of-view
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-wagering-guaranteed-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pittsburgh-privateers-take-sports-wagering-trustworthiness-expense-to
https://sportsbettingindustry.blogspot.com/2024/03/game-changing-strategy-sports-betting.html
https://casino999.mystrikingly.com/blog/greatest-chances-movers-after-first-rounds-of-world-cup
https://articleinsportsbetting.blogspot.com/2024/03/Winwinning-strategies-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/monitoring-west-virginia-s-games-wagering-plans-and-send-off
https://bestsportsbettingarticles.blogspot.com/2024/03/art-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/oregon-lottery-preparing-portable-application-with-versatile-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/level-up-betting-game.html
https://daddys-lucky000.mystrikingly.com/blog/rhode-island-makes-stride-nearer-to-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/03/excel-sports-wagering-like-pro.html
https://lucky-razi-777.mystrikingly.com/blog/seriously-fighting-yet-no-democratic-in-new-york-state
https://gameswageringarticles.blogspot.com/2024/03/psychological-tricks-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/choctaw-intend-to-be-first-in-quite-a-while-to-offer-games-wagering
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/murphy-makes-sports-wagering-lawful-in-new-jersey
https://sportsbettingindustry.blogspot.com/2024/03/key-success-sports-betting.html
https://casino999.mystrikingly.com/blog/stage-set-for-decision-on-legitimate-new-york-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/why-is-murphy-delayed-to-make-nj-sports-wagering-regulation
https://bestsportsbettingarticles.blogspot.com/2024/03/mental-aspect-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/at-any-rate-so-what-in-blazes-is-uprightness-checking
https://bettingstratigicarticle.blogspot.com/2024/03/power-mind-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/lead-representative-wagers-on-phillies-to-start-up-lawful-delaware-sports
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-mental-mastery.html
https://lucky-razi-777.mystrikingly.com/blog/mailbag-mythbusters-uigea-and-sports-wagering-made-sense
https://gameswageringarticles.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/massachusetts-preparing-to-make-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-divisional-round-weekend-sportsbooks-say-victories-are-coming
https://sportsbettingindustry.blogspot.com/2024/03/successful-sports-betting.html
https://casino999.mystrikingly.com/blog/pennsylvania-closures-notable-year-with-record-month-to-month-sports-betting
https://articleinsportsbetting.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/somewhere-around-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bestsportsbettingarticles.blogspot.com/2024/03/Comprehensiveuide-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting-success.html
https://daddys-lucky000.mystrikingly.com/blog/versatile-games-betting-authoritatively-dispatches-in-vermont
https://sportswageringinfo.blogspot.com/2024/03/strategy-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-administrator-once-again-introduces-sports-wagering-bill
https://gameswageringarticles.blogspot.com/2024/03/mastering-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-sharps-activity-giving-prime-games-looked-for-in-ohio
https://onlinesportsbettingstory.blogspot.com/2024/03/world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-legislators-might-put-destiny-of-sports-wagering-in-citizens-grasp
https://sportsbettingindustry.blogspot.com/2024/03/blog-post_18.html
https://casino999.mystrikingly.com/blog/draftkings-perspectives-sports-wagering-as-tremendous-learning-experience
https://articleinsportsbetting.blogspot.com/2024/03/mental-side-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/kansas-legislators-get-educated-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-mind-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-seeking-after-different-roads-to-sanction-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-mindset-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/could-face-sports-book-become-a-thing
https://sportswageringinfo.blogspot.com/2024/03/mastering-mind-game-betting.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-lead-representative-expresses-associations-a-desire-for-peace
https://gameswageringarticles.blogspot.com/2024/03/mastering-psychology-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-bill-russell-denounces-any-kind-of-authority-on-miami
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting_01715168387.html
https://site-6828921-2394-9309.mystrikingly.com/blog/high-court-strikes-down-government-sports-wagering-boycott-opening-entryway
https://sportsbettingindustry.blogspot.com/2024/03/strategies-betting-success.html
https://casino999.mystrikingly.com/blog/ancestral-worries-supposedly-easing-back-michigan-on-sports-wagering-regulation
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mental-game-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-fiend-examines-strife-recuperation
https://bestsportsbettingarticles.blogspot.com/2024/03/secrets-strategic-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-tangle
https://bettingstratigicarticle.blogspot.com/2024/03/expert-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/the-most-effective-method-to-explore-the-games-wagering-data-and-promote
https://sportswageringinfo.blogspot.com/2024/03/key-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/connecticut-bill-escapes-council-yet-clans-in-conflict-with-state
https://gameswageringarticles.blogspot.com/2024/03/winning-mindset-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/a-terrible-bet-slighting-sanctioned-betting-s-expected-new-clients
https://onlinesportsbettingstory.blogspot.com/2024/03/mastering-mind-game-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/monetary-examination-the-amount-will-connecticut-advantage-from-sports
https://sportsbettingindustry.blogspot.com/2024/03/mastery-sports-betting-success.html
https://casino999.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://articleinsportsbetting.blogspot.com/2024/03/sports-bet-wins-redefine-strategy.html
https://site-4763676-9520-5980.mystrikingly.com/blog/what-precisely-is-a-games-wagering-promote
https://bestsportsbettingarticles.blogspot.com/2024/03/ultimate-guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/possibilities-obscure-for-entry-of-connecticut-sports-wagering-bill
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/how-a-sex-embarrassment-is-easing-back-missouri-s-capacity-to-legitimize
https://sportswageringinfo.blogspot.com/2024/03/dark-side-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/players-associations-stake-guarantee-for-cut-of-sports-wagering-pie-now-as-well
https://onlinesportsbettingstory.blogspot.com/2024/03/winning-sports-bets-every-time.html
https://site-6828921-2394-9309.mystrikingly.com/blog/massachusetts-deliveries-extensive-survey-of-state-s-games-wagering-an
https://sportsbettingindustry.blogspot.com/2024/03/psychology-behind-successful-sports-betting.html
https://casino999.mystrikingly.com/blog/the-wire-demonstration-of-1961-when-the-web-met-the-bedrock-government
https://articleinsportsbetting.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-not-keen-on-paying-associations-a-games-wagering-sovereignty
https://bestsportsbettingarticles.blogspot.com/2024/03/beginners-guide-making-money-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/laissez-les-bons-temps-rouler-louisiana-opens-sports-wagering-discussion
https://bettingstratigicarticle.blogspot.com/2024/03/revolutionizing-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/connecticut-dems-squeezing-for-legitimized-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-strategies-work.html
https://lucky-razi-777.mystrikingly.com/blog/oklahoma-ancestral-games-wagering-bill-clears-board-heads-to-full-house
https://gameswageringarticles.blogspot.com/2024/03/sports-outcomes-beat-odds.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-jersey-tolerating-sports-wagering-applications-chief-affirms
https://onlinesportsbettingstory.blogspot.com/2024/03/tips-tricks-insider-knowledge-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-prepares-crazy-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/03/art-sports-betting-like-champ.html
https://casino999.mystrikingly.com/blog/west-virginia-official-examines-state-s-games-wagering-bill-and-future-of
https://articleinsportsbetting.blogspot.com/2024/03/biggest-sports-bet-wins.html
https://site-4763676-9520-5980.mystrikingly.com/blog/iowa-bill-to-legitimize-sports-wagering-advances-subtleties-need-pressing
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-art-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/charge-presented-in-missouri-pointed-toward-sanctioning-games-wagering-in-state
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post_25.html
https://daddys-lucky000.mystrikingly.com/blog/ball-gets-going-on-iowa-authorization-of-sports-wagering-with-hsb-592
https://sportswageringinfo.blogspot.com/2024/03/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-sports-wagering-bill-would-allow-betting-at-five-club-and-on
https://gameswageringarticles.blogspot.com/2024/03/enhance-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-legitimate-games-wagering-regulation-temporary-fad
https://onlinesportsbettingstory.blogspot.com/2024/03/sharpening-your-skills-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-junkie-talks-about-disturbance-recuperation
https://sportsbettingindustry.blogspot.com/2024/03/level-up-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/instructions-to-explore-the-games-wagering-data-and-promote-industry
https://bestsportsbettingarticles.blogspot.com/2024/04/advanced-sports-betting-triumph.html
https://site-6861241-1631-6047.mystrikingly.com/blog/in-the-event-that-sports-wagering-made-lawful-mississippi-intends-to
https://bettingstratigicarticle.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/a-terrible-bet-disregarding-legitimized-betting-s-expected-new-clients
https://sportswageringinfo.blogspot.com/2024/04/improving-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/monetary-investigation-the-amount-will-connecticut-advantage-from-sports
https://gameswageringarticles.blogspot.com/2024/04/refining-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://onlinesportsbettingstory.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maine-senate-supersedes-sports-wagering-blackball-sets-state-on-way-for
https://sportsbettingindustry.blogspot.com/2024/04/supercharge-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/wading-into-controversy-dialing-back-kentucky-games-wagering-vote
https://articleinsportsbetting.blogspot.com/2024/04/improving-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nonattendances-defer-maine-blackball-supersede-decision-on-legitimate-games
https://bestsportsbettingarticles.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/colorado-inches-nearer-to-may-sports-wagering-send-off-notwithstanding
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/in-the-midst-of-epic-u-s-sports-wagering-turf-war-penn-public-plants
https://sportswageringinfo.blogspot.com/2024/04/secrets-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-principles-among-plan-things-for-illinois-gaming-executive
https://gameswageringarticles.blogspot.com/2024/04/elevate-skills-rookie-pro.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maine-lead-representative-denials-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/04/essential-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/report-ncaa-has-found-175-games-betting-infringement-beginning-around-2018
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-skills-today.html
https://casino999.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/04/elevate-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/mlb-rule-changes-lead-to-additional-wagering-on-taken-bases
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sportsbetting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/concentrate-on-shows-huge-flood-in-wagering-among-ladies-on-ladies-games
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-skills-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/crisis-kentucky-games-wagering-guidelines-preclude-deluding-publicizing
https://sportswageringinfo.blogspot.com/2024/04/sharpen-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/three-occasion-betting-licenses-destined-to-be-available-for-anyone-in-arizona
https://gameswageringarticles.blogspot.com/2024/04/strategies-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/seminoles-will-not-have-the-option-to-send-off-sports-betting-in-florida
https://onlinesportsbettingstory.blogspot.com/2024/04/improve-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/hold-rate-for-new-york-sports-betting-remaining-parts-stuck-on-high
https://sportsbettingindustry.blogspot.com/2024/04/essential-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/most-recent-surveying-recommends-backing-for-california-betting-drives
https://articleinsportsbetting.blogspot.com/2024/04/enhance-sports-betting-skills-now.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-gaming-commission-sitting-tight-for-public-remarks
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/elys-game-innovation-uses-d-c-as-retail-sports-betting-model
https://bettingstratigicarticle.blogspot.com/2024/04/polishing-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/capitalizing-on-changing-out-bookmaker-dishes-on-money-out-apparatus
https://sportswageringinfo.blogspot.com/2024/04/mastering-sports-betting-pro.html
https://lucky-razi-777.mystrikingly.com/blog/maryland-lottery-chief-offers-versatile-send-off-course-of-events-subtleties
https://gameswageringarticles.blogspot.com/2024/04/master-sports-betting-5-steps.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-in-possessing-mahowny-the-activity-is-the-juice
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-insider-tips.html
https://site-6828921-2394-9309.mystrikingly.com/blog/oregon-lottery-carries-out-application-sports-wagering-coming-the-following
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/sports-wagering-and-wounds-in-school-football-isolating-great-data-from-clamor
https://articleinsportsbetting.blogspot.com/2024/04/mastering-art-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/elite-athletics-associations-proceed-with-full-court-push-on-west-virginia
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-wins.html
https://site-6861241-1631-6047.mystrikingly.com/blog/where-the-midwest-is-at-on-sports-wagering-regulation
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/aga-to-schumer-sports-wagering-doesn-t-need-government-oversight
https://sportswageringinfo.blogspot.com/2024/04/mastering-science-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/arkansas-gaming-sports-wagering-polling-form-drive-enduring-an-onslaught
https://gameswageringarticles.blogspot.com/2024/04/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-expert-will-congress-engage-with-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/mind-master-sports-bettor.html
https://site-6828921-2394-9309.mystrikingly.com/blog/intelligent-bettors-ought-to-pay-attention-to-the-buzz-prior-to-wagering
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-management-techniques.html
https://casino999.mystrikingly.com/blog/maine-officials-move-toward-legitimate-games-wagering
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-analytics.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-s-conflict-over-betting-gets-more-sizzling
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/tennessee-posts-370-million-games-wagering-handle-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://daddys-lucky000.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://sportswageringinfo.blogspot.com/2024/04/master-sports-betting-intelligence.html
https://lucky-razi-777.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-bill-bites-the-dust-in-panel-on-conclusive-day-of-kentucky
https://onlinesportsbettingstory.blogspot.com/2024/04/master-sports-betting-like-pro.html
https://site-6828921-2394-9309.mystrikingly.com/blog/kentucky-crisis-sports-wagering-regs-could-make-worry-among-administrators
https://sportsbettingindustry.blogspot.com/2024/04/mastering-art-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-report-records-conceivable-dependable-betting-upgrades
https://articleinsportsbetting.blogspot.com/2024/04/journey-sports-betting-succes.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-draws-nearer-to-inking-manage-new-games-betting-accomplice
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-techniques.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-controller-endorses-most-recent-games-betting-methods
https://bettingstratigicarticle.blogspot.com/2024/04/become-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://sportswageringinfo.blogspot.com/2024/04/tips-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/concentrate-on-shows-critical-flood-in-wagering-among-ladies-on-ladies-games
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-maximum-returns.html
https://site-6861405-2997-4499.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://onlinesportsbettingstory.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://site-6828921-2394-9309.mystrikingly.com/blog/is-vermont-getting-ready-to-consider-sanctioning-games-wagering
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://casino999.mystrikingly.com/blog/espn-refreshing-growing-focusing-on-sports-betting-substance
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies_01879442689.html
https://site-4763676-9520-5980.mystrikingly.com/blog/survey-says-most-ohioans-don-t-want-to-bet-on-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pointsbet-plans-to-handle-wagers-in-less-than-a-second-as-shift-to-in-game
https://bettingstratigicarticle.blogspot.com/2024/04/strategies-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/online-sportsbooks-are-playing-get-up-to-speed-with-regards-to-ada-consistence
https://sportswageringinfo.blogspot.com/2024/04/becoming-master-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/bally-s-applies-to-offer-versatile-games-betting-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesotans-aren-t-in-with-no-reservations-on-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/future-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/yasiel-puig-pulls-out-blameworthy-request-in-unlawful-games-wagering-case
https://sportsbettingindustry.blogspot.com/2024/04/revolutionizing-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-affirms-postpone-in-sports-wagering-send-off-as-iger-behaviors-key-audit
https://articleinsportsbetting.blogspot.com/2024/04/top-sports-betting-trends-2024.html
https://site-4763676-9520-5980.mystrikingly.com/blog/will-sports-wagering-twitter-endure-musk-s-fart-mode-second
https://bestsportsbettingarticles.blogspot.com/2024/04/inside-world-in-play-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/massachusetts-gaming-commission-consents-to-acknowledge-mgm-s-late-application
https://bettingstratigicarticle.blogspot.com/2024/04/rise-mobile-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-computerized-betting-authorizing-contest-not-really-solid
https://sportswageringinfo.blogspot.com/2024/04/data-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-times-fiercely-comes-up-short-on-sports-wagering-story
https://gameswageringarticles.blogspot.com/2024/04/blog-post_12.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-might-ftx-s-crash-affect-crypto-in-sports-wagering-5cbefb65-9344-4f22-bb25-1dcf520d525a
https://onlinesportsbettingstory.blogspot.com/2024/04/AI-transforming-sports-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/versatile-sportsbooks-will-go-live-in-maryland-on-wednesday-lottery-reports
https://sportsbettingindustry.blogspot.com/2024/04/traditional-sports-betting.html
https://casino999.mystrikingly.com/blog/california-betting-drives-made-the-most-awful-sort-of-history
https://articleinsportsbetting.blogspot.com/2024/04/deep-dive-prop-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://bestsportsbettingarticles.blogspot.com/2024/04/popularity-virtual-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-wrongfully-on-sports
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-beginners.html
https://daddys-lucky000.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://sportswageringinfo.blogspot.com/2024/04/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/california-betting-drives-made-the-most-obviously-awful-sort-of-history
https://gameswageringarticles.blogspot.com/2024/04/blockchain-sports-betting-security.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-responsible-gaming.html
https://site-6828921-2394-9309.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-unlawfully-on-sports
https://sportsbettingindustry.blogspot.com/2024/04/trends-live-betting.html
https://casino999.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://articleinsportsbetting.blogspot.com/2024/04/dynamic-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/maryland-portable-send-off-unavoidable-as-betting-commission-grants-10-licenses
https://bestsportsbettingarticles.blogspot.com/2024/04/cross-section-sports-betting-social-media.html
https://site-6861241-1631-6047.mystrikingly.com/blog/caesars-offering-100-in-credits-in-front-of-maryland-sports-wagering-send-off
https://bettingstratigicarticle.blogspot.com/2024/04/latest-mobile-betting-apps.html
https://daddys-lucky000.mystrikingly.com/blog/texas-representative-acquaints-goal-with-permit-sports-betting-club-betting
https://sportswageringinfo.blogspot.com/2024/04/predictive-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/holey-strokes-little-golf-betting-endorsed-in-colorado-wyoming
https://gameswageringarticles.blogspot.com/2024/04/sports-betting-regulations.html
https://site-6861405-2997-4499.mystrikingly.com/blog/one-day-on-one-free-day-plan-for-maryland-send-off-certain-to-befuddle-bettors
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-markets-rise.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sportsbook-celebrity-projects-ain-t-what-they-used-to-be-bettors-say
https://sportsbettingindustry.blogspot.com/2024/04/leverage-data-smart-betting-decisions.html
https://casino999.mystrikingly.com/blog/month-to-month-stock-watch-sports-wagering-stocks-keep-afloat-in-front-of
https://articleinsportsbetting.blogspot.com/2024/04/popularity-niche-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/not-dead-yet-sports-wagering-restored-in-missouri
https://bestsportsbettingarticles.blogspot.com/2024/04/influence-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ohio-sports-betting-application-window-opens-june-15
https://bettingstratigicarticle.blogspot.com/2024/04/live-streaming-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/the-usfl-needs-to-embrace-sports-bettors-and-dfs-players-right-away
https://sportswageringinfo.blogspot.com/2024/04/sports-betting-trends-social-media.html
https://lucky-razi-777.mystrikingly.com/blog/best-games-wagering-scenes-riding-the-borail-with-mine-that-bird-in-50-to-1
https://gameswageringarticles.blogspot.com/2024/04/tips-sports-betting-beginners.html
https://site-6861405-2997-4499.mystrikingly.com/blog/arizona-sports-wagering-handle-falls-barely-short-of-500m-for-december
https://onlinesportsbettingstory.blogspot.com/2024/04/evolution-parlay-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pennsylvania-posts-800-million-games-wagering-handle-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/mind-betting-rofessional.html
https://casino999.mystrikingly.com/blog/nba-hands-lifetime-boycott-to-jontay-watchman-for-conspicuous-betting
https://articleinsportsbetting.blogspot.com/2024/04/think-like-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/draftkings-and-enthusiasts-go-head-to-head-in-court-over-leader-abandonment
https://bestsportsbettingarticles.blogspot.com/2024/04/journey-becoming-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-changing-game.html
https://daddys-lucky000.mystrikingly.com/blog/espn-unlv-declare-beneficiaries-of-first-dependable-betting-partnerships
https://sportswageringinfo.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://gameswageringarticles.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-decrees-bettors-need-to-follow-for-capable-parlay-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/secrets-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-sports-wagering-handle-aggregates-1-07-billion-for-february
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-playbook.html
https://casino999.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-sanctioning-endeavors-fizzled
https://articleinsportsbetting.blogspot.com/2024/04/insights-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-bettors-bound-to-knock-back-the-firewater-than-different-players
https://bestsportsbettingarticles.blogspot.com/2024/04/evolution-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/iowa-south-carolina-establishes-ladies-games-wagering-standards
https://bettingstratigicarticle.blogspot.com/2024/04/true-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/caitlin-clark-previously-carrying-gigantic-wagering-interest-to-the-wnba
https://sportswageringinfo.blogspot.com/2024/04/world-inside-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-bill-spotlights-on-issue-betting-third-minnesota
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-vs-casual-bettors.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesota-sports-wagering-sanctioning-discussion-warms-up-in-house-advisory
https://onlinesportsbettingstory.blogspot.com/2024/04/psychology-behind-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-recruits-lori-kalani-as-first-boss-dependable-gaming-official
https://sportsbettingindustry.blogspot.com/2024/04/winning-like-betting-professional.html
https://casino999.mystrikingly.com/blog/more-secure-betting-conversation-addresses-late-embarrassments
https://articleinsportsbetting.blogspot.com/2024/04/consistent-success-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ontario-sportsbooks-not-permitted-to-take-wagers-on-wba-boxing
https://bestsportsbettingarticles.blogspot.com/2024/04/truth-behind-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-sports-wagering-polling-form-drive-outperforms-300-000-marks
https://bettingstratigicarticle.blogspot.com/2024/04/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/washington-d-c-sports-wagering-handle-under-16-million-for-spring
https://sportswageringinfo.blogspot.com/2024/04/life-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://onlinesportsbettingstory.blogspot.com/2024/04/tools-resources-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-jersey-sports-wagering-handle-tops-1-3-billion-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/transitioning-betting-professional.html
https://casino999.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://articleinsportsbetting.blogspot.com/2024/04/financial-realities-professional-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/the-precepts-bettors-need-to-follow-for-dependable-parlay-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/betting-professionals-trends-make-decisions.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-guide.html
https://daddys-lucky000.mystrikingly.com/blog/2024-nba-season-finisher-wagering-review
https://sportswageringinfo.blogspot.com/2024/04/betting-professionals-stay-top.html
https://lucky-razi-777.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-future.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-becomes-essential-games-wagering-application-in-washington-d-c
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crusade-for-more-pleasant-betting-billions-bet-illicitly-on-ncaa-competitions
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-balancing-luck-skill.html
https://casino999.mystrikingly.com/blog/bally-bet-plans-for-june-sports-wagering-send-off-in-massachusetts
https://articleinsportsbetting.blogspot.com/2024/04/takes-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/tennessee-games-wagering-handle-hits-472-million-for-spring
https://bestsportsbettingarticles.blogspot.com/2024/04/ethical-dilemmas-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/here-s-to-you-mr-robinson-nate-robinson-dishes-on-the-knicks-sports
https://bettingstratigicarticle.blogspot.com/2024/04/betting-like-pro.html
https://daddys-lucky000.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-authorization-endeavors-fizzled
https://sportswageringinfo.blogspot.com/2024/04/secrets-behind-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/sports-bettors-bound-to-hit-the-bottle-hard-than-different-speculators
https://gameswageringarticles.blogspot.com/2024/04/psychology-betting-professionals.html
https://site-6861405-2997-4499.mystrikingly.com/blog/caitlin-clark-previously-carrying-monstrous-wagering-interest-to-the-wnba
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post_25.html
https://site-6828921-2394-9309.mystrikingly.com/blog/more-modest-games-associations-hustling-for-legitimate-games-wagering-open-doors
https://sportsbettingindustry.blogspot.com/2024/05/betting-change-perspective.html
https://casino999.mystrikingly.com/blog/football-is-top-dog-ms-september-sports-wagering-handle-ranges-31-77-million
https://articleinsportsbetting.blogspot.com/2024/04/betting-blunders-brilliance.html
https://site-4763676-9520-5980.mystrikingly.com/blog/after-lively-hearing-indiana-legislators-will-keep-on-investigating-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/lessons-learned-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://bettingstratigicarticle.blogspot.com/2024/04/lessons-from-world-betting.html
https://daddys-lucky000.mystrikingly.com/blog/study-mlb-nba-to-yield-joined-1-7-billion-from-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/04/essential-life-lessons-learned-betting.html
https://lucky-razi-777.mystrikingly.com/blog/hearing-uncovers-illinois-legislators-advancing-toward-legitimizing-sports
https://onlinesportsbettingstory.blogspot.com/2024/04/Career-betting-professional.html
https://site-6828921-2394-9309.mystrikingly.com/blog/the-arrangements-keep-comin-new-york-planes-mgm-figure-out-advertising
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-top-predictions.html
https://casino999.mystrikingly.com/blog/looking-at-replies-to-enter-sports-wagering-inquiries-in-illinois
https://articleinsportsbetting.blogspot.com/2024/04/mysteries-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pgcb-to-hold-hearings-decision-on-three-additional-games-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/highly-effective-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/minnesota-administrator-hopeful-but-still-guarded-about-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-exposed.html
https://daddys-lucky000.mystrikingly.com/blog/mgm-resorts-turns-into-nhl-s-true-games-wagering-accomplice-in-memorable-new
https://sportswageringinfo.blogspot.com/2024/04/lessons-learned-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/with-significant-declaration-impending-nhl-supposedly-betting-everything-on
https://gameswageringarticles.blogspot.com/2024/04/betting-will-blow-your-mind.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncaa-declares-foundation-of-new-advisory-group-to-look-at-sports-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/unconventional-lessons-learned-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/louisiana-officials-looking-at-sports-wagering-legitimization
https://sportsbettingindustry.blogspot.com/2024/04/lessons-learned-betting-strategies.html
https://casino999.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://articleinsportsbetting.blogspot.com/2024/04/unveiling-betting-wisdom.html
https://site-4763676-9520-5980.mystrikingly.com/blog/more-modest-games-associations-dashing-for-legitimate-games-wagering-open-doors
https://bestsportsbettingarticles.blogspot.com/2024/04/essential-lessons-every-risk-taker.html
https://site-6861241-1631-6047.mystrikingly.com/blog/football-is-above-all-else-ms-september-sports-wagering-handle-ranges-31-77
https://bettingstratigicarticle.blogspot.com/2024/04/betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/after-energetic-hearing-indiana-officials-will-keep-on-investigating-sports
https://sportswageringinfo.blogspot.com/2024/04/blog-post_29.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/blog-post_26.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://onlinesportsbettingstory.blogspot.com/2024/05/practical-lessons-smarter-decision-making.html
https://site-6828921-2394-9309.mystrikingly.com/blog/legislators-pushing-for-legitimate-games-wagering-in-washington-d-c
https://sportsbettingindustry.blogspot.com/2024/05/betting-lessons-kickstart-journey.html
https://casino999.mystrikingly.com/blog/sports-wagering-dispatches-in-new-mexico-we-anticipate-a-major-weekend
https://articleinsportsbetting.blogspot.com/2024/05/betting-lessons-learned-strategic-living.html
https://site-4763676-9520-5980.mystrikingly.com/blog/where-do-gubernatorial-competitors-stand-west-version
https://bestsportsbettingarticles.blogspot.com/2024/05/essential-lessons-every-gambler.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-examiner-in-game-betting-one-of-smartest-choices-to-make
https://bettingstratigicarticle.blogspot.com/2024/05/lessons-learned-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-on-plan-this-week-in-indiana-illinois-and-washington-d-c
https://sportswageringinfo.blogspot.com/2024/05/betting-secrets-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/september-new-jersey-sports-wagering-handle-leaps-to-184m-income-24m
https://gameswageringarticles.blogspot.com/2024/05/lessons-learned-high-stakes-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/where-do-gubernatorial-up-and-comers-stand-on-sports-wagering-east-version
해외배팅사이트 추천 https://spo337.com/
해외스포츠배팅사이트 추천 https://spo337.com/
해외배팅사이트에이전시 추천 https://spo337.com/
머니라인247 https://spo337.com/ml247/
황룡카지노 https://spo337.com/gdcasino/
아시안커넥트 https://spo337.com/asianconnect/
스보벳 https://spo337.com/sbobet/
피나클 https://spo337.com/pinbet88/
맥스벳 https://spo337.com/maxbet/
파워볼 https://spo337.com/category/powerball/
BTI Sports https://spo337.com/btisports/
https://rb.gy/4y0o7
https://sites.google.com/view/spo337/
https://bit.ly/45dAsE6
https://cutt.ly/dwbyj5Dq
https://ln.run/Cl2oj
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/iv97s
https://cutt.ly/2wbyki06
https://sites.google.com/view/xn—b60by94a1wbra414jbsf-/
https://ln.run/VribE
https://sites.google.com/view/hdcasino/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/3vh3j
https://cutt.ly/Cwbykk2t
https://sites.google.com/view/hdcasino/
https://ln.run/6Bw_0
https://sites.google.com/view/moneyline247/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://rb.gy/03f4u
https://sites.google.com/view/moneyline247/
https://duckduckgo.com/?q=https%3A%2F%2Fspo337.com&t=h_&ia=web
mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
materinstvo.ru/forward?link=http%3A%2F%2Fspo337.com%2F
http://computer-chess.org/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
https://enchantedcottageshop.com/shop/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
https://buist-keatch.org/sphider/include/click_counter.php?url=http%3A%2F%2Fspo337.com%2F
www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
rel.chubu-gu.ac.jp/soumokuji/cgi-bin/go.cgi?http%3A%2F%2Fspo337.com%2F
fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
www.veloxbox.us/link/?h=http%3A%2F%2Fspo337.com%2F
www.adult-plus.com/ys/rank.php?mode=link&id=592&url=http%3A%2F%2Fspo337.com%2F
mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
sparktime.justclick.ru/lms/api-login/?hash=MO18szcRUQdzpT%2FrstSCW5K8Gz6ts1NvTJLVa34vf1A%3D&authBhvr=1&email=videotrend24%40mail.ru&expire=1585462818&lms%5BrememberMe%5D=1&targetPath=http%3A%2F%2Fspo337.com%2F
http://vcteens.com/cgi-bin/at3/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.bquest.org/Links/Redirect.aspx?ID=164&url=http%3A%2F%2Fspo337.com%2F
www.stipendije.info/phpAdsNew/adclick.php?bannerid=129&zoneid=1&source=&dest=http%3A%2F%2Fspo337.com%2F
today.od.ua/redirect.php?url=http%3A%2F%2Fspo337.com%2F
search.kcm.co.kr/jump.php?url=http%3A%2F%2Fspo337.com%2F
laskma.megastart-slot.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
www.mir-stalkera.ru/go?http%3A%2F%2Fspo337.com%2F
duhocphap.edu.vn/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.millerovo161.ru/go?http%3A%2F%2Fspo337.com%2F
www.naturaltranssexuals.com/cgi-bin/a2/out.cgi?id=97&l=toplist&u=http%3A%2F%2Fspo337.com%2F
https://amanaimages.com/lsgate/?lstid=pM6b0jdQgVM-Y9ibFgTe6Zv1N0oD2nYuMA&lsurl=http%3A%2F%2Fspo337.com%2F
planszowkiap.pl/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://covenantpeoplesministry.org/cpm/wp/sermons/?show&url=http%3A%2F%2Fspo337.com%2F
https://mightypeople.asia/link.php?id=M0ZGNHFISkd2bFh0RmlwSFU4bDN4QT09&destination=http%3A%2F%2Fspo337.com%2F
www.chinaleatheroid.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
i.s0580.cn/module/adsview/content/?action=click&bid=5&aid=163&url=http%3A%2F%2Fspo337.com%2F&variable=&source=https%3A%2F%2Fcutepix.info%2Fsex%2Friley-reyes.php
jsv3.recruitics.com/redirect?rx_cid=506&rx_jobId=39569207&rx_url=http%3A%2F%2Fspo337.com%2F
www.supermoto8.com/sidebanner/62?href=http%3A%2F%2Fspo337.com%2F
http://www.youtube.de/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.es/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ca/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.nl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ch/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.be/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.se/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.dk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.pt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.no/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.gr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cl/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.at/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.bg/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.sk/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.rs/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lt/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.si/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.hr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ee/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.lu/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.tn/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.ke/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.co.cr/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.kz/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.cat/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.ge/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?event=channel_description&q=http%3A%2F%2Fspo337.com%2F&gl=ml
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=DE
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=IT
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AR
https://www.youtube.at/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ch/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.fr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.es/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.jp/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.co.uk/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ru/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.pl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.gr/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.nl/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.ca/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.cz/redirect?q=http%3A%2F%2Fspo337.com%2F
https://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F&gl=AU
https://www.youtube.com.tw/redirect?q=http%3A%2F%2Fspo337.com%2F
www.mexicolore.co.uk/click.php?url=http%3A%2F%2Fspo337.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=http%3A%2F%2Fspo337.com%2F
www.invisalign-doctor.in/api/redirect?url=http%3A%2F%2Fspo337.com%2F
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=http%3A%2F%2Fspo337.com%2F
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=http%3A%2F%2Fspo337.com%2F
trackingapp4.embluejet.com/Mod_Campaigns/tracking.asp?idem=31069343&em=larauz@untref.edu.ar&ca=73143&ci=0&me=72706&of=581028&adirecta=0&url=http%3A%2F%2Fspo337.com%2F
smils.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=http%3A%2F%2Fspo337.com/
cipresso.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.vacacionartravel.com/DTCSpot/public/banner_redirect.aspx?idca=286&ids=75665176&cp=167&idcli=0&ci=2&p=http%3A%2F%2Fspo337.com%2F
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=http%3A%2F%2Fspo337.com%2F
www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=http%3A%2F%2Fspo337.com%2F
www.cccowe.org/lang.php?lang=en&url=http%3A%2F%2Fspo337.com%2F
www.joeshouse.org/booking?link=http%3A%2F%2Fspo337.com%2F&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://okane-antena.com/redirect/index/fid100269/?u=http%3A%2F%2Fspo337.com%2F
www.gmwebsite.com/web/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
swra.backagent.net/ext/rdr/?http%3A%2F%2Fspo337.com%2F
www.mytokachi.jp/index.php?type=click&mode=sbm&code=2981&url=http%3A%2F%2Fspo337.com%2F
www.wqketang.com/logout?goto=http%3A%2F%2Fspo337.com%2F
access.bridges.com/externalRedirector.do?url=http%3A%2F%2Fspo337.com%2F
www.dolgin.net/zen_dolgin/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
bot.buymeapie.com/recipe?url=http%3A%2F%2Fspo337.com%2F
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=http%3A%2F%2Fspo337.com%2F
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
http://blackwhitepleasure.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
www.knet-web.net/m/pRedirect.php?uID=2&iID=259&iURL=http%3A%2F%2Fspo337.com%2F
azlan.techdata.com/InTouch/GUIBnrT3/BnrTrackerPublic.aspx?CountryCode=18&BannerLangCulture=nl-nl&URL=http%3A%2F%2Fspo337.com%2F&Target=2&BannerId=41919&Zoneid=281&Parameters=&cos=Azlan
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44__zoneid=1__cb=7743e8d201__oadest=http%3A%2F%2Fspo337.com%2F
www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=http%3A%2F%2Fspo337.com%2F
www.lzmfjj.com/Go.asp?URL=http%3A%2F%2Fspo337.com%2F
http://alexmovs.com/cgi-bin/atx/out.cgi?id=148&tag=topatx&trade=http%3A%2F%2Fspo337.com%2F
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2__bannerid=40__zoneid=16__cb=fc1d72225c__oadest=http%3A%2F%2Fspo337.com%2F
psylive.ru/success.aspx?id=0&goto=spo337.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=http%3A%2F%2Fspo337.com%2F
www.mybunnies.net/te3/out.php?u=http%3A%2F%2Fspo337.com%2F
lubaczowskie.pl/rdir/?l=http%3A%2F%2Fspo337.com%2F&lid=1315
www.kowaisite.com/bin/out.cgi?id=kyouhuna&url=http%3A%2F%2Fspo337.com%2F
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=http%3A%2F%2Fspo337.com%2F
www.wave24.net/cgi-bin/linkrank/out.cgi?id=106248&cg=1&url=spo337.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
zh-hk.guitarians.com/home/redirect/ubid/1015?r=http%3A%2F%2Fspo337.com%2F
www.cumshoter.com/cgi-bin/at3/out.cgi?id=98&tag=top&trade=http%3A%2F%2Fspo337.com%2F
shp.hu/hpc_uj/click.php?ml=5&url=http%3A%2F%2Fspo337.com%2F
lrnews.ru/xgo.php?url=http%3A%2F%2Fspo337.com%2F
www.dive-international.net/places/redirect.php?b=797&web=www.http%3A%2F%2Fspo337.com%2F
www.themza.com/redirect.php?r=http%3A%2F%2Fspo337.com%2F
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://http%3A%2F%2Fspo337.com%2F
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=http%3A%2F%2Fspo337.com%2F
spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
forest.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
reefcentral.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=http%3A%2F%2Fspo337.com%2F
https://trackdaytoday.com/redirect-out?url=http%3A%2F%2Fspo337.com%2F
https://bigjobslittlejobs.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=4__zoneid=4__cb=68dbdae1d1__oadest=http%3A%2F%2Fspo337.com%2F
www.obertaeva.com/include/get.php?go=http%3A%2F%2Fspo337.com%2F
www.quanmama.com/t/goto.aspx?url=http%3A%2F%2Fspo337.com%2F
quartiernetz-friesenberg.ch/links-go.php?to=http%3A%2F%2Fspo337.com%2F
http://anorexicpornmovies.com/cgi-bin/atc/out.cgi?id=20&u=http%3A%2F%2Fspo337.com%2F
www.maultalk.com/url.php?to=http%3A%2F%2Fspo337.com%2F
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=http%3A%2F%2Fspo337.com%2F
www.e-expo.net/category/click_url.html?url=http%3A%2F%2Fspo337.com%2F
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=http%3A%2F%2Fspo337.com%2F
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=http%3A%2F%2Fspo337.com%2F
http://getdatasheet.com/url.php?url=http%3A%2F%2Fspo337.com%2F
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=http%3A%2F%2Fspo337.com%2F
www.aldolarcher.com/tools/esstat/esdown.asp?File=http%3A%2F%2Fspo337.com%2F
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&no_chat=1&domain=http%3A%2F%2Fspo337.com%2F
cs.payeasy.com.tw/click?url=http%3A%2F%2Fspo337.com%2F
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=http%3A%2F%2Fspo337.com%2F
http://tswzjs.com/go.asp?url=http%3A%2F%2Fspo337.com%2F
asstomouth.guru/out.php?url=http%3A%2F%2Fspo337.com%2F
advtest.exibart.com/adv/adv.php?id_banner=7201&link=http%3A%2F%2Fspo337.com%2F
https://thairesidents.com/l.php?b=85&p=2,5&l=http%3A%2F%2Fspo337.com%2F
www.latestnigeriannews.com/link_channel.php?channel=http%3A%2F%2Fspo337.com%2F
www.haogaoyao.com/proad/default.aspx?url=http%3A%2F%2Fspo337.com%2F
globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
citysafari.nl/Home/setCulture?language=en&returnUrl=http%3A%2F%2Fspo337.com%2F
es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
lorena-kuhni.kz/redirect?link=http%3A%2F%2Fspo337.com%2F
www.webshoptrustmark.fr/Change/en?returnUrl=http%3A%2F%2Fspo337.com%2F
https://processon.com/setting/locale?language=zh&back=http%3A%2F%2Fspo337.com%2F
c.yam.com/srh/wsh/r.c?http%3A%2F%2Fspo337.com%2F
www.jolletorget.no/J/l.php?l=http%3A%2F%2Fspo337.com%2F
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=http%3A%2F%2Fspo337.com%2F
www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
www.sparetimeteaching.dk/forward.php?link=http%3A%2F%2Fspo337.com%2F
https://paspn.net/default.asp?p=90&gmaction=40&linkid=52&linkurl=http%3A%2F%2Fspo337.com%2F
www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
www.poddebiczak.pl/?action=set-desktop&url=http%3A%2F%2Fspo337.com%2F
ant53.ru/file/link.php?url=http%3A%2F%2Fspo337.com%2F
www.docin.com/jsp_cn/mobile/tip/android_v1.jsp?forward=http%3A%2F%2Fspo337.com%2F
old.magictower.ru/cgi-bin/redir/redir.pl?http%3A%2F%2Fspo337.com%2F
go.flx1.com/click?id=1&m=11&pl=113&dmcm=16782&euid=16603484876&out=http%3A%2F%2Fspo337.com%2F
moba-hgh.de/link2http.php?href=http%3A%2F%2Fspo337.com%2F
https://gazetablic.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=34__zoneid=26__cb=0e0dfef92b__oadest=http%3A%2F%2Fspo337.com%2F
watchvideo.co/go.php?url=http%3A%2F%2Fspo337.com%2F
www.todoku.info/gpt/rank.cgi?mode=link&id=29649&url=http%3A%2F%2Fspo337.com%2F
www.fotochki.com/redirect.php?go=http%3A%2F%2Fspo337.com%2F
bayerwald.tips/plugins/bannerverwaltung/bannerredirect.php?bannerid=1&url=http%3A%2F%2Fspo337.com%2F
ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=http%3A%2F%2Fspo337.com%2F
www.widgetinfo.net/read.php?sym=FRA_LM&url=http%3A%2F%2Fspo337.com%2F
arctic.nyheter24.se/rdb/nyheter24_eed6ad4b451f2fb8193922f832bc91ed/5?url=http%3A%2F%2Fspo337.com%2F
www.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
school.mosreg.ru/soc/moderation/abuse.aspx?link=http%3A%2F%2Fspo337.com%2F
affiliates.kanojotoys.com/affiliate/scripts/click.php?a_aid=widdi77&desturl=http%3A%2F%2Fspo337.com%2F
www.gotomypctech.com/affiliates/scripts/click.php?a_aid=ed983915&a_bid=&desturl=http%3A%2F%2Fspo337.com%2F
www.my-sms.ru/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F&rel=external
www.genderpsychology.com/http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
www.360wichita.com/amp-banner-tracking?adid=192059&url=http%3A%2F%2Fspo337.com%2F
www.tsv-bad-blankenburg.de/cms/page/mod/url/url.php?eid=16&urlpf=http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://zerlong.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
catalog.flexcom.ru/go?z=36047&i=55&u=http%3A%2F%2Fspo337.com%2F
www.wellvit.nl/response/forward/c1e41491e30c5af3c20f80a2af44e440.php?link=0&target=http%3A%2F%2Fspo337.com%2F
www.ident.de/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=76__zoneid=2__cb=8a18c95a9e__oadest=http%3A%2F%2Fspo337.com%2F
www.fertilab.net/background_manager.aspx?ajxName=link_banner&id_banner=50&url=http%3A%2F%2Fspo337.com%2F
shop-uk.fmworld.com/Queue/Index?url=http%3A%2F%2Fspo337.com%2F
www.weddinginlove.com/redirect/?url=http%3A%2F%2Fspo337.com%2F
nieuws.rvent.nl/bitmailer/statistics/mailstatclick/42261?link=http%3A%2F%2Fspo337.com%2F
https://jobanticipation.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobanticipation.com
www.xsbaseball.com/tracker/index.html?t=ad&pool_id=3&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
eos.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
b.sm.su/click.php?bannerid=56&zoneid=10&source=&dest=http%3A%2F%2Fspo337.com%2F
https://bethlehem-alive.com/abnrs/countguideclicks.cfm?targeturl=http%3A%2F%2Fspo337.com%2F&businessid=29579
www.bookmark-favoriten.com/?goto=http%3A%2F%2Fspo337.com%2F
shop.yuliyababich.eu/RU/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
www.depmode.com/go.php?http%3A%2F%2Fspo337.com%2F
https://urgankardesler.com/anasayfa/yonlen?link=http%3A%2F%2Fspo337.com%2F
www.metalindex.ru/netcat/modules/redir/?&site=http%3A%2F%2Fspo337.com%2F
www.rprofi.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://darudar.org/external/?link=http%3A%2F%2Fspo337.com%2F
www.postsabuy.com/autopost4/page/generate/?link=http%3A%2F%2Fspo337.com%2F&list=PL9d7lAncfCDSkF4UPyhzO59Uh8cOoD-8q&fb_node=942812362464093&picture&name=%E0%B9%82%E0%B8%9B%E0%B8%A3%E0%B9%81%E0%B8%81%E0%B8%A3%E0%B8%A1%E0%B9%82%E0%B8%9E%E0%B8%AA%E0%B8%82%E0%B8%B2%E0%B8%A2%E0%B8%AA%E0%B8%B4%E0%B8%99%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%AD%E0%B8%AD%E0%B8%99%E0%B9%84%E0%B8%A5%E0%B8%99%E0%B9%8C+&caption=%E0%B9%80%E0%B8%A5%E0%B8%82%E0%B8%B2%E0%B8%AA%E0%B9%88%E0%B8%A7%E0%B8%99%E0%B8%95%E0%B8%B1%E0%B8%A7+%E0%B8%97%E0%B8%B5%E0%B8%84%E0%B8%B8%E0%B8%93%E0%B8%A5%E0%B8%B7%E0%B8%A1%E0%B9%84%E0%B8%A1%E0%B9%88%E0%B8%A5%E0%B8%87+Line+%40postsabuy&description=%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%B2%E0%B8%96%E0%B8%B9%E0%B8%81%E0%B8%97%E0%B8%B5%E0%B9%88%E0%B8%AA%E0%B8%B8%E0%B8%94%E0%B9%83%E0%B8%99+3+%E0%B9%82%E0%B8%A5%E0%B8%81+%E0%B8%AD%E0%B8%B4%E0%B8%AD%E0%B8%B4
www.perimeter.org/track.pdf?url=http%3A%2F%2Fspo337.com%2F
forum.darievna.ru/go.php?http%3A%2F%2Fspo337.com%2F
techlab.rarus.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.spiritualforums.com/vb/redir.php?link=http%3A%2F%2Fspo337.com%2F
www.review-mag.com/cdn/www/delivery/view.php?ct=1&oaparams=2__bannerid=268__zoneid=1__cb=8c1317f219__oadest=http%3A%2F%2Fspo337.com%2F
belantara.or.id/lang/s/ID?url=http%3A%2F%2Fspo337.com%2F
ele-market.ru/consumer.php?url=http%3A%2F%2Fspo337.com%2F
https://www.хорошие-сайты.рф/r.php?r=http%3A%2F%2Fspo337.com%2F
https://jobsflagger.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://gpoltava.com/away/?go=http%3A%2F%2Fspo337.com%2F
www.cardexchange.com/index.php/tools/packages/tony_mailing_list/services/?mode=link&mlm=62&mlu=0&u=2&url=http%3A%2F%2Fspo337.com%2F
services.nfpa.org/Authentication/GetSSOSession.aspx?return=http%3A%2F%2Fspo337.com%2F
http://spaceup.org/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
yarko-zhivi.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
365sekretov.ru/redirect.php?action=url&goto=http%3A%2F%2Fspo337.com%2F%20
www.sgdrivingtest.com/redirect.php?page=http%3A%2F%2Fspo337.com%2F
www.jxren.com/news/link/link.asp?id=7&url=http%3A%2F%2Fspo337.com%2F
particularcareers.co.uk/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
https://bsaonline.com/MunicipalDirectory/SelectUnit?unitId=411&returnUrl=http%3A%2F%2Fspo337.com%2F&sitetransition=true
www.elit-apartament.ru/go?http%3A%2F%2Fspo337.com%2F
sendai.japansf.net/rank.cgi?mode=link&id=1216&url=http%3A%2F%2Fspo337.com%2F
www.bmwfanatics.ru/goto.php?l=http%3A%2F%2Fspo337.com%2F
www.saabsportugal.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
cms.sive.it/Jump.aspx?gotourl=http%3A%2F%2Fspo337.com%2F
largusladaclub.ru/go/url=https:/http%3A%2F%2Fspo337.com%2F
https://kekeeimpex.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=GBP&cRate=77.86247
mycounter.com.ua/go.php?http%3A%2F%2Fspo337.com%2F
l2base.su/go?http%3A%2F%2Fspo337.com%2F
https://freeseotool.org/url/?q=http%3A%2F%2Fspo337.com%2F
www.duomodicagliari.it/reg_link.php?link_ext=http%3A%2F%2Fspo337.com%2F&prov=1
assine.hostnet.com.br/cadastro/?rep=17&url=http%3A%2F%2Fspo337.com%2F
www.dvnlp.de/profile/gruppe/redirect/5?url=http%3A%2F%2Fspo337.com%2F
http://hotmaturegirlfriends.com/cl.php?porno=MzB4MzB4NjEw&url=http%3A%2F%2Fspo337.com%2F
www.smkn5pontianak.sch.id/redirect/?alamat=http%3A%2F%2Fspo337.com%2F
www.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
bilometro.brksedu.com.br/tracking?url=http%3A%2F%2Fspo337.com%2F&zorigem=hotsite-blackfriday
www.omschweiz.ch/select-your-country?publicUrl=http%3A%2F%2Fspo337.com%2F
https://anacolle.net/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
www.cubamusic.com/Home/ChangeLanguage?lang=es-ES&returnUrl=http%3A%2F%2Fspo337.com%2F
expoclub.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.deypenburgschecourant.nl/reklame/www/delivery/ck.php?oaparams=2__bannerid=44__zoneid=11__cb=078c2a52ea__oadest=http%3A%2F%2Fspo337.com%2F
tramplintk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
cnc.extranet.gencat.cat/treball_cnc/AppJava/FileDownload.do?pdf=http%3A%2F%2Fspo337.com%2F&codi_cnv=9998045
www.gamecollections.co.uk/search/redirect.php?retailer=127&deeplink=http%3A%2F%2Fspo337.com%2F
bearcong.no1.sexy/hobby-delicious/rank.cgi?mode=link&id=19&url=http%3A%2F%2Fspo337.com%2F
dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
www.surinenglish.com/backend/conectar.php?url=http%3A%2F%2Fspo337.com%2F
www.reference-cannabis.com/interface/sortie.php?adresse=http%3A%2F%2Fspo337.com%2F
login.0×69416d.co.uk/sso/logout?tenantId=tnl&gotoUrl=http%3A%2F%2Fspo337.com%2F&domain=0×69416d.co.uk
blog.link-usa.jp/emi?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://damki.net/go/?http%3A%2F%2Fspo337.com%2F
opensesame.wellymulia.zaxaa.com/b/66851136?s=1&redir=http%3A%2F%2Fspo337.com%2F
ism3.infinityprosports.com/ismdata/2009100601/std-sitebuilder/sites/200901/www/en/tracker/index.html?t=ad&pool_id=1&ad_id=112&url=http%3A%2F%2Fspo337.com%2F
apps.cancaonova.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=149__zoneid=20__cb=87d2c6208d__oadest=http%3A%2F%2Fspo337.com%2F
www.lespritjardin.be/?advp_click_bimage_id=19&url=http%3A%2F%2Fspo337.com%2F&shortcode_id=10
www.dresscircle-net.com/psr/rank.cgi?mode=link&id=14&url=http%3A%2F%2Fspo337.com%2F
www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
fms.csonlineschool.com.au/changecurrency/1?returnurl=http%3A%2F%2Fspo337.com%2F
www.v-archive.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.jagat.co.jp/analysis/analysis.php?url=http%3A%2F%2Fspo337.com%2F
https://vse-doski.com/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.karatetournaments.net/link7.asp?LRURL=http%3A%2F%2Fspo337.com%2F&LRTYP=O
simracing.su/go/?http%3A%2F%2Fspo337.com%2F
click.cheshi.com/go.php?proid=218&clickid=1393306648&url=http%3A%2F%2Fspo337.com%2F
fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
flypoet.toptenticketing.com/index.php?url=http%3A%2F%2Fspo337.com%2F
www.horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
d-click.fiemg.com.br/u/18081/131/75411/137_0/82cb7/?url=http%3A%2F%2Fspo337.com%2F
www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
www.packmage.net/uc/goto/?url=http%3A%2F%2Fspo337.com%2F
my.9991.com/login_for_index_0327.php?action=logout&forward=http%3A%2F%2Fspo337.com%2F
www.sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=986&redirect=http%3A%2F%2Fspo337.com%2F
www.naturum.co.jp/ad/linkshare/?siteID=p_L785d6UQY-V4Fh4Rxs7wNzOPgtzv95Tg&lsurl=http%3A%2F%2Fspo337.com%2F
www.d-e-a.eu/newsletter/redirect.php?link=http%3A%2F%2Fspo337.com%2F
www.bom.ai/goweburl?go=http%3A%2F%2Fspo337.com%2F
enews2.sfera.net/newsletter/redirect.php?id=sabricattani@gmail.com_0000006566_144&link=http%3A%2F%2Fspo337.com%2F
underwater.com.au/redirect_url/id/7509/?redirect=http%3A%2F%2Fspo337.com%2F
https://eqsoftwares.com/languages/setlanguage?languagesign=en&redirect=http%3A%2F%2Fspo337.com%2F
www.jagdambasarees.com/Home/ChangeCurrency?urls=http%3A%2F%2Fspo337.com%2F&cCode=MYR&cRate=14.554
www.priegeltje.nl/gastenboek/go.php?url=http%3A%2F%2Fspo337.com%2F
www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.rz114.cn/url.html?url=http%3A%2F%2Fspo337.com%2F
www.greatdealsindia.com/redirects/infibeam.aspx?url=http%3A%2F%2Fspo337.com%2F
rs.345kei.net/rank.php?id=37&mode=link&url=http%3A%2F%2Fspo337.com%2F
webapp.jgz.la/?c=scene&a=link&id=8665466&url=http%3A%2F%2Fspo337.com%2F
www.erotiikkalelut.com/url.php?link=http%3A%2F%2Fspo337.com%2F
vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
https://fachowiec.com/zliczanie-bannera?id=24&url=http%3A%2F%2Fspo337.com%2F
ieea.ir/includes/change_lang.php?lang=en&goto=http%3A%2F%2Fspo337.com%2F
scribe.mmonline.io/click?evt_nm=Clicked+Registration+Completion&evt_typ=clickEmail&app_id=m4marry&eml_sub=Registration+Successful&usr_did=4348702&cpg_sc=NA&cpg_md=email&cpg_nm=&cpg_cnt=&cpg_tm=NA&link_txt=Live+Chat&em_type=Notification&url=http%3A%2F%2Fspo337.com%2F
polo-v1.feathr.co/v1/analytics/crumb?flvr=email_link_click&rdr=http%3A%2F%2Fspo337.com%2F
sintesi.provincia.mantova.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F
https://careerchivy.com/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F
shinsekai.type.org/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
https://maned.com/scripts/lm/lm.php?tk=CQkJZWNuZXdzQGluZm90b2RheS5jb20JW05ld3NdIE1FSSBBbm5vdW5jZXMgUGFydG5lcnNoaXAgV2l0aCBUd2l4bCBNZWRpYQkxNjcyCVBSIE1lZGlhIENvbnRhY3RzCTI1OQljbGljawl5ZXMJbm8=&url=http%3A%2F%2Fspo337.com%2F
www.etaigou.com/turn2.php?ad_id=276&link=http%3A%2F%2Fspo337.com%2F
crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
www.tagirov.org/out.php?url=http%3A%2F%2Fspo337.com%2F
https://startlist.club/MSF/Language/Set?languageIsoCode=en&returnUrl=http%3A%2F%2Fspo337.com%2F
www.tetsumania.net/search/rank.cgi?mode=link&id=947&url=http%3A%2F%2Fspo337.com%2F
https://imperial-info.net/link?idp=125&url=http%3A%2F%2Fspo337.com%2F
www.brainlanguage-sa.com/setcookie.php?lang=en&file=http%3A%2F%2Fspo337.com%2F
www.asensetranslations.com/modules/babel/redirect.php?newlang=en_US&newurl=http%3A%2F%2Fspo337.com%2F
gameshock.jeez.jp/rank.cgi?mode=link&id=307&url=http%3A%2F%2Fspo337.com%2F
https://tripyar.com/go.php?http%3A%2F%2Fspo337.com%2F
www.floridafilmofficeinc.com/?goto=http%3A%2F%2Fspo337.com%2F
tsvc1.teachiworld.com/bin/checker?mode=4&module=11&mailidx=19130&dmidx=0&emidx=0&service=0&cidx=&etime=20120328060000&seqidx=3&objidx=22&encoding=0&url=http%3A%2F%2Fspo337.com%2F
rechner.atikon.at/lbg.at/newsletter/linktracking?subscriber=&delivery=38116&url=http%3A%2F%2Fspo337.com%2F
www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
go.eniro.dk/lg/ni/cat-2611/http:/http%3A%2F%2Fspo337.com%2F
www.fisherly.com/redirect?type=website&ref=listing_detail&url=http%3A%2F%2Fspo337.com%2F
bio-pack.ru/bitrix/redirect.php?goto=http://http%3A%2F%2Fspo337.com%2F
fid.com.ua/redirect/?go=http%3A%2F%2Fspo337.com%2F
www.modernipanelak.cz/?b=618282165&redirect=http%3A%2F%2Fspo337.com%2F
h5.hbifeng.com/index.php?c=scene&a=link&id=14240604&url=http%3A%2F%2Fspo337.com%2F
www.bkdc.ru/bitrix/redirect.php?event1=news_out&event2=32reg.roszdravnadzor.ru/&event3=A0A0B5A09180D0%A09582A0BBA1A085%D0E2A084D0D1C2D0%A085+A0A0B5A182B0A0%C2D0D0D096+A1A0BBA0B180D0%A09795+A0A0B0A09582A1%D1D0D0D0A182B5+A0A091A08695A0%D1D0A6A185A0A085%D0D1D0D082A1A085%D0D0D1D0A095B1A0%C2D0D0D091&goto=http%3A%2F%2Fspo337.com%2F
record.affiliatelounge.com/WS-jvV39_rv4IdwksK4s0mNd7ZgqdRLk/7/?deeplink=http%3A%2F%2Fspo337.com%2F
d-click.artenaescola.org.br/u/3806/290/32826/1416_0/53052/?url=http%3A%2F%2Fspo337.com%2F
www.morroccoaffiliate.com/aff.php?id=883&url=http%3A%2F%2Fspo337.com%2F
www.flooble.com/cgi-bin/clicker.pl?id=grabbadl&url=http%3A%2F%2Fspo337.com%2F
www.sportsbook.ag/ctr/acctmgt/pl/openLink.ctr?ctrPage=http%3A%2F%2Fspo337.com%2F
www.ra2d.com/directory/redirect.asp?id=596&url=http%3A%2F%2Fspo337.com%2F
www.anorexicnudes.net/cgi-bin/atc/out.cgi?u=http%3A%2F%2Fspo337.com%2F
www.zjjiajiao.com.cn/ad/adredir.asp?url=http%3A%2F%2Fspo337.com%2F
https://hakobo.com/wp/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
t.agrantsem.com/tt.aspx?cus=216&eid=1&p=216-2-71016b553a1fa2c9.3b14d1d7ea8d5f86&d=http%3A%2F%2Fspo337.com%2F
moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
www.cheek.co.jp/location/location.php?id=keibaseminar&url=http%3A%2F%2Fspo337.com%2F
www.quantixtickets3.com/php-bin-8/kill_session_and_redirect.php?redirect=http%3A%2F%2Fspo337.com%2F
www.photokonkurs.com/cgi-bin/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.nbda.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.thislife.net/cgi-bin/webcams/out.cgi?id=playgirl&url=http%3A%2F%2Fspo337.com%2F
https://www.dans-web.nu/klick.php?url=http%3A%2F%2Fspo337.com%2F
https://voobrajulya.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.nafta-him.com/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
www.kyoto-osaka.com/search/rank.cgi?mode=link&id=9143&url=http%3A%2F%2Fspo337.com%2F
www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
red.ribbon.to/~zkcsearch/zkc-search/rank.cgi?mode=link&id=156&url=http%3A%2F%2Fspo337.com%2F
akademik.tkyd.org/Home/SetCulture?culture=en-US&returnUrl=http%3A%2F%2Fspo337.com%2F
www.rencai8.com/web/jump_to_ad_url.php?id=642&url=http%3A%2F%2Fspo337.com%2F
https://www.baby22.com.tw/Web/turn.php?ad_id=160&link=http%3A%2F%2Fspo337.com%2F
https://www.kichink.com/home/issafari?uri=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=1032500&url=http%3A%2F%2Fspo337.com%2F
5cfxm.hxrs6.servertrust.com/v/affiliate/setCookie.asp?catId=1180&return=http%3A%2F%2Fspo337.com%2F
http://3dcreature.com/cgi-bin/at3/out.cgi?id=187&trade=http%3A%2F%2Fspo337.com%2F
https://nowlifestyle.com/redir.php?k=9a4e080456dabe5eebc8863cde7b1b48&url=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
www.acutenet.co.jp/cgi-bin/lcount/lcounter.cgi?link=http%3A%2F%2Fspo337.com%2F
http://news-matome.com/method.php?method=1&url=http%3A%2F%2Fspo337.com%2F
https://lifecollection.top/site/gourl?url=http%3A%2F%2Fspo337.com%2F
https://www.toscanapiu.com/web/lang.php?lang=DEU&oldlang=ENG&url=http%3A%2F%2Fspo337.com%2F
speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.sainttropeztourisme.com/en/bannieres/redirection/index.html?id=649&lien=http%3A%2F%2Fspo337.com%2F
www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
www.interracialhall.com/cgi-bin/atx/out.cgi?trade=http%3A%2F%2Fspo337.com%2F
https://www.tumimusic.com/link.php?url=http%3A%2F%2Fspo337.com%2F
www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
mail.resen.gov.mk/redir.hsp?url=http%3A%2F%2Fspo337.com%2F
https://www.pompengids.net/followlink.php?id=495&link=http%3A%2F%2Fspo337.com%2F
https://www.hirforras.net/scripts/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://lens-club.ru/link?go=http%3A%2F%2Fspo337.com%2F
https://t.raptorsmartadvisor.com/.lty?url=http%3A%2F%2Fspo337.com%2F&loyalty_id=14481&member_id=b01bbee6-4592-4345-a0ee-5d71ed6f1929
https://evoautoshop.com/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://beautycottageshop.com/change.php?lang=cn&url=http%3A%2F%2Fspo337.com%2F
http://1000love.net/lovelove/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.ffw-ellar.de/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://www.contactlenshouse.com/currency.asp?c=CAD&r=http%3A%2F%2Fspo337.com%2F
https://sogo.i2i.jp/link_go.php?url=http%3A%2F%2Fspo337.com%2F
https://ceb.bg/catalog/statistic/?id=61&location=http%3A%2F%2Fspo337.com%2F
https://malehealth.ie/redirect/?age=40&part=waist&illness=obesity&refer=http%3A%2F%2Fspo337.com%2F
https://magicode.me/affiliate/go?url=http%3A%2F%2Fspo337.com%2F
https://jobregistry.net/jobclick/?RedirectURL=http%3A%2F%2Fspo337.com%2F&Domain=jobregistry.net&rgp_m=title13&et=4495
https://www.goatzz.com/adredirect.aspx?adType=SiteAd&ItemID=9595&ReturnURL=http%3A%2F%2Fspo337.com%2F
https://miyagi.lawyer-search.tv/details/linkchk.aspx?type=o&url=http%3A%2F%2Fspo337.com%2F
https://www.snwebcastcenter.com/event/page/count_download_time.php?url=http%3A%2F%2Fspo337.com%2F
https://go.onelink.me/v1xd?pid=Patch&c=Mobile%20Footer&af_web_dp=http%3A%2F%2Fspo337.com%2F
https://www.weather.net/cgi-bin/redir?http%3A%2F%2Fspo337.com%2F
https://gaggedtop.com/cgi-bin/top/out.cgi?ses=sBZFnVYGjF&id=206&url=http%3A%2F%2Fspo337.com%2F
https://e-bike-test.net/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://www.luckyplants.com/cgi-bin/toplist/out.cgi?id=rmontero&url=http%3A%2F%2Fspo337.com%2F
https://webankety.cz/dalsi.aspx?site=http%3A%2F%2Fspo337.com%2F
https://runkeeper.com/apps/authorize?redirect_uri=http%3A%2F%2Fspo337.com%2F
https://www.emiratesvoice.com/footer/comment_like_dislike_ajax/?code=like&commentid=127&redirect=http%3A%2F%2Fspo337.com%2F
https://envios.uces.edu.ar/control/click.mod.php?id_envio=8147&email=gramariani@gmail.com&url=http%3A%2F%2Fspo337.com%2F
https://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://www.ab-search.com/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.studyrama.be/tracking.php?origine=ficheform5683&lien=http://http%3A%2F%2Fspo337.com%2F
https://shop.merchtable.com/users/authorize?return_url=http%3A%2F%2Fspo337.com%2F
https://nudewwedivas.forumcommunity.net/m/ext.php?url=http%3A%2F%2Fspo337.com%2F
https://cztt.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://mientaynet.com/advclick.php?o=textlink&u=15&l=http%3A%2F%2Fspo337.com%2F
https://atlanticleague.com/tracker/index.html?t=ad&pool_id=11&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
https://www.podcastone.com/site/rd?satype=40&said=4&aaid=email&camid=-4999600036534929178&url=http%3A%2F%2Fspo337.com%2F
https://www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=http%3A%2F%2Fspo337.com%2F
https://monarchbeachmembers.play18.com/ViewSwitcher/SwitchView?mobile=False&returnUrl=http%3A%2F%2Fspo337.com%2F
https://wayi.com.tw/wayi_center.aspx?flag=banner&url=http%3A%2F%2Fspo337.com%2F&idno=443
https://d.agkn.com/pixel/2389/?che=2979434297&col=22204979,1565515,238211572,435508400,111277757&l1=http%3A%2F%2Fspo337.com%2F
https://mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=http%3A%2F%2Fspo337.com%2F
https://bemidji.bigdealsmedia.net/include/sort.php?return_url=http%3A%2F%2Fspo337.com%2F&sort=a:3:{s:9:%E2%80%9Ddirection%E2%80%9D;s:3:%E2%80%9DASC%E2%80%9D;s:5:%E2%80%9Dfield%E2%80%9D;s:15:%E2%80%9DItems.PriceList%E2%80%9D;s:5:%E2%80%9Dlabel%E2%80%9D;s:9:%E2%80%9Dvalue_asc%E2%80%9D;}
https://hslda.org/content/a/LinkTracker.aspx?id=4015475&appeal=385&package=36&uri=http%3A%2F%2Fspo337.com%2F
https://panarmenian.net/eng/tofv?tourl=http%3A%2F%2Fspo337.com%2F
https://lavoro.provincia.como.it/portale/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=935
https://www.dodeley.com/?action=show_ad&url=http%3A%2F%2Fspo337.com%2F
https://www.ignicaodigital.com.br/affiliate/?idev_id=270&u=http%3A%2F%2Fspo337.com%2F
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=http%3A%2F%2Fspo337.com%2F
https://m.wedkuje.pl/mobile/redirect.php?redir=http%3A%2F%2Fspo337.com%2F
https://area51.to/go/out.php?s=100&l=site&u=http%3A%2F%2Fspo337.com%2F
https://games4ever.3dn.ru/go?http%3A%2F%2Fspo337.com%2F
https://de.reasonable.shop/SetCurrency.aspx?currency=CNY&returnurl=http%3A%2F%2Fspo337.com%2F
https://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
https://mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.shop-bell.com/out.php?id=kibocase&category=ladies&url=http%3A%2F%2Fspo337.com%2F
https://m.addthis.com/live/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.pcreducator.com/Common/SSO.aspx?returnUrl=http%3A%2F%2Fspo337.com%2F
https://horsesmouth.com/LinkTrack.aspx?u=http%3A%2F%2Fspo337.com%2F
https://soom.cz/projects/get2mail/redir.php?id=c2e52da9ad&url=http%3A%2F%2Fspo337.com%2F
https://www.iaai.com/VehicleInspection/InspectionProvidersUrl?name=AA%20Transit%20Pros%20Inspection%20Service&url=http%3A%2F%2Fspo337.com%2F
https://cingjing.fun-taiwan.com/AdRedirector.aspx?padid=303&target=http%3A%2F%2Fspo337.com%2F
https://rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=http%3A%2F%2Fspo337.com%2F
https://campaign.unitwise.com/click?emid=31452&emsid=ee720b9f-a315-47ce-9552-fd5ee4c1c5fa&url=http%3A%2F%2Fspo337.com%2F
https://www.swipeclock.com/sc/cookie.asp?sitealias=79419397&redirect=http%3A%2F%2Fspo337.com%2F
https://www.gutscheinaffe.de/wp-content/plugins/AND-AntiBounce/redirector.php?url=http%3A%2F%2Fspo337.com%2F
https://home.uceusa.com/Redirect.aspx?r=http%3A%2F%2Fspo337.com%2F
https://www.seankenney.com/include/jump.php?num=http%3A%2F%2Fspo337.com%2F
https://runningcheese.com/go?url=http%3A%2F%2Fspo337.com%2F
http://chtbl.com/track/118167/http%3A%2F%2Fspo337.com%2F
https://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
https://crewroom.alpa.org/SAFETY/LinkClick.aspx?link=http%3A%2F%2Fspo337.com%2F&mid=12872
http://dstats.net/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://union.591.com.tw/stats/event/redirect?e=eyJpdiI6IjdUd1B5Z2FPTmNWQzBmZk1LblR2R0E9PSIsInZhbHVlIjoiQTI4TnVKMzdjMkxrUjcrSWlkcXdzbjRQeGRtZ0ZGbXdNSWxkSkVieENwNjQ1cHF5aDZmWmFobU92ZGVyUk5jRTlxVnI2TG5pb0dJVHZSUUlHcXFTbGo3UDliYWU5UE5MSjlMY0xOQnFmbVRQSFNoZDRGd2dqVDZXZEU4WFoyajJ0S0JITlQ2XC9SXC9jRklPekdmcnFGb09vRllqNHVtTHlYT284ZmN3d0ozOHFkclRYYnU5UlY2NTFXSGRheW5SbGxJb3BmYjQ2Mm9TWUFCTEJuXC9iT25nYkg4QXpOd2pHVlBWTWxWXC91aWRQMVhKQmVJXC9qMW9IdlZaVVlBdWlCYW4rS0JualhSMElFeVZYN3NnUW1qcUdxcWUrSlFROFhKbWttdkdvMUJ3aWVRa2I3MVV5TXpER3doa2ZuekFWNWd3OGpuQ1VSczFDREVKaklaUks0TTRIY2pUeXYrQmdZYUFTK1F4RWpTY0RRaW5Nc0krdVJ2N2VUT1wvSUxVVWVKN3hnQU92QmlCbjQyMUpRdTZKVWJcL0RCSVFOcWl0azl4V2pBazBHWmVhdWptZGREVXh0VkRNWWxkQmFSYXhBRmZtMHA5dTlxMzIzQzBVaWRKMEFqSG0wbGkxME01RDBaaElTaU5QKzIxbSswaUltS0FYSzViZlFmZjZ1XC9Yclg0U2VKdHFCc0pTNndcL09FWklUdjlQM2dcL2RuN0szQ3plWmcyYWdpQzJDQ2NIcWROczVua3dIM1Q3OXpJY3Z0XC93MVk3SHUyODZHU3Z5aHFVbWEwRFU1ZFdyMGt0YWpsb3BkQitsZER5aWk4YWMrZWYzSFNHNERhOGdDeUJWeEtoSm9wQ0hQb2EzOHZ3eHFGVTQ2Mk1QSEZERzlXZWxRRTJldjJkdnZUM0ZwaW1KcEVVc3ZXWjRHaTZWRDJOK0YxR3d4bXhMR3BhWmZBNkJ6eUYxQjR4ODVxc0d0YkFpYU8yZ2tuWGdzelBpU3dFUjJVYUVtYUlpZllSUTVpaHZMbjhySFp4VEpQR3EyYnRLTmdcLzRvKzQwRmtGNUdWWnQ0VjFpcTNPc0JubEdWenFiajRLRFg5a2dRZFJOZ1wvaUEwVHR3ZnYzakpYVmVtT294aFk1TXBUZ3FmVnF2dnNSVWJ5VEE0WGZpV3o3Y0k2SjJhM2RDK2hoQ0FvV2YrSW9QWnhuZG5QN1hlOEFaTVZrcFZ3c0pXVHhtNkRTUkpmenpibG8zdTM0cGF6Q3oxTEJsdDdiOUgwWXFOUkNHWjlEbTBFYzdIRUcyalYrcW4wYklFbnlGYlZJUG00R1VDQTZLZEVJRklIbFVNZFdpS3RkeCt5bVpTNUkrOXE3dDlxWmZ2bitjSGlSeE9wZTg5Yk9wS0V6N1wvd1EzUTNVenNtbjlvQUJhdGsxMzNkZTdjTU1LNkd4THJMYTBGUEJ4elEycFNTNGZabEJnalhJc0pYZit1c1wvWDBzSm1JMzRad3F3PT0iLCJtYWMiOiI4MjNhNDJlYTMwOTlmY2VlYzgxNmU1N2JiM2QzODk5YjI5MDFhYThhMDBkYzNhODljOTRmMTMzMzk0YTM5OGIzIn0=&source=BANNER.2913&url=http%3A%2F%2Fspo337.com%2F
https://fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=http%3A%2F%2Fspo337.com%2F
https://pdcn.co/e/http%3A%2F%2Fspo337.com%2F
https://api.webconnex.com/v1/postmaster/track/click/4f8036d14ee545798599c8921fbfcd22/db005310dba511e89fb606f49a4ee876?url=http%3A%2F%2Fspo337.com%2F
https://www.lecake.com/stat/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://www.im-harz.com/counter/counter.php?url=http%3A%2F%2Fspo337.com%2F
https://pzz.to/click?uid=8571&target_url=http%3A%2F%2Fspo337.com%2F
https://www.ricacorp.com/Ricapih09/redirect.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.tremblant.ca/Shared/LanguageSwitcher/ChangeCulture?culture=en&url=http%3A%2F%2Fspo337.com%2F
https://www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=http%3A%2F%2Fspo337.com%2F
https://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://igert2011.videohall.com/to_client?target=http%3A%2F%2Fspo337.com%2F
https://sete.gr/app_plugins/newsletterstudio/pages/tracking/trackclick.aspx?nid=218221206039243162226109144018149003034132019130&e=000220142174231130224127060133189018075115154134&url=http%3A%2F%2Fspo337.com%2F
https://ovatu.com/e/c?url=http%3A%2F%2Fspo337.com%2F
https://primepartners.globalprime.com/afs/wcome.php?c=427|0|1&e=GP204519&url=http%3A%2F%2Fspo337.com%2F
https://ltp.org/home/setlocale?locale=en&returnUrl=http%3A%2F%2Fspo337.com%2F
https://www.morhipo.com/shared/partnercookie?k=gort&url=http%3A%2F%2Fspo337.com%2F
https://www.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bg/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://maps.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://za.zalo.me/v3/verifyv2/pc?token=OcNsmjfpL0XY2F3BtHzNRs4A-hhQ5q5sPXtbk3O&continue=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://scanmail.trustwave.com/?c=8510&d=4qa02KqxZJadHuhFUvy7ZCUfI_2L10yeH0EeBz7FGQ&u=http%3A%2F%2Fspo337.com%2F
https://images.google.co.kr/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://animal.doctorsfile.jp/redirect/?ct=doctor&id=178583&url=http%3A%2F%2Fspo337.com%2F
https://hr.pecom.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://cse.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://jump2.bdimg.com/mo/q/checkurl?url=http%3A%2F%2Fspo337.com%2F
https://severeweather.wmo.int/cgi-bin/goto?where=http%3A%2F%2Fspo337.com%2F
https://beam.jpn.org/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://blogranking.fc2.com/out.php?id=414788&url=http%3A%2F%2Fspo337.com%2F
https://wtk.db.com/777554543598768/optout?redirect=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=http%3A%2F%2Fspo337.com%2F
https://images.google.lv/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://m.caijing.com.cn/member/logout?referer=http%3A%2F%2Fspo337.com%2F
https://jamesattorney.agilecrm.com/click?u=http%3A%2F%2Fspo337.com%2F
https://wikimapia.org/external_link?url=http%3A%2F%2Fspo337.com%2F
https://rsv.nta.co.jp/affiliate/set/af100101.aspx?site_id=66108024&redi_url=http%3A%2F%2Fspo337.com%2F
https://adengine.old.rt.ru/go.jsp?to=http%3A%2F%2Fspo337.com%2F
https://thediplomat.com/ads/books/ad.php?i=4&r=http%3A%2F%2Fspo337.com%2F
https://mitsui-shopping-park.com/lalaport/iwata/redirect.html?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.de/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.es/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.it/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.br/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ru/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.au/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.be/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.at/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.se/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ch/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.my/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.co/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ie/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ph/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.no/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pe/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ae/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ee/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.eg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.si/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ec/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.qa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.li/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.uy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ba/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.is/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.az/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ng/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.np/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.by/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.as/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.do/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.jo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.lk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ai/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ni/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.la/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.jm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ps/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bo/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.af/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.fj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.kw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.lb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ms/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ci/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sb/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.dj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.py/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.am/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ad/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.gh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.bn/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.sh/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ly/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.na/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.me/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.je/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ge/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ht/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.im/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.sl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bj/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ml/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cv/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.tl/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.mm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ac/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.st/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.td/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.pg/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.gp/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.nf/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.ne/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.pn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://images.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mn/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://www.google.mn/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.com.om/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.dz/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.kg/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.ga/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.nu/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?sa=i&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.bt/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://asia.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://www.macro.ua/out.php?link=http%3A%2F%2Fspo337.com%2F
http://www.aurki.com/jarioa/redirect?id_feed=510&url=http%3A%2F%2Fspo337.com%2F
http://www.cnainterpreta.it/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.site-navi.net/sponavi/rank.cgi?mode=link&id=890&url=http%3A%2F%2Fspo337.com%2F
https://www.mattias.nu/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
http://teenstgp.us/cgi-bin/out.cgi?u=http%3A%2F%2Fspo337.com%2F
http://congovibes.com/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.humaniplex.com/jscs.html?hj=y&ru=http%3A%2F%2Fspo337.com%2F
http://www.qlt-online.de/cgi-bin/click/clicknlog.pl?link=http%3A%2F%2Fspo337.com%2F
http://ww.sdam-snimu.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.uktrademarkregistration.co.uk/JumpTo.aspx?url=http%3A%2F%2Fspo337.com%2F
http://fosteringsuccessmichigan.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fotos24.org/url?q=http%3A%2F%2Fspo337.com%2F
http://fouillez-tout.com/cgi-bin/redirurl.cgi?http%3A%2F%2Fspo337.com%2F
http://frag-den-doc.de/index.php?s=verlassen&url=http%3A%2F%2Fspo337.com%2F
http://freethailand.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://freshcannedfrozen.ca/?URL=http%3A%2F%2Fspo337.com%2F
http://funkhouse.de/url?q=http%3A%2F%2Fspo337.com%2F
http://ga.naaar.nl/link/?url=http%3A%2F%2Fspo337.com%2F
http://gb.poetzelsberger.org/show.php?c453c4=http%3A%2F%2Fspo337.com%2F
http://getmethecd.com/?URL=http%3A%2F%2Fspo337.com%2F
http://goldankauf-oberberg.de/out.php?link=http%3A%2F%2Fspo337.com%2F
http://distributors.hrsprings.com/?URL=http%3A%2F%2Fspo337.com%2F
http://dorf-v8.de/url?q=http%3A%2F%2Fspo337.com%2F
http://doverwx.com/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://dr-guitar.de/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://drdrum.biz/quit.php?url=http%3A%2F%2Fspo337.com%2F
http://ds-media.info/url?q=http%3A%2F%2Fspo337.com%2F
http://excitingperformances.com/?URL=http%3A%2F%2Fspo337.com%2F
http://familie-huettler.de/link.php?link=http%3A%2F%2Fspo337.com%2F
http://forum.vizslancs.hu/lnks.php?uid=net&url=http%3A%2F%2Fspo337.com%2F
http://forum.wonaruto.com/redirection.php?redirection=http%3A%2F%2Fspo337.com%2F
https://www.fairsandfestivals.net/?URL=http%3A%2F%2Fspo337.com%2F
http://chatx2.whocares.jp/redir.jsp?u=http%3A%2F%2Fspo337.com%2F
http://chuanroi.com/Ajax/dl.aspx?u=http%3A%2F%2Fspo337.com%2F
http://club.dcrjs.com/link.php?url=http%3A%2F%2Fspo337.com%2F
http://conny-grote.de/url?q=http%3A%2F%2Fspo337.com%2F
http://crazyfrag91.free.fr/?URL=http%3A%2F%2Fspo337.com%2F
http://crewe.de/url?q=http%3A%2F%2Fspo337.com%2F
http://cwa4100.org/uebimiau/redir.php?http%3A%2F%2Fspo337.com%2F
http://d-quintet.com/i/index.cgi?id=1&mode=redirect&no=494&ref_eid=33&url=http%3A%2F%2Fspo337.com%2F
http://data.allie.dbcls.jp/fct/rdfdesc/usage.vsp?g=http%3A%2F%2Fspo337.com%2F
http://data.linkedevents.org/describe/?url=http%3A%2F%2Fspo337.com%2F
http://dayviews.com/externalLinkRedirect.php?url=http%3A%2F%2Fspo337.com%2F
http://db.cbservices.org/cbs.nsf/forward?openform&http%3A%2F%2Fspo337.com%2F
http://simvol-veri.ru/xp/?goto=http%3A%2F%2Fspo337.com%2F
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=http%3A%2F%2Fspo337.com%2F
https://stroim100.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://kakaku-navi.net/items/detail.php?url=http%3A%2F%2Fspo337.com%2F
http://www.paladiny.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://tido.al/vazhdo.php?url=http%3A%2F%2Fspo337.com%2F
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=http%3A%2F%2Fspo337.com%2F
https://www.usjournal.com/go.php?campusID=190&url=http%3A%2F%2Fspo337.com%2F
https://temptationsaga.com/buy.php?url=http%3A%2F%2Fspo337.com%2F
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=http%3A%2F%2Fspo337.com%2F
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=http%3A%2F%2Fspo337.com%2F
https://spb-medcom.ru/redirect.php?http%3A%2F%2Fspo337.com%2F
https://www.info-realty.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.sermemole.com/public/serbook/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hradycz.cz/redir.php?b=445&t=http%3A%2F%2Fspo337.com%2F
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=http%3A%2F%2Fspo337.com%2F
https://uk.kindofbook.com/redirect.php/?red=http%3A%2F%2Fspo337.com%2F
http://m.17ll.com/apply/tourl/?url=http%3A%2F%2Fspo337.com%2F
http://auyttrv.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bartos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://cllfather.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littlejohnny.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://semesmemos.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://faultypirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mmurugesamnfo.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://katihfmaxtron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ikkemandar.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://googlejfgdlenewstoday.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrecadominicana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://anupam-bestprice89.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bhrepublica.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allinspirations.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://weallfreieds.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://shanmegurad.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://manualmfuctional.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://verybeayurifull.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prasat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://meri.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://prabu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://easehipranaam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://nidatyi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://krodyit.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://naine.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://breajwasi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://beithe.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://allthingnbeyondblog.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://usafunworldt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://financialallorner.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mjghouthernmatron.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://chandancomputers.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingshopping.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptritam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tech-universes.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://littleboy.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://correctinspiration.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://esujkamien.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ujkaeltnx.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ausnangck2809.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://supermu.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://buyfcyclingteam.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tumari.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hariomm.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://lejano.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://jotumko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bestandroid.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://discoverable.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://puriagatratt.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://azizlemon.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ptrfsiitan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://boardgame-breking.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://flowwergulab.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bingcreater-uk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://skinnyskin.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://amil.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mohan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://dilip.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://avinasg.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://virat.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hsadyttk.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://ashu-quality.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://tinko.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://konkaruna.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://udavhav.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://matura.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://patana.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://bopal.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gwl.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://delhi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://gopi.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kripa.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://charanraj.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pream.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://sang.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://pasas.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://kanchan.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://assadaaka.nl/?URL=http%3A%2F%2Fspo337.com%2F
http://atchs.jp/jump?u=http%3A%2F%2Fspo337.com%2F
http://away3d.com/?URL=http%3A%2F%2Fspo337.com%2F
http://blackberryvietnam.net/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://boogiewoogie.com/?URL=http%3A%2F%2Fspo337.com%2F
http://bsumzug.de/url?q=http%3A%2F%2Fspo337.com%2F
http://business.eatonton.com/list?globaltemplate=http%3A%2F%2Fspo337.com%2F
http://archives.midweek.com/?URL=http%3A%2F%2Fspo337.com%2F
http://armdrag.com/mb/get.php?url=http%3A%2F%2Fspo337.com%2F
http://asai-kota.com/acc/acc.cgi?REDIRECT=http%3A%2F%2Fspo337.com%2F
http://ass-media.de/wbb2/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://cdiabetes.com/redirects/offer.php?URL=http%3A%2F%2Fspo337.com%2F
https://padletcdn.com/cgi/fetch?disposition=attachment&url=http%3A%2F%2Fspo337.com%2F
http://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
http://centuryofaction.org/?URL=http%3A%2F%2Fspo337.com%2F
http://Somewh.a.T.dfqw@www.newsdiffs.org/article-history/www.findabeautyschool.com/map.aspx?url=http%3A%2F%2Fspo337.com%2F
http://actontv.org/?URL=http%3A%2F%2Fspo337.com%2F
http://bigbarganz.com/g/?http%3A%2F%2Fspo337.com%2F
http://andreasgraef.de/url?q=http%3A%2F%2Fspo337.com%2F
http://app.espace.cool/ClientApi/SubscribeToCalendar/1039?url=http%3A%2F%2Fspo337.com%2F
http://archive.paulrucker.com/?URL=http%3A%2F%2Fspo337.com%2F
http://baseballpodcasts.net/Feed2JS/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
http://bbs.mottoki.com/index?bbs=hpsenden&act=link&page=94&linkk=http%3A%2F%2Fspo337.com%2F
http://bernhardbabel.com/url?q=http%3A%2F%2Fspo337.com%2F
http://albertaaerialapplicators.com/?URL=http%3A%2F%2Fspo337.com%2F
http://alexanderroth.de/url?q=http%3A%2F%2Fspo337.com%2F
http://alt.baunetzwissen.de/rd/nl-track/?obj=bnw&cg1=redirect&cg2=wissen-newsletter:beton&cg3=partner&datum=2017-01&titel=partnerklick-beton&firma=informationszentrumbeton&link=http%3A%2F%2Fspo337.com%2F
https://duckduckgo.com/?q=https%3A%2F%2Fspo1top.com&t=h_&ia=web
https://www.google.com/search?q=https%3A%2F%2Fspo1top.com&sca_esv=567933587&sxsrf=AM9HkKkLNk4hFHvgRG-dTHcW476TZakh3Q%3A1695527512326&ei=WLIPZZPIE66t4-EPwYOS4AE&ved=0ahUKEwiT1NCYrMKBAxWu1jgGHcGBBBwQ4dUDCBA&uact=5&oq=https%3A%2F%2Fspo1top.com&gs_lp=Egxnd3Mtd2l6LXNlcnAiE2h0dHBzOi8vc3BvMXRvcC5jb20yBBAjGCdI2x9QkAtY2xpwAXgBkAEAmAGHAaABygeqAQMwLji4AQPIAQD4AQHCAgoQABhHGNYEGLAD4gMEGAAgQYgGAZAGBg&sclient=gws-wiz-serp
http://client.paltalk.com/client/webapp/client/External.wmt?url=http%3A%2F%2Fspo337.com%2F
http://community.nxp.com/t5/custom/page/page-id/ExternalRedirect?url=http%3A%2F%2Fspo337.com%2F
http://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://ceskapozice.lidovky.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
http://mitsui-shopping-park.com/lalaport/ebina/redirect.html?url=http%3A%2F%2Fspo337.com%2F
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=http%3A%2F%2Fspo337.com%2F
http://fishbiz.seagrant.uaf.edu/click-thru.html?url=http%3A%2F%2Fspo337.com%2F
http://kupiauto.zr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://ipx.bcove.me/?url=http%3A%2F%2Fspo337.com%2F
https://toolbarqueries.google.td/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
https://home.guanzhuang.org/link.php?url=http%3A%2F%2Fspo337.com%2F
http://dvd24online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://twindish-electronics.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.beigebraunapartment.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.mix-choice.com/yomi/rank.cgi?mode=link&id=391&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
http://www.1soft-tennis.com/search/rank.cgi?mode=link&id=17&url=http%3A%2F%2Fspo337.com%2F
http://big-data-fr.com/linkedin.php?lien=http%3A%2F%2Fspo337.com%2F
http://reg.kost.ru/cgi-bin/go?http%3A%2F%2Fspo337.com%2F
http://www.kirstenulrich.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.inkwell.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.co.je/url?q=http%3A%2F%2Fspo337.com%2F
https://n1653.funny.ge/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://maps.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://clients1.google.de/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
http://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
http://www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=http%3A%2F%2Fspo337.com%2F
https://www.google.to/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.bi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.nickl-architects.com/url?q=http%3A%2F%2Fspo337.com%2F
https://www.ocbin.com/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lobenhausen.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.bs/url?q=http%3A%2F%2Fspo337.com%2F
http://redirect.me/?http%3A%2F%2Fspo337.com%2F
http://kank.o.oo7.jp/cgi-bin/ys4/rank.cgi?mode=link&id=569&url=http%3A%2F%2Fspo337.com%2F
http://ruslog.com/forum/noreg.php?http%3A%2F%2Fspo337.com%2F
http://forum.ahigh.ru/away.htm?link=http%3A%2F%2Fspo337.com%2F
http://www.wildner-medien.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.tifosy.de/url?q=http%3A%2F%2Fspo337.com%2F
https://image.google.co.ck/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://ads.icorp.ro/others/STS/?t=CeNortjKxUjK0NDFVsgZcMBADAm4-&g=http%3A%2F%2Fspo337.com%2F
http://deai-ranking.org/search/rank.cgi?mode=link&id=28&url=http%3A%2F%2Fspo337.com%2F
https://izispicy.com/go.php?url=http%3A%2F%2Fspo337.com%2F
http://demo.vieclamcantho.vn/baohiemthatnghiep/Redirect.aspx?sms=90bb20bb20tbb20thc3%B4ng&link=http%3A%2F%2Fspo337.com%2F
http://www.city-fs.de/url?q=http%3A%2F%2Fspo337.com%2F
http://p.profmagic.com/urllink.php?url=http%3A%2F%2Fspo337.com%2F
https://www.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.pa/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.ee/url?sa=j&source=web&rct=j&url=http%3A%2F%2Fspo337.com%2F
http://www.arndt-am-abend.de/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.com.vc/url?q=http%3A%2F%2Fspo337.com%2F
http://maxnetworks.org/searchlink/rank.cgi?mode=link&id=321&url=http%3A%2F%2Fspo337.com%2F
https://image.google.com.ng/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
https://www.kenzai-navi.com/location/location_topbanner.php?bs=238&m=899&href=http%3A%2F%2Fspo337.com%2F
https://www.anybeats.jp/jump/?http%3A%2F%2Fspo337.com%2F
http://url-collector.appspot.com/positiveVotes?topic=Hate%20speech&url=http%3A%2F%2Fspo337.com%2F
https://cse.google.com.cy/url?q=http%3A%2F%2Fspo337.com%2F
https://maps.google.be/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://top.hange.jp/linkdispatch/dispatch?targetUrl=http%3A%2F%2Fspo337.com%2F
http://www.link.gokinjyo-eikaiwa.com/rank.cgi?mode=link&id=5&url=http%3A%2F%2Fspo337.com%2F
http://www.air-dive.com/au/mt4i.cgi?mode=redirect&ref_eid=697&url=http%3A%2F%2Fspo337.com%2F
http://okozukai.j-web.jp/j-web/okozukai/ys4/rank.cgi?mode=link&id=6817&url=http%3A%2F%2Fspo337.com%2F
https://sfmission.com/rss/feed2js.php?src=http%3A%2F%2Fspo337.com%2F
https://www.watersportstaff.co.uk/extern.aspx?src=http%3A%2F%2Fspo337.com%2F&cu=60096&page=1&t=1&s=42"
http://siamcafe.net/board/go/go.php?http%3A%2F%2Fspo337.com%2F
http://www.whitening-navi.info/cgi/search-smartphone/rank.cgi?mode=link&id=1431&url=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?q=http%3A%2F%2Fspo337.com%2F
http://www.youtube.com/redirect?event=channeldescription&q=http%3A%2F%2Fspo337.com%2F
http://www.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://go.e-frontier.co.jp/rd2.php?uri=http%3A%2F%2Fspo337.com%2F
http://adchem.net/Click.aspx?url=http%3A%2F%2Fspo337.com%2F
http://www.reddotmedia.de/url?q=http%3A%2F%2Fspo337.com%2F
https://10ways.com/fbredir.php?orig=http%3A%2F%2Fspo337.com%2F
https://emailtrackerapi.leadforensics.com/api/URLOpen?EmailSentRecordID=17006&URL=http%3A%2F%2Fspo337.com%2F
http://search.haga-f.net/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://7ba.ru/out.php?url=http%3A%2F%2Fspo337.com%2F
http://www.51queqiao.net/link.php?url=http%3A%2F%2Fspo337.com%2F
https://cse.google.dm/url?q=http%3A%2F%2Fspo337.com%2F
https://rsyosetsu.bookmarks.jp/ys4/rank.cgi?mode=link&id=3519&url=http%3A%2F%2Fspo337.com%2F
https://ulfishing.ru/forum/go.php?http%3A%2F%2Fspo337.com%2F
https://underwood.ru/away.html?url=http%3A%2F%2Fspo337.com%2F
https://unicom.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.unifin.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://uogorod.ru/feed/520?redirect=http%3A%2F%2Fspo337.com%2F
http://uvbnb.ru/go?http%3A%2F%2Fspo337.com%2F
https://skibaza.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://smartservices.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.topkam.ru/gtu/?url=http%3A%2F%2Fspo337.com%2F
https://torggrad.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://torgi-rybinsk.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://tpprt.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://clients1.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://images.google.al/url?q=http%3A%2F%2Fspo337.com%2F
http://toolbarqueries.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.al/url?q=http%3A%2F%2Fspo337.com%2F
https://www.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.capitalbikepark.se/bok/go.php?url=http%3A%2F%2Fspo337.com%2F
http://cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
https://joomlinks.org/?url=http%3A%2F%2Fspo337.com%2F
https://managementportal.de/modules/mod_jw_srfr/redir.php?url=http%3A%2F%2Fspo337.com%2F
http://www.green-cross.pro/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://m.adlf.jp/jump.php?l=http%3A%2F%2Fspo337.com%2F
https://www.moonbbs.com/dm/dmlink.php?dmurl=http%3A%2F%2Fspo337.com%2F
http://www.mac52ipod.cn/urlredirect.php?go=http%3A%2F%2Fspo337.com%2F
http://chrison.net/ct.ashx?id=dad2849a-8862-4a6d-b842-ef628cbfd3c3&url=http%3A%2F%2Fspo337.com%2F
http://datasheetcatalog.biz/url.php?url=http%3A%2F%2Fspo337.com%2F
http://minlove.biz/out.html?id=nhmode&go=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/win/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://request-response.com/blog/ct.ashx?url=http%3A%2F%2Fspo337.com%2F
https://turbazar.ru/url/index?url=http%3A%2F%2Fspo337.com%2F
http://members.asoa.org/sso/logout.aspx?returnurl=http%3A%2F%2Fspo337.com%2F
http://login.mediafort.ru/autologin/mail/?code=14844×02ef859015x290299&url=http%3A%2F%2Fspo337.com%2F
https://www.sports-central.org/cgi-bin/axs/ax.pl?http%3A%2F%2Fspo337.com%2F
http://dot.boss.sk/o/rs.php?ssid=1&szid=4&dsid=12&dzid=32&url=http%3A%2F%2Fspo337.com%2F
http://www.americanstylefridgefreezer.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.newzealandgirls.co.nz/auckland/escorts/clicks.php?click_id=NavBar_AF_AdultDirectory&target=http%3A%2F%2Fspo337.com%2F
https://wdesk.ru/go?http%3A%2F%2Fspo337.com%2F
http://edcommunity.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://page.yicha.cn/tp/j?url=http%3A%2F%2Fspo337.com%2F
http://www.internettrafficreport.com/cgi-bin/cgirdir.exe?http%3A%2F%2Fspo337.com%2F
http://www.interfacelift.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
https://good-surf.ru/r.php?g=http%3A%2F%2Fspo337.com%2F
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=http%3A%2F%2Fspo337.com%2F
https://gektor-nsk.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://lekoufa.ru/banner/go?banner_id=4&link=http%3A%2F%2Fspo337.com%2F
https://forsto.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.gigatran.ru/go?url=http%3A%2F%2Fspo337.com%2F
http://www.gigaalert.com/view.php?h=&s=http%3A%2F%2Fspo337.com%2F
http://www.jkes.tyc.edu.tw/dyna/webs/gotourl.php?id=357&url=http%3A%2F%2Fspo337.com%2F
http://www.laopinpai.com/gourl.asp?url=/gourl.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.humanbrainmapping.org/i4a/etrack/track.cfm?rType=2&campaignID=3572&contactID=4524&origURL=http%3A%2F%2Fspo337.com%2F
https://gcup.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.gearguide.ru/phpbb/go.php?http%3A%2F%2Fspo337.com%2F
https://es.catholic.net/ligas/ligasframe.phtml?liga=http%3A%2F%2Fspo337.com%2F
https://passport.sfacg.com/LoginOut.aspx?Returnurl=http%3A%2F%2Fspo337.com%2F
https://offers.sidex.ru/stat_ym_new.php?redir=http%3A%2F%2Fspo337.com%2F&hash=1577762
https://globalmedia51.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://ramset.com.au/document/url/?url=http%3A%2F%2Fspo337.com%2F
https://www.vicsport.com.au/analytics/outbound?url=http%3A%2F%2Fspo337.com%2F
http://www.cyprus-net.com/banner_click.php?banid=4&link=http%3A%2F%2Fspo337.com%2F
http://a.gongkong.com/db/adredir.asp?id=16757&url=http%3A%2F%2Fspo337.com%2F
http://gfaq.ru/go?http%3A%2F%2Fspo337.com%2F
http://gbi-12.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://www.global-flat.com/mobile/mobile_switch.php?dir=tofull&url=http%3A%2F%2Fspo337.com%2F
https://golden-resort.ru/out.php?out=http%3A%2F%2Fspo337.com%2F
http://avalon.gondor.ru/away.php?link=http%3A%2F%2Fspo337.com%2F
http://www.laosubenben.com/home/link.php?url=http%3A%2F%2Fspo337.com%2F
http://www.lifeshow.com.tw/show.php?ty5_id=1599&url=http%3A%2F%2Fspo337.com%2F
http://games.cheapdealuk.co.uk/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.netwalkerstore.com/redirect.asp?accid=18244&adcampaignid=2991&adcampaigntype=2&affduration=30&url=http%3A%2F%2Fspo337.com%2F
http://tsugarubrand.jp/blog/?wptouch_switch=mobile&redirect=http%3A%2F%2Fspo337.com%2F
http://blackhistorydaily.com/black_historylinks/link.asp?linkid=5&URL=http%3A%2F%2Fspo337.com%2F
http://www.kitchencabinetsdirectory.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://cityprague.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://www.hachimantaishi.com/click3/click3.cgi?cnt=c5&url=http%3A%2F%2Fspo337.com%2F
http://earnupdates.com/goto.php?url=http%3A%2F%2Fspo337.com%2F
http://www.careerbuilder.com.cn/cn/tallyredirector.aspx?task=Ushi&objName=SnsShare&redirectUrl=http%3A%2F%2Fspo337.com%2F&methodName=SetSnsShareLink
http://banners.knollenstein.com/adnetwork/servlet/advertBeans.TrackingServlet?seid=27709655&t=100&redirectTo=http%3A%2F%2Fspo337.com%2F&cid=DECONL&vid=986809&externalsource=jobbsquareppc
https://meyeucon.org/ext-click.php?url=http%3A%2F%2Fspo337.com%2F
http://www.cnmhe.fr/spip.php?action=cookie&url=http%3A%2F%2Fspo337.com%2F
https://perezvoni.com/blog/away?url=http%3A%2F%2Fspo337.com%2F
https://www.ciymca.org/af/register-redirect/71104?url=http%3A%2F%2Fspo337.com%2F
https://whizpr.nl/tracker.php?u=http%3A%2F%2Fspo337.com%2F
http://bw.irr.by/knock.php?bid=252583&link=http%3A%2F%2Fspo337.com%2F
http://www.beeicons.com/redirect.php?site=http%3A%2F%2Fspo337.com%2F
http://www.diwaxx.ru/hak/redir.php?redir=http%3A%2F%2Fspo337.com%2F
http://www.actuaries.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMVFIELD]EMAIL[EMV/FIELD]&cat=Techniquesculturales&url=http%3A%2F%2Fspo337.com%2F
https://www.jukujo.gs/bin/out.cgi?id=kimiomof&url=http%3A%2F%2Fspo337.com%2F
http://hansolav.net/blog/ct.ashx?id=0af6301b-e71f-44be-838f-905709eee792&url=http%3A%2F%2Fspo337.com%2F
http://bitrix.adlr.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://m.snek.ai/redirect?url=http%3A%2F%2Fspo337.com%2F
http://www.officeservice.com.br/trackMKT/trackerFolha.aspx?DESCRIPTION=HotSite_EcrmContabil&FROM=Workshop&DESTINATION=http%3A%2F%2Fspo337.com%2F
https://www.arbsport.ru/gotourl.php?url=http%3A%2F%2Fspo337.com%2F
http://www.strana.co.il/finance/redir.aspx?site=http%3A%2F%2Fspo337.com%2F
https://www.tourplanisrael.com/redir/?url=http%3A%2F%2Fspo337.com%2F
http://www.mutualgravity.com/archangel/webcontact/d_signinsimple.php?action=signup&CID=240&EID=&S=default.css&return=http%3A%2F%2Fspo337.com%2F
http://buildingreputation.com/lib/exe/fetch.php?media=http%3A%2F%2Fspo337.com%2F
http://gals.graphis.ne.jp/mkr/out.cgi?id=04489&go=http%3A%2F%2Fspo337.com%2F
http://dir.tetsumania.net/search/rank.cgi?mode=link&id=3267&url=http%3A%2F%2Fspo337.com%2F
http://www.project24.info/mmview.php?dest=http%3A%2F%2Fspo337.com%2F
http://tfads.testfunda.com/TFServeAds.aspx?strTFAdVars=4a086196-2c64-4dd1-bff7-aa0c7823a393,TFvar,00319d4f-d81c-4818-81b1-a8413dc614e6,TFvar,GYDH-Y363-YCFJ-DFGH-5R6H,TFvar,http%3A%2F%2Fspo337.com%2F
http://www.request-response.com/blog/ct.ashx?id=d827b163-39dd-48f3-b767-002147c94e05&url=http%3A%2F%2Fspo337.com%2F
http://www.cooltgp.org/tgp/click.php?id=370646&u=http%3A%2F%2Fspo337.com%2F
http://sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=785&redirect=http%3A%2F%2Fspo337.com%2F
http://www.kyoto-osaka.com/search/rank.cgi?mode=link&url=http%3A%2F%2Fspo337.com%2F
http://metalist.co.il/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://i-marine.eu/pages/goto.aspx?link=http%3A%2F%2Fspo337.com%2F
http://www.teenlove.biz/cgibin/atc/out.cgi?id=24&u=http%3A%2F%2Fspo337.com%2F
http://www.altoona.com/newsletter/click.php?volume=52&url=http%3A%2F%2Fspo337.com%2F
http://www.katjushik.ru/link.php?to=http%3A%2F%2Fspo337.com%2F
http://gondor.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
http://proekt-gaz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.tricitiesapartmentguide.com/MobileDefault.aspx?reff=http%3A%2F%2Fspo337.com%2F
http://www.vietnamsingle.com/vn/banners/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.voxlocalis.net/enlazar/?url=http%3A%2F%2Fspo337.com%2F
http://no-smok.net/nsmk/InterWiki?action=goto&oe=EUC-KR&url=http%3A%2F%2Fspo337.com%2F
http://www.stalker-modi.ru/go?http%3A%2F%2Fspo337.com%2F
http://www.p1-uranai.com/rank.cgi?mode=link&id=538&url=http%3A%2F%2Fspo337.com%2F
http://earthsciencescanada.com/modules/babel/redirect.php?newlang=en_us&newurl=http%3A%2F%2Fspo337.com%2F
http://psykodynamiskt.nu/?wptouch_switch=desktop&redirect=http%3A%2F%2Fspo337.com%2F
http://www.pcbheaven.com/forum/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.castellodivezio.it/lingua.php?lingua=EN&url=http%3A%2F%2Fspo337.com%2F
http://moscowdesignmuseum.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.fairpoint.net/~jensen1242/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://lirasport.com.ar/bloques/bannerclick.php?id=7&url=http%3A%2F%2Fspo337.com%2F
http://www.knabstrupper.se/guestbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.pba.ph/redirect?url=http%3A%2F%2Fspo337.com%2F&id=3&type=tab
https://bondage-guru.net/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.westbloomfieldlibrary.org/includes/statistics.php?StatType=Link&StatID=Facebook&weblink=http%3A%2F%2Fspo337.com%2F
https://tenisnews.com.br/clickTracker/clickTracker.php?u=http%3A%2F%2Fspo337.com%2F
http://www.stevestechspot.com/ct.ashx?id=9fdcf4a4-6e09-4807-bc31-ac1adf836f6c&url=http%3A%2F%2Fspo337.com%2F
http://rcoi71.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://www.laselection.net/redir.php3?cat=int&url=http%3A%2F%2Fspo337.com%2F
http://www.masai-mara.com/cgi-bin/link2.pl?grp=mm&link=http%3A%2F%2Fspo337.com%2F
http://evenemangskalender.se/redirect/?id=15723&lank=http%3A%2F%2Fspo337.com%2F
http://anifre.com/out.html?go=http%3A%2F%2Fspo337.com%2F
http://www.restavracije-gostilne.si/banner.php?id=44&url=http%3A%2F%2Fspo337.com%2F
http://hobbyplastic.co.uk/trigger.php?r_link=http%3A%2F%2Fspo337.com%2F
http://ads.pukpik.com/myads/click.php?banner_id=316&banner_url=http%3A%2F%2Fspo337.com%2F
https://www.adminer.org/redirect/?sa=t&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackmamas.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://primorye.ru/go.php?id=19&url=http%3A%2F%2Fspo337.com%2F
https://www.123gomme.it/it/ViewSwitcher/SwitchView?mobile=True&returnUrl=http%3A%2F%2Fspo337.com%2F
http://youmydaddy.com/cgi-bin/at3/out.cgi?id=88&trade=http%3A%2F%2Fspo337.com%2F
https://naviking.localking.com.tw/about/redirect.aspx?mid=7&url=http%3A%2F%2Fspo337.com%2F
http://sms-muzeji.si/Home/ChangeCulture?lang=sl&returnUrl=http%3A%2F%2Fspo337.com%2F
http://r.emeraldexpoinfo.com/s.ashx?ms=EXI2:125462_115115&e=krubin723@aol.com&eId=440186886&c=h&url=http%3A%2F%2Fspo337.com%2F
https://apps.firstrain.com/service/openurl.jsp?action=titleclick&src=rss&u=http%3A%2F%2Fspo337.com%2F
http://cdp.thegoldwater.com/click.php?id=101&url=http%3A%2F%2Fspo337.com%2F
http://www.appenninobianco.it/ads/adclick.php?bannerid=159&zoneid=8&source=&dest=http%3A%2F%2Fspo337.com%2F
https://www.eduplus.hk/special/emailalert/goURL.jsp?clickURL=http%3A%2F%2Fspo337.com%2F
http://www.japan.road.jp/navi/navi.cgi?jump=226&url=http%3A%2F%2Fspo337.com%2F
http://www.arch.iped.pl/artykuly.php?id=1&cookie=1&url=http%3A%2F%2Fspo337.com%2F
http://hirlevelcenter.eu/click.php?hirlevel_id=13145441085508&url=http%3A%2F%2Fspo337.com%2F
https://birds.cz/avif/redirect.php?from=avif.obs.php&url=http%3A%2F%2Fspo337.com%2F
http://www.bigblackbootywatchers.com/cgi-bin/sites/out.cgi?url=http%3A%2F%2Fspo337.com%2F
http://barykin.com/go.php?http%3A%2F%2Fspo337.com%2F
https://forum.everleap.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
http://www.mosig-online.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hccincorporated.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fatnews.com/?URL=http%3A%2F%2Fspo337.com%2F
https://csirealty.com/?URL=http%3A%2F%2Fspo337.com%2F
http://asadi.de/url?q=http%3A%2F%2Fspo337.com%2F
http://treblin.de/url?q=http%3A%2F%2Fspo337.com%2F
https://kentbroom.com/?URL=http%3A%2F%2Fspo337.com%2F
http://0845.boo.jp/cgi/mt3/mt4i.cgi?id=24&mode=redirect&no=15&ref_eid=3387&url=http%3A%2F%2Fspo337.com%2F
http://110.164.66.211/ULIB6//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://110.164.92.12/ULIB//dublin.linkout.php?url=http%3A%2F%2Fspo337.com%2F
http://198.54.125.86.myopenlink.net/describe/?url=http%3A%2F%2Fspo337.com%2F
http://202.144.225.38/jmp?url=http%3A%2F%2Fspo337.com%2F
http://2cool2.be/url?q=http%3A%2F%2Fspo337.com%2F
http://4coma.net/cgi/mt4/mt4i.cgi?cat=12&mode=redirect&ref_eid=3231&url=http%3A%2F%2Fspo337.com%2F
http://4travel.jp/dynamic/redirect.php?mode=dm_tour&url=http%3A%2F%2Fspo337.com%2F
http://imaginingourselves.globalfundforwomen.org/pb/External.aspx?url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.cd/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ag/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.et/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.fm/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.md/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.mw/url?q=http%3A%2F%2Fspo337.com%2F
https://clients1.google.nu/url?sa=j&url=http%3A%2F%2Fspo337.com%2F
https://clients1.google.rw/url?q=http%3A%2F%2Fspo337.com%2F
http://search.pointcom.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.cercasostituto.it/index.php?name=GestBanner&file=counter&idbanner=40&dir_link=http%3A%2F%2Fspo337.com%2F
http://vsvejr.dk/mt/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.meteo-leran.fr/meteotemplate/template/plugins/deviations/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.eurobichons.com/fda%20alerts.php?url=http%3A%2F%2Fspo337.com%2F
https://mydojo.at/de_AT/karate/weiterleitung?redirect=http%3A%2F%2Fspo337.com%2F
http://click.phosphodiesterase4.com/k.php?ai=&url=http%3A%2F%2Fspo337.com%2F
http://www.mariahownersclub.com/forum/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://www.wildromance.com/buy.php?url=http%3A%2F%2Fspo337.com%2F&store=iBooks&book=omk-ibooks-us
http://mapleriverweather.com/mobile/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.archijob.co.il/index/comp_website.asp?companyId=1469&website=http%3A%2F%2Fspo337.com%2F
https://student-helpr.rminds.dev/redirect?redirectTo=http%3A%2F%2Fspo337.com%2F
http://www.kalinna.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.hartmanngmbh.de/url?q=http%3A%2F%2Fspo337.com%2F
https://www.the-mainboard.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://www.betamachinery.com/?URL=http%3A%2F%2Fspo337.com%2F
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=http%3A%2F%2Fspo337.com%2F
http://www.sprang.net/url?q=http%3A%2F%2Fspo337.com%2F
https://img.2chan.net/bin/jump.php?http%3A%2F%2Fspo337.com%2F
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=http%3A%2F%2Fspo337.com%2F
http://local.rongbachkim.com/rdr.php?url=http%3A%2F%2Fspo337.com%2F
http://bachecauniversitaria.it/link/frm_top.php?url=http%3A%2F%2Fspo337.com%2F
https://www.stcwdirect.com/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://forum.vcoderz.com/externalredirect.php?url=http%3A%2F%2Fspo337.com%2F
https://www.momentumstudio.com/?URL=http%3A%2F%2Fspo337.com%2F
https://s-p.me/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://ww.thesteelbrothers.com/buy.php?store=iBooks&url=http%3A%2F%2Fspo337.com%2F
http://thdt.vn/convert/convert.php?link=http%3A%2F%2Fspo337.com%2F
http://www.doitweb365.de/scripts/doitweb.exe/rasklickzaehler2?http%3A%2F%2Fspo337.com%2F
https://www.guadamur.eu/template/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://weather.cube.com.gr/pages/station/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.fimmgviterbo.org/mobfimmgviterbo/index.php?nametm=counter&idbanner=4&dir_link=http%3A%2F%2Fspo337.com%2F
http://www.mckinneyfarm.com/template/plugins/stationExtremes/redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://utmagazine.ru/r?url=http%3A%2F%2Fspo337.com%2F
http://www.evrika41.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
http://expomodel.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://facto.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://fallout3.ru/utils/ref.php?url=http%3A%2F%2Fspo337.com%2F
https://fc-zenit.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://forum-region.ru/forum/away.php?s=http%3A%2F%2Fspo337.com%2F
http://forumdate.ru/redirect-to/?redirect=http%3A%2F%2Fspo337.com%2F
http://shckp.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://shinglas.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sibran.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://sintez-oka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://app.espace.cool/clientapi/subscribetocalendar/974?url=http%3A%2F%2Fspo337.com%2F
http://111056.net/yomisearch/rank.cgi?mode=link&id=6205&url=http%3A%2F%2Fspo337.com%2F
http://stanko.tw1.ru/redirect.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sozialemoderne.de/url?q=http%3A%2F%2Fspo337.com%2F
http://www.showb.com/search/ranking.cgi?mode=link&id=7083&url=http%3A%2F%2Fspo337.com%2F
http://imagelibrary.asprey.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://ime.nu/http%3A%2F%2Fspo337.com%2F
http://interflex.biz/url?q=http%3A%2F%2Fspo337.com%2F
http://ivvb.de/url?q=http%3A%2F%2Fspo337.com%2F
http://j.lix7.net/?http%3A%2F%2Fspo337.com%2F
http://jacobberger.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jahn.eu/url?q=http%3A%2F%2Fspo337.com%2F
http://jamesvelvet.com/?URL=www.http%3A%2F%2Fspo337.com%2F
http://jla.drmuller.net/r.php?url=http%3A%2F%2Fspo337.com%2F
http://jump.pagecs.net/http%3A%2F%2Fspo337.com%2F
http://kagarin.net/cgi/mt/mt4i.cgi?id=2&mode=redirect&no=330&ref_eid=103&url=http%3A%2F%2Fspo337.com%2F
http://karkom.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kens.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinderundjugendpsychotherapie.de/url?q=http%3A%2F%2Fspo337.com%2F
http://kinhtexaydung.net/redirect/?url=http%3A%2F%2Fspo337.com%2F
http://neoromance.info/link/rank.cgi?mode=link&id=26&url=http%3A%2F%2Fspo337.com%2F
http://www.hainberg-gymnasium.com/url?q=http%3A%2F%2Fspo337.com%2F
https://befonts.com/checkout/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.usap.gov/externalsite.cfm?http%3A%2F%2Fspo337.com%2F
https://maps.google.com.ua/url?rct=j&sa=t&url=http%3A%2F%2Fspo337.com%2F
http://images.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.com.mx/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://www.shinobi.jp/etc/goto.html?http%3A%2F%2Fspo337.com%2F
http://images.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.hu/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.com.hk/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.sg/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.pt/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ar/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.ua/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.no/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
http://cse.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
http://images.google.com.tr/url?q=http%3A%2F%2Fspo337.com%2F
http://maps.google.dk/url?q=http%3A%2F%2Fspo337.com%2F
http://www.google.fi/url?q=http%3A%2F%2Fspo337.com%2F
https://supplier-portal-uat.daimler.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://strelmag.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://spb90.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://spartak.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://sovaisova.ru/bitrix/redirect.php?event1=2009_mainpage&event2=go_www&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://slashwrestling.com/cgi-bin/redirect.cgi?http%3A%2F%2Fspo337.com%2F
https://seoandme.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://s-online.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://rssfeeds.wtsp.com/~/t/0/0/wtsp/home/~http%3A%2F%2Fspo337.com%2F
https://rostovmama.ru/redirect?url=http%3A%2F%2Fspo337.com%2F
https://rev1.reversion.jp/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.star174.ru/redir.php?url=http%3A%2F%2Fspo337.com%2F
https://staten.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://stopcran.ru/go?http%3A%2F%2Fspo337.com%2F
http://studioad.ru/go?http%3A%2F%2Fspo337.com%2F
http://swepub.kb.se/setattribute?language=en&redirect=http%3A%2F%2Fspo337.com%2F
https://www.licnioglasi.org/index.php?thememode=full;redirect=http%3A%2F%2Fspo337.com%2F
http://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=http%3A%2F%2Fspo337.com%2F
http://www2.smartmail.com.ar/tl.php?p=hqf/f94/rs/1fp/4c0/rs//http%3A%2F%2Fspo337.com%2F
http://old.roofnet.org/external.php?link=http%3A%2F%2Fspo337.com%2F
http://www.bucatareasa.ro/link.php?url=http%3A%2F%2Fspo337.com%2F
http://mosprogulka.ru/go?http%3A%2F%2Fspo337.com%2F
https://uniline.co.nz/Document/Url/?url=http%3A%2F%2Fspo337.com%2F
https://tracker.onrecruit.net/api/v1/redirect/?redirect_to=http%3A%2F%2Fspo337.com%2F
http://slipknot1.info/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.samovar-forum.ru/go?http%3A%2F%2Fspo337.com%2F
http://staldver.ru/go.php?go=http%3A%2F%2Fspo337.com%2F
https://posts.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://plus.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://naruto.su/link.ext.php?url=http%3A%2F%2Fspo337.com%2F
https://justpaste.it/redirect/172fy/http%3A%2F%2Fspo337.com%2F
https://jt-pr-dot-yamm-track.appspot.com/Redirect?ukey=1iXi3dF8AuzUIsgifbcVnqqx-anF4B8R-9PC3UGhHO3E-1131306248&key=YAMMID-79725512&link=http%3A%2F%2Fspo337.com%2F
https://ipv4.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://forum.solidworks.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://foro.infojardin.com/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://ditu.google.com/url?q=http%3A%2F%2Fspo337.com%2F
https://de.flavii.de/index.php?flavii=linker&link=http%3A%2F%2Fspo337.com%2F
https://dakke.co/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_attribute_to_url=http%3A%2F%2Fspo337.com%2F
https://contacts.google.com/url?sa=t&url=http%3A%2F%2Fspo337.com%2F
https://community.rsa.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.nxp.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.esri.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://community.cypress.com/external-link.jspa?url=http%3A%2F%2Fspo337.com%2F
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=http%3A%2F%2Fspo337.com%2F
https://cdn.iframe.ly/api/iframe?url=http%3A%2F%2Fspo337.com%2F
https://bukkit.org/proxy.php?link=http%3A%2F%2Fspo337.com%2F
https://branch.app.link/?$deeplink_path=article%2Fjan%2F123&$fallback_url=http%3A%2F%2Fspo337.com%2F&channel=facebook&feature=affiliate
https://boowiki.info/go.php?go=http%3A%2F%2Fspo337.com%2F
https://blaze.su/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
https://bbs.pku.edu.cn/v2/jump-to.php?url=http%3A%2F%2Fspo337.com%2F
https://bares.blog.idnes.cz/redir.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.flyingsamaritans.net/Startup/SetupSite.asp?RestartPage=http%3A%2F%2Fspo337.com%2F
https://www.ewind.cz/index.php?page=home/redirect&url=http%3A%2F%2Fspo337.com%2F
https://www.eas-racing.se/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
https://www.direkt-einkauf.de/includes/refer.php?id=170&url=http%3A%2F%2Fspo337.com%2F
https://www.dialogportal.com/Services/Forward.aspx?link=http%3A%2F%2Fspo337.com%2F
https://www.curseforge.com/linkout?remoteUrl=http%3A%2F%2Fspo337.com%2F
https://www.counterwelt.com/charts/click.php?user=14137&link=http%3A%2F%2Fspo337.com%2F
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=http%3A%2F%2Fspo337.com%2F
https://www.autopartskart.com/buyfromamzon.php?url=http%3A%2F%2Fspo337.com%2F
https://www.autoandrv.com/linkout.aspx?websiteurl=http%3A%2F%2Fspo337.com%2F
https://www.art-prizes.com/AdRedirector.aspx?ad=MelbPrizeSculpture_2017&target=http%3A%2F%2Fspo337.com%2F
https://wirelessestimator.com/advertise/newsletter_redirect.php?url=http%3A%2F%2Fspo337.com%2F
https://wasitviewed.com/index.php?href=http%3A%2F%2Fspo337.com%2F
https://tvtropes.org/pmwiki/no_outbounds.php?o=http%3A%2F%2Fspo337.com%2F
https://trello.com/add-card?source=mode=popup&name=click+here&desc=http%3A%2F%2Fspo337.com%2F
https://sutd.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
https://abiznes.com.ua/bitrix/redirect.php?event1=&event2=&event3=&goto=http%3A%2F%2Fspo337.com%2F
http://www.sv-mama.ru/shared/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.sofion.ru/banner.php?r1=41&r2=2234&goto=http%3A%2F%2Fspo337.com%2F
http://www.rss.geodles.com/fwd.php?url=http%3A%2F%2Fspo337.com%2F
http://www.nuttenzone.at/jump.php?url=http%3A%2F%2Fspo337.com%2F
http://www.myhottiewife.com/cgi-bin/arpro/out.cgi?id=Jojo&url=http%3A%2F%2Fspo337.com%2F
http://www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=http%3A%2F%2Fspo337.com%2F
http://www.johnvorhees.com/gbook/go.php?url=http%3A%2F%2Fspo337.com%2F
http://www.imsnet.at/LangChange.aspx?uri=http%3A%2F%2Fspo337.com%2F
http://www.hon-cafe.net/cgi-bin/re.cgi?lid=hmw&url=http%3A%2F%2Fspo337.com%2F
http://www.glorioustronics.com/redirect.php?link=http%3A%2F%2Fspo337.com%2F
http://www.etis.ford.com/externalURL.do?url=http%3A%2F%2Fspo337.com%2F
http://www.chungshingelectronic.com/redirect.asp?url=http%3A%2F%2Fspo337.com%2F
http://www.bdsmandfetish.com/cgi-bin/sites/out.cgi?id=mandymon&url=http%3A%2F%2Fspo337.com%2F
http://visits.seogaa.ru/redirect/?g=http%3A%2F%2Fspo337.com%2F
http://tharp.me/?url_to_shorten=http%3A%2F%2Fspo337.com%2F
http://speakrus.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://spbstroy.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://solo-center.ru/links.php?go=http%3A%2F%2Fspo337.com%2F
http://sennheiserstore.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://school364.spb.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://sc.sie.gov.hk/TuniS/http%3A%2F%2Fspo337.com%2F
http://rzngmu.ru/go?http%3A%2F%2Fspo337.com%2F
http://rostovklad.ru/go.php?http%3A%2F%2Fspo337.com%2F
http://portalnp.snauka.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
http://markiza.me/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://jump.5ch.net/?http%3A%2F%2Fspo337.com%2F
http://imperialoptical.com/news-redirect.aspx?url=http%3A%2F%2Fspo337.com%2F
http://hellothai.com/wwwlink/wwwredirect.asp?hp_id=1242&url=http%3A%2F%2Fspo337.com%2F
http://guru.sanook.com/?URL=http%3A%2F%2Fspo337.com%2F
http://fr.knubic.com/redirect_to?url=http%3A%2F%2Fspo337.com%2F
http://branch.app.link/?$deeplink_path=article/jan/123&$fallback_url=http%3A%2F%2Fspo337.com%2F
http://biz-tech.org/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
http://2010.russianinternetweek.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://xat.com/web_gear/chat/linkvalidator.php?link=http%3A%2F%2Fspo337.com%2F
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=http%3A%2F%2Fspo337.com%2F
https://www.woodlist.us/delete-company?nid=13964&element=http%3A%2F%2Fspo337.com%2F
https://www.viecngay.vn/go?to=http%3A%2F%2Fspo337.com%2F
https://www.uts.edu.co/portal/externo.php?id=http%3A%2F%2Fspo337.com%2F
https://www.talgov.com/Main/exit.aspx?url=http%3A%2F%2Fspo337.com%2F
https://www.serie-a.ru/bitrix/redirect.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.russianrobotics.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.ruchnoi.ru/ext_link?url=http%3A%2F%2Fspo337.com%2F
https://www.rprofi.ru/bitrix/rk.php?goto=http%3A%2F%2Fspo337.com%2F
https://www.roccotube.com/cgi-bin/at3/out.cgi?id=27&tag=toplist&trade=http%3A%2F%2Fspo337.com%2F
https://www.nyl0ns.com/cgi-bin/a2/out.cgi?id=43&l=btop&u=http%3A%2F%2Fspo337.com%2F
https://www.meetme.com/apps/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=http%3A%2F%2Fspo337.com%2F
https://www.interpals.net/url_redirect.php?href=http%3A%2F%2Fspo337.com%2F
https://www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=http%3A%2F%2Fspo337.com%2F
https://www.hottystop.com/cgi-bin/at3/out.cgi?id=12&trade=http%3A%2F%2Fspo337.com%2F
https://www.hobowars.com/game/linker.php?url=http%3A%2F%2Fspo337.com%2F
https://www.hentainiches.com/index.php?id=derris&tour=http%3A%2F%2Fspo337.com%2F
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=http%3A%2F%2Fspo337.com%2F
https://www.greencom.ru/catalog/irrigation_systems.html?jumpsite=2008&url=http%3A%2F%2Fspo337.com%2F
https://www.girlznation.com/cgi-bin/atc/out.cgi?id=50&l=side&u=http%3A%2F%2Fspo337.com%2F
https://www.funeralunion.org/delete-company?nid=39&element=http%3A%2F%2Fspo337.com%2F
https://www.fudbal91.com/tz.php?zone=America/Iqaluit&r=http%3A%2F%2Fspo337.com%2F
https://www.frodida.org/BannerClick.php?BannerID=29&LocationURL=http%3A%2F%2Fspo337.com%2F
https://kryvbas.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://google.cat/url?q=http%3A%2F%2Fspo337.com%2F
https://joomluck.com/go/?http%3A%2F%2Fspo337.com%2F
https://www.anonymz.com/?http%3A%2F%2Fspo337.com%2F
https://weburg.net/redirect?url=http%3A%2F%2Fspo337.com%2F
https://tw6.jp/jump/?url=http%3A%2F%2Fspo337.com%2F
https://www.spainexpat.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.fotka.pl/link.php?u=http%3A%2F%2Fspo337.com%2F
https://www.lolinez.com/?http%3A%2F%2Fspo337.com%2F
https://nanos.jp/jmp?url=http%3A%2F%2Fspo337.com%2F
https://www.fca.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://savvylion.com/?bmDomain=http%3A%2F%2Fspo337.com%2F
https://www.soyyooestacaido.com/http%3A%2F%2Fspo337.com%2F
https://www.gta.ru/redirect/www.http%3A%2F%2Fspo337.com%2F
https://directx10.org/go?http%3A%2F%2Fspo337.com%2F
https://mejeriet.dk/link.php?id=http%3A%2F%2Fspo337.com%2F
https://ezdihan.do.am/go?http%3A%2F%2Fspo337.com%2F
https://fishki.net/click?http%3A%2F%2Fspo337.com%2F
https://hiddenrefer.com/?http%3A%2F%2Fspo337.com%2F
https://romhacking.ru/go?http%3A%2F%2Fspo337.com%2F
https://turion.my1.ru/go?http%3A%2F%2Fspo337.com%2F
https://kassirs.ru/sweb.asp?url=http%3A%2F%2Fspo337.com%2F
https://www.allods.net/redirect/http%3A%2F%2Fspo337.com%2F
https://icook.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.pasco.k12.fl.us/?URL=http%3A%2F%2Fspo337.com%2F
https://anolink.com/?link=http%3A%2F%2Fspo337.com%2F
https://www.questsociety.ca/?URL=http%3A%2F%2Fspo337.com%2F
https://www.disl.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://holidaykitchens.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.mbcarolinas.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.anibox.org/go?http%3A%2F%2Fspo337.com%2F
https://google.info/url?q=http%3A%2F%2Fspo337.com%2F
https://atlantis-tv.ru/go?http%3A%2F%2Fspo337.com%2F
https://otziv.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.sgvavia.ru/go?http%3A%2F%2Fspo337.com%2F
https://element.lv/go?url=http%3A%2F%2Fspo337.com%2F
https://karanova.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://789.ru/go.php?url=http%3A%2F%2Fspo337.com%2F
https://krasnoeselo.su/go?http%3A%2F%2Fspo337.com%2F
https://game-era.do.am/go?http%3A%2F%2Fspo337.com%2F
https://kudago.com/go/?to=http%3A%2F%2Fspo337.com%2F
https://after.ucoz.net/go?http%3A%2F%2Fspo337.com%2F
https://kinteatr.at.ua/go?http%3A%2F%2Fspo337.com%2F
https://nervetumours.org.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://kopyten.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://www.taker.im/go/?u=http%3A%2F%2Fspo337.com%2F
https://usehelp.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://sepoa.fr/wp/go.php?http%3A%2F%2Fspo337.com%2F
https://world-source.ru/go?http%3A%2F%2Fspo337.com%2F
https://mail2.mclink.it/SRedirect/http%3A%2F%2Fspo337.com%2F
https://www.swleague.ru/go?http%3A%2F%2Fspo337.com%2F
https://nazgull.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.rosbooks.ru/go?http%3A%2F%2Fspo337.com%2F
https://beskuda.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://richmonkey.biz/go/?http%3A%2F%2Fspo337.com%2F
https://vlpacific.ru/?goto=http%3A%2F%2Fspo337.com%2F
https://google.co.ck/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ls/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zm/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ve/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.zw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.uk/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ao/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.cr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.nz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.th/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ug/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ma/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.za/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.kr/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.mz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.vi/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.ke/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.hu/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.tz/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.jp/url?q=http%3A%2F%2Fspo337.com%2F
https://eric.ed.gov/?redir=http%3A%2F%2Fspo337.com%2F
https://www.usich.gov/?URL=http%3A%2F%2Fspo337.com%2F
https://sec.pn.to/jump.php?http%3A%2F%2Fspo337.com%2F
https://www.earth-policy.org/?URL=http%3A%2F%2Fspo337.com%2F
https://www.silverdart.co.uk/?URL=http%3A%2F%2Fspo337.com%2F
https://pr-cy.ru/jump/?url=http%3A%2F%2Fspo337.com%2F
https://google.co.bw/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.id/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.in/url?q=http%3A%2F%2Fspo337.com%2F
https://google.co.il/url?q=http%3A%2F%2Fspo337.com%2F
https://pikmlm.ru/out.php?p=http%3A%2F%2Fspo337.com%2F
https://regnopol.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://mss.in.ua/go.php?to=http%3A%2F%2Fspo337.com%2F
https://owohho.com/away?url=http%3A%2F%2Fspo337.com%2F
https://www.youa.eu/r.php?u=http%3A%2F%2Fspo337.com%2F
https://cool4you.ucoz.ru/go?http%3A%2F%2Fspo337.com%2F
https://rg4u.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://dawnofwar.org.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.de-online.ru/go?http%3A%2F%2Fspo337.com%2F
https://www.allpn.ru/redirect/?url=http%3A%2F%2Fspo337.com%2F
https://click.start.me/?url=http%3A%2F%2Fspo337.com%2F
https://prizraks.clan.su/go?http%3A%2F%2Fspo337.com%2F
https://flyd.ru/away.php?to=http%3A%2F%2Fspo337.com%2F
https://risunok.ucoz.com/go?http%3A%2F%2Fspo337.com%2F
https://www.google.ca/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.fr/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.mk/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.ki/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sn/url?q=http%3A%2F%2Fspo337.com%2F
https://cse.google.sr/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.so/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.cl/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.sc/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.iq/url?q=http%3A%2F%2Fspo337.com%2F
https://www.semanticjuice.com/site/http%3A%2F%2Fspo337.com%2F
https://cse.google.kz/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.gy/url?q=http%3A%2F%2Fspo337.com%2F
https://s79457.gridserver.com/?URL=http%3A%2F%2Fspo337.com%2F
https://cdl.su/redirect?url=http%3A%2F%2Fspo337.com%2F
https://www.fondbtvrtkovic.hr/?URL=http%3A%2F%2Fspo337.com%2F
https://lostnationarchery.com/?URL=http%3A%2F%2Fspo337.com%2F
https://www.booktrix.com/live/?URL=http%3A%2F%2Fspo337.com%2F
https://www.google.ro/url?q=http%3A%2F%2Fspo337.com%2F
https://www.google.tm/url?q=http%3A%2F%2Fspo337.com%2F
https://www.marcellusmatters.psu.edu/?URL=http%3A%2F%2Fspo337.com%2F
https://onlinesportsbettingstory.blogspot.com/2024/01/asian-handicap-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-creates-absurd-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/01/fantasy-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/connecticut-considering-new-advertisement-club-and-sports-wagering-bill
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-profit.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-official-talks-about-state-s-games-wagering-bill-and-future-of
https://bestsportsbettingarticles.blogspot.com/2024/01/consideration-sports-betting-best-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/why-sports-expectation-markets-are-like-onions-and-film-industry-film-receipts
https://bettingstratigicarticle.blogspot.com/2024/01/technology-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/iowa-bill-to-authorize-sports-wagering-advances-subtleties-need-pressing
https://sportswageringinfo.blogspot.com/2024/01/future-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://gameswageringarticles.blogspot.com/2024/01/blog-post_31.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-lawful-games-wagering-regulation-trend
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-pro-level.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-bankroll-the-executives-benefits-victors-and-discipline-1623a8ef-e6da-4dd6-bedb-5596e78ec1af
https://sportsbettingindustry.blogspot.com/2024/01/game-changing-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/sports-wagering-nfl-lines-chances-point-spreads-and-then-some
https://articleinsportsbetting.blogspot.com/2024/01/optimal-wins-sports-betting-.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-jersey-representative-pallone-submits-amicus-brief-in-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/best-odds-earnings-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/american-gaming-affiliation-records-brief-in-sports-wagering-case-in-high-court
https://bettingstratigicarticle.blogspot.com/2024/02/essential-tips-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/n-j-representative-leonard-spear-on-sports-wagering-jersey-merits
https://sportswageringinfo.blogspot.com/2024/02/sports-wagering-triumph.html
https://lucky-razi-777.mystrikingly.com/blog/7-of-the-greatest-possible-advantages-of-lawful-managed-sports-wagering
https://gameswageringarticles.blogspot.com/2024/02/games-wagering-victory-tips.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettor-at-focus-of-alabama-baseball-embarrassment-has-to-deal-with-upwards-of
https://onlinesportsbettingstory.blogspot.com/2024/02/mastering-online-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-sports-wagering-bill-starts-development-through-senate
https://sportsbettingindustry.blogspot.com/2024/02/successful-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/looking-for-bettors-and-administrators-for-wide-arriving-at-industry-reviews
https://articleinsportsbetting.blogspot.com/2024/02/savvy-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bestsportsbettingarticles.blogspot.com/2024/02/winning-streak-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/something-like-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups-f8de9e02-c6a6-495d-9ec9-14a31daf9d51
https://onlinesportsbettingstory.blogspot.com/2024/02/changing-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportsbettingindustry.blogspot.com/2024/02/mastering-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/rocha-california-drive-will-assist-harm-the-brand-of-portable-games-wagering
https://articleinsportsbetting.blogspot.com/2024/02/mastering-revolutionary-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/is-america-crummy-with-awful-games-bettors
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tip-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-s-three-portable-sportsbooks-all-set-with-send-off-advancements
https://bettingstratigicarticle.blogspot.com/2024/02/expert-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/media-note-pad-where-might-the-telecom-companies-be-without-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/successful-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/yearlings-texans-same-game-parlay-expects-pass-cheerful-undertaking
https://gameswageringarticles.blogspot.com/2024/02/secret-success-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maturing-superteams-among-wagering-top-choices-to-bring-home-nba-championship
https://onlinesportsbettingstory.blogspot.com/2024/02/proven-winnings-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/climate-abbreviated-pga-visit-occasion-sparkles-sports-wagering-discussion
https://sportsbettingindustry.blogspot.com/2024/02/mastering%20strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/an-hour-endeavors-to-handle-sports-wagering-thrashes-about-all-things-being
https://articleinsportsbetting.blogspot.com/2024/02/art-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ncaa-solicitations-restriction-on-school-player-prop-wagers-in-ohio
https://bestsportsbettingarticles.blogspot.com/2024/02/unbeatable-sports%20betting-tips.html
https://site-6861241-1631-6047.mystrikingly.com/blog/liv-golf-wagering-markets-presented-in-additional-states-for-2024-season
https://bettingstratigicarticle.blogspot.com/2024/02/profit-expert-betting-stratigic.html
https://daddys-lucky000.mystrikingly.com/blog/mississippi-online-games-wagering-bill-clears-house-missouri-bill-escapes-panel
https://sportswageringinfo.blogspot.com/2024/02/Winning-Sports-Wagering.html
https://lucky-razi-777.mystrikingly.com/blog/swift-at-the-super-bowl-can-t-wager-on-it
https://gameswageringarticles.blogspot.com/2024/02/strategies-excel-ports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-betr-getting-ready-to-leave-massachusetts-market
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/record-promotion-offers-aided-lift-pennsylvanians-wagering-the-month-before
https://sportsbettingindustry.blogspot.com/2024/02/guide-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/lexi-thompson-thought-about-a-major-wagering-longshot-in-vegas-pga-visit
https://articleinsportsbetting.blogspot.com/2024/02/successful-guide-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-controllers-acclimate-to-new-difficulties-in-betting-business
https://bestsportsbettingarticles.blogspot.com/2024/02/psychology-winning-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/greatest-administrators-amazed-to-catch-wind-of-potential-california-sports
https://bettingstratigicarticle.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-remains-school-football-wagering-number-one-after-flimsy-september
https://sportswageringinfo.blogspot.com/2024/02/proven-tactics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/fanduel-keeps-on-ruling-virginia-sports-betting-scene
https://gameswageringarticles.blogspot.com/2024/02/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/travis-kelce-prop-wagering-detonates-as-taylor-swift-gives-a-shout-out-to-him
https://onlinesportsbettingstory.blogspot.com/2024/02/tips-win-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-dark-horse-dream-asset-will-support-dependable-betting-advancement
https://sportsbettingindustry.blogspot.com/2024/02/strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-bet-is-a-lock-to-send-off-by-this-date
https://articleinsportsbetting.blogspot.com/2024/02/mastering-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-week-5-mahomes-slides-bettors-cry-coolio-rides-wan-dale-skims
https://bestsportsbettingarticles.blogspot.com/2024/02/best-sports-wagering-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ncaa-hopes-to-return-to-sports-betting-infringement-discipline-framework
https://bettingstratigicarticle.blogspot.com/2024/02/sports-wagering-skills.html
https://daddys-lucky000.mystrikingly.com/blog/finally-around-is-prepared-to-play-in-illinois
https://onlinesportsbettingstory.blogspot.com/2024/02/%20strategies-sports-wagering-triumph.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-controllers-look-for-proactive-reaction-to-digital-hacks-at
https://sportsbettingindustry.blogspot.com/2024/02/expert-strategies-sports-wagering.html
https://casino999.mystrikingly.com/blog/caesars-enters-developing-microbetting-business-sector-with-simplebet
https://bestsportsbettingarticles.blogspot.com/2024/02/crushing-online-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/bally-bet-wynnbet-granted-maryland-versatile-wagering-licenses
https://bettingstratigicarticle.blogspot.com/2024/02/online-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/wynnbet-gets-massachusetts-computerized-sports-wagering-permit
https://sportswageringinfo.blogspot.com/2024/02/dominate-online-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-once-more-sets-month-to-month-sports-betting-income-record
https://gameswageringarticles.blogspot.com/2024/02/sports-betting-revealed.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettors-bet-more-than-200-million-in-maryland-in-november
https://onlinesportsbettingstory.blogspot.com/2024/02/online-sports-betting-hacks.html
https://site-6828921-2394-9309.mystrikingly.com/blog/fan-commitment-media-organizations-will-drive-sports-wagering-dealmaking
https://sportsbettingindustry.blogspot.com/2024/02/thrills-online-sports-betting.html
https://casino999.mystrikingly.com/blog/return-of-enormous-ten-football-gives-knock-to-sportsbooks
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-strategies-pay-off.html
https://site-4763676-9520-5980.mystrikingly.com/blog/development-in-us-helps-pad-william-slope-s-q3-decrease-in-incomes
https://bestsportsbettingarticles.blogspot.com/2024/02/best-online-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/d-c-s-first-free-sportsbook-preparing-for-opening-as-neighbors-stand-up-to
https://bettingstratigicarticle.blogspot.com/2024/02/successful-online-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/star-card-sharks-blame-nv-bookmaker-for-undermining-in-game-wagers
https://sportswageringinfo.blogspot.com/2024/02/sports-betting-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/the-week-in-sports-wagering-record-breaking-month-to-month-750m-new-jersey
https://gameswageringarticles.blogspot.com/2024/02/profits-online-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/turner-sports-makes-fanduel-official-games-wagering-accomplice-for-nba
https://onlinesportsbettingstory.blogspot.com/2024/02/skill-meets-fortune-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-gambling-clubs-planned-to-return-july-1
https://sportsbettingindustry.blogspot.com/2024/02/online-sports-betting-pros.html
https://casino999.mystrikingly.com/blog/gold-silver-and-betting-how-colorado-s-gaming-towns-became
https://articleinsportsbetting.blogspot.com/2024/02/online-sports-betting-hobby.html
https://site-4763676-9520-5980.mystrikingly.com/blog/arlington-park-itha-at-long-last-have-arrangement-for-horse-racing-in-illinois
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tips-every-sports-bettor%20.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-moving-again-in-georgia
https://bettingstratigicarticle.blogspot.com/2024/02/guide-safe-responsible-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://sportswageringinfo.blogspot.com/2024/02/safety-sports-betting-online.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-survey-expansive-help-for-club-gaming-not-such-a-huge-amount-for
https://gameswageringarticles.blogspot.com/2024/02/secure-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sporttrade-is-a-genuine-games-wagering-roller-coaster
https://onlinesportsbettingstory.blogspot.com/2024/02/%20online-sports-betting-enthusiasts.html
https://site-6828921-2394-9309.mystrikingly.com/blog/everything-that-bet365-s-colorado-send-off-says-to-us-about-the
https://sportsbettingindustry.blogspot.com/2024/02/tips-secure-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration
https://articleinsportsbetting.blogspot.com/2024/02/priority-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-unquestionable
https://bestsportsbettingarticles.blogspot.com/2024/02/safely-world-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/regardless-of-udoka-suspension-celtics-remain-title-top-choices-all-things
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-wins.html
https://daddys-lucky000.mystrikingly.com/blog/maryland-posts-sports-wagering-handle-of-32-5m-in-january
https://onlinesportsbettingstory.blogspot.com/2024/02/enjoying-online-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nevada-controllers-caution-sportsbooks-that-detailing-postpones-won-t-go-on
https://sportsbettingindustry.blogspot.com/2024/02/safety-measures-sports-betting.html
https://casino999.mystrikingly.com/blog/college-of-memphis-handles-issue-betting-exploration-a8c987a8-5921-4dd4-8953-8ddc50b1486a
https://articleinsportsbetting.blogspot.com/2024/02/world-sports-wagering-confidence.html
https://site-4763676-9520-5980.mystrikingly.com/blog/while-wagering-longshot-parlays-line-shopping-is-an-outright-absolute
https://bestsportsbettingarticles.blogspot.com/2024/02/secrets-secure-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/alberta-s-controllers-actually-haggling-with-potential-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/world-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/illinois-bettor-hits-3-million-parlay-thanks-to-late-energize-by-horses
https://sportswageringinfo.blogspot.com/2024/02/finances-well-being-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/betfair-applies-for-sports-betting-permit-at-joined-center
https://gameswageringarticles.blogspot.com/2024/02/security-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/top-sportsbooks-turn-on-prop-27-promotion-system-in-california
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-dominate-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/wynnbet-draws-nearer-to-new-york-send-off
https://sportsbettingindustry.blogspot.com/2024/02/sports-betting-strategies-revealed.html
https://casino999.mystrikingly.com/blog/ohio-gambling-club-control-commission-sets-sports-wagering-application-charges
https://articleinsportsbetting.blogspot.com/2024/02/tips-live-betting-triumphs.html
https://site-4763676-9520-5980.mystrikingly.com/blog/a-bettor-s-manual-for-the-wild-and-weird-at-the-colder-time-of-year-olympics
https://bestsportsbettingarticles.blogspot.com/2024/02/strategies-work-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/month-to-month-stock-watch-m-a-reports-whirl-as-sports-wagering-stocks-stay
https://bettingstratigicarticle.blogspot.com/2024/02/live-sports-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/will-arkansas-versatile-betting-get-february-endorsement
https://sportswageringinfo.blogspot.com/2024/02/live-betting-tactics-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-in-louisiana-gets-off-major-areas-of-strength-for-to
https://gameswageringarticles.blogspot.com/2024/02/strategies-guaranteed-wins-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-plans-to-open-sportsbook-parlor-at-joined-center-in-chicago
https://onlinesportsbettingstory.blogspot.com/2024/02/online-betting-strategies-unleashed.html
https://site-6828921-2394-9309.mystrikingly.com/blog/april-brings-newbie-ohio-its-most-minimal-wagering-action-so-far
https://sportsbettingindustry.blogspot.com/2024/02/live-betting-strategies-exposed.html
https://casino999.mystrikingly.com/blog/issue-betting-subsidizing-authoritatively-cut-from-d-c-spending-plan
https://articleinsportsbetting.blogspot.com/2024/02/strategies-real-time-live-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://bestsportsbettingarticles.blogspot.com/2024/02/sports-betting-strategies-victory.html
https://site-6861241-1631-6047.mystrikingly.com/blog/report-senate-decision-on-north-carolina-portable-wagering-bill-just-around
https://bettingstratigicarticle.blogspot.com/2024/02/live-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/neglecting-to-report-betting-infringement-a-no-ncaa-says
https://sportswageringinfo.blogspot.com/2024/02/live-sports-betting-techniques.html
https://lucky-razi-777.mystrikingly.com/blog/scientists-go-after-making-sense-of-card-sharks-conduct-at-vegas-social-event
https://gameswageringarticles.blogspot.com/2024/02/Live%20Betting%20Strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/zensports-to-offer-money-back-remunerations-to-tennessee-games-bettors
https://onlinesportsbettingstory.blogspot.com/2024/02/successful-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-keeps-on-kicking-the-tires-on-the-streaming-conflicts
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting-5-simple-steps.html
https://casino999.mystrikingly.com/blog/charge-documented-to-give-each-arizona-clan-an-occasion-betting-permit
https://articleinsportsbetting.blogspot.com/2024/02/blog-post.html
https://site-4763676-9520-5980.mystrikingly.com/blog/perceptions-from-a-visit-to-betmgm-sportsbook-at-nats-park
https://bestsportsbettingarticles.blogspot.com/2024/02/world-best-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/georgia-partners-hopeful-2022-is-the-year-for-lawful-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/02/complex-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/locationsmart-an-option-in-contrast-to-geocomply-for-ontario-igaming
https://sportswageringinfo.blogspot.com/2024/02/conquering-world-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/illinois-sportsbooks-see-wagering-income-plunge-in-december
https://gameswageringarticles.blogspot.com/2024/02/tactics-sports-betting-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/proline-retail-sports-bettors-baffled-with-chances-disparities
https://onlinesportsbettingstory.blogspot.com/2024/02/succeed-world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-s-betting-bonus-at-last-endorses-5-club-however-go-live-date-muddled
https://sportsbettingindustry.blogspot.com/2024/02/exciting-world-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-club-baffled-by-absence-of-sports-wagering-endorsement
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-smarter.html
https://site-4763676-9520-5980.mystrikingly.com/blog/bclc-reports-single-game-games-wagering-income-is-watching-ontario-market
https://bestsportsbettingarticles.blogspot.com/2024/02/mastering-sports-betting-worldwide.html
https://site-6861241-1631-6047.mystrikingly.com/blog/wash-st-sports-wagering-scientist-talks-bootleg-market-relocation-portable
https://bettingstratigicarticle.blogspot.com/2024/02/sports-betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/a-story-of-three-new-games-wagering-states
https://sportswageringinfo.blogspot.com/2024/02/success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/entain-promotes-strong-tech-stack-with-draftkings-romance-having-blurred
https://onlinesportsbettingstory.blogspot.com/2024/02/strategies-online-sports-betting_01573407901.html
https://site-6828921-2394-9309.mystrikingly.com/blog/florida-high-court-expresses-no-to-taking-seminoles-hard-rock-stage-3e9c4744-c515-4536-a0c8-7bb56cf957b7
https://sportsbettingindustry.blogspot.com/2024/02/world-sports-betting.html
https://casino999.mystrikingly.com/blog/california-ancestral-chief-board-officially-goes-against-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/wisconsin-adolescent-confesses-to-scheme-in-sports-wagering-hacking-case
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/record-u-s-wagering-handle-anticipated-for-las-vegas-excellent-prix
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/draftkings-reports-moderate-parlay-element-coming-soon-to-online-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/proven-strategies-success.html
https://lucky-razi-777.mystrikingly.com/blog/ontario-controller-partners-start-conversations-around-new-promotion-rules
https://gameswageringarticles.blogspot.com/2024/02/foolproof-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncpg-s-new-board-president-a-hero-with-an-arrangement
https://onlinesportsbettingstory.blogspot.com/2024/02/ultimate-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-york-turns-out-to-be-first-state-to-top-2-billion-in-month-to-month-handle
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-professional.html
https://casino999.mystrikingly.com/blog/california-s-clans-open-to-novel-thoughts-however-don-t-have-any-desire-to
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-guaranteed-success.html
https://site-4763676-9520-5980.mystrikingly.com/blog/florida-parimutuels-look-for-court-request-driving-seminoles-to-bring-down
https://bestsportsbettingarticles.blogspot.com/2024/02/Proven-strategies-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-greatest-pr-challenge-espn-faces-when-espn-bet-goes-live
https://bettingstratigicarticle.blogspot.com/2024/02/innovative-betting-strategies.html
https://daddys-lucky000.mystrikingly.com/blog/north-carolina-sports-wagering-board-of-trustees-offers-second-bunch-of-rules
https://sportswageringinfo.blogspot.com/2024/02/transformative-strategies-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/expert-association-organization-wagering-on-carrom-to-take-u-s-by-tempest
https://gameswageringarticles.blogspot.com/2024/02/effective-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/illinois-the-state-with-the-20-million-games-betting-permit-and-no-takers-dbbfc4dc-8a1d-4f1d-96bc-1334337be2c0
https://onlinesportsbettingstory.blogspot.com/2024/02/advanced-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/genius-associations-informing-on-sports-wagering-respectability-may-blow-up
https://sportsbettingindustry.blogspot.com/2024/02/betting-strategies-success.html
https://casino999.mystrikingly.com/blog/a-few-gubernatorial-up-and-comers-take-solid-position-on-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/02/sports-betting-strategies-dominate.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-officials-gradual-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/professional-betting-strategies.html
https://site-6861241-1631-6047.mystrikingly.com/blog/paddy-power-betfair-declares-procurement-of-fanduel
https://bettingstratigicarticle.blogspot.com/2024/02/inside-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/new-york-state-sports-wagering-plans-picture-it-s-a-piece-confounded
https://sportswageringinfo.blogspot.com/2024/02/mastering-betting-strategies.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-and-liquor-a-story-of-two-restrictions
https://onlinesportsbettingstory.blogspot.com/2024/03/betting-strategies-consistent-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-dependable-betting-bill-language-rejected-in-everyday-get-together
https://sportsbettingindustry.blogspot.com/2024/03/revolutionize-betting-game.html
https://casino999.mystrikingly.com/blog/virginia-tops-10-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/03/elevate-betting-game.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-senate-supports-revised-sports-wagering-bill
https://bestsportsbettingarticles.blogspot.com/2024/03/proven-sports-betting-strategies-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maryland-lottery-issues-versatile-betting-permit-to-aficionados-sportsbook
https://bettingstratigicarticle.blogspot.com/2024/03/elevate-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/april-brings-novice-ohio-its-most-minimal-wagering-action-hitherto
https://sportswageringinfo.blogspot.com/2024/03/ultimate-guide-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/issue-betting-financing-formally-cut-from-d-c-spending-plan
https://gameswageringarticles.blogspot.com/2024/03/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/north-carolina-s-versatile-games-wagering-bill-going-to-senate-floor
https://onlinesportsbettingstory.blogspot.com/2024/03/transform-betting-experience.html
https://site-6828921-2394-9309.mystrikingly.com/blog/media-note-pad-kelce-siblings-sound-off-on-individual-nfl-players
https://sportsbettingindustry.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/best-wagering-scenes-getting-stoned-in-the-music-of-possibility
https://articleinsportsbetting.blogspot.com/2024/03/mastering-betting-pro-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ohio-has-turned-into-the-most-intriguing-state-with-regards-to-the-sportsbook
https://bestsportsbettingarticles.blogspot.com/2024/03/betting-strategies-serious-players.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-regulator-approves-latest-sports-wagering-procedures
https://bettingstratigicarticle.blogspot.com/2024/03/Betting%20Strategies%20for%20Victory.html
https://daddys-lucky000.mystrikingly.com/blog/three-new-administrators-could-be-live-in-arizona-by-mid-2024
https://sportswageringinfo.blogspot.com/2024/03/unveiling-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://gameswageringarticles.blogspot.com/2024/03/journey-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/administrators-push-back-on-proposed-betting-methods-in-vermont
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crab-sports-dispatches-wagering-stage-in-maryland-application-not-yet
https://sportsbettingindustry.blogspot.com/2024/03/deep-dive-world-wagering.html
https://casino999.mystrikingly.com/blog/pieces-prosperity-assists-colorado-bettors-with-avoiding-may-pattern-of
https://articleinsportsbetting.blogspot.com/2024/03/journey-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://bestsportsbettingarticles.blogspot.com/2024/03/dollars-world-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/maine-betting-control-boss-back-working-after-neglected-suspension
https://bettingstratigicarticle.blogspot.com/2024/03/wagering-journey-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/is-most-recent-florida-choice-actually-an-ancestral-gaming-major-advantage
https://sportswageringinfo.blogspot.com/2024/03/art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-virtuoso-games-expand-sports-wagering-information-association-through
https://onlinesportsbettingstory.blogspot.com/2024/03/wagering-journey-success.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-first-to-attempt-to-boycott-bettors-who-undermine-competitors
https://sportsbettingindustry.blogspot.com/2024/03/unconventional-wagering-journey.html
https://casino999.mystrikingly.com/blog/sportradar-inks-sports-wagering-information-concurrence-with-south-america-s
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-wizardry-mastering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-virtuoso-games-broaden-sports-wagering-information-organization-through
https://bestsportsbettingarticles.blogspot.com/2024/03/unveiling-wagering-journey.html
https://site-6861241-1631-6047.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://bettingstratigicarticle.blogspot.com/2024/03/navigating-world-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/how-truly-do-cultivate-children-need-to-manage-the-fate-of-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/lessons-wagering-journey.html
https://lucky-razi-777.mystrikingly.com/blog/how-in-all-actuality-do-encourage-children-need-to-manage-the-eventual-fate
https://gameswageringarticles.blogspot.com/2024/03/journey-fortunes-made-lost.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ohio-officials-twofold-games-betting-expense
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-betting-strategies-winning-big.html
https://site-6828921-2394-9309.mystrikingly.com/blog/five-nfl-groups-with-ideal-prospects-chances
https://sportsbettingindustry.blogspot.com/2024/03/blog-post.html
https://casino999.mystrikingly.com/blog/football-challenges-of-old-made-the-present-huge-games-wagering-prominence
https://articleinsportsbetting.blogspot.com/2024/03/sports-wagering-like-champion.html
https://site-4763676-9520-5980.mystrikingly.com/blog/one-more-influx-of-gambling-clubs-apply-to-join-new-jersey-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/tips-excel-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/fanduel-sportsbook-send-off-imprints-another-lawful-games-wagering-achievement
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-controllers-authorities-urge-persistence-to-try-not-to-bumble
https://sportswageringinfo.blogspot.com/2024/03/inner-champion-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/cheerful-commemoration-delaware-sports-wagering-a-champ-one-month-in
https://gameswageringarticles.blogspot.com/2024/03/sports-wagering-strategies-success.html
https://site-6861405-2997-4499.mystrikingly.com/blog/excitement-is-high-as-mississippi-sports-wagering-arrangements-in-progress
https://onlinesportsbettingstory.blogspot.com/2024/03/Navigate-art-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-wagering-lines-problem-what-happens-when-everybody-risks-everything-group
https://sportsbettingindustry.blogspot.com/2024/03/decoding-art-sports-wagering.html
https://casino999.mystrikingly.com/blog/report-ny-will-have-greatest-games-wagering-business-sector-in-u-s
https://articleinsportsbetting.blogspot.com/2024/03/maximizing-odds-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pga-visit-concurs-it-d-be-stupid-to-restrict-portable-wagering-to-on-premises
https://bestsportsbettingarticles.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/kentucky-putting-money-on-sports-wagering-to-assist-with-fixing-annuity-issue
https://sportswageringinfo.blogspot.com/2024/03/dominate-art-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/legitimate-games-wagering-now-regulation-in-rhode-island-and-state-gets-51
https://gameswageringarticles.blogspot.com/2024/03/comprehensive-guide-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-nearby-according-to-an-understudy-s-point-of-view
https://onlinesportsbettingstory.blogspot.com/2024/03/sports-wagering-guaranteed-wins.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pittsburgh-privateers-take-sports-wagering-trustworthiness-expense-to
https://sportsbettingindustry.blogspot.com/2024/03/game-changing-strategy-sports-betting.html
https://casino999.mystrikingly.com/blog/greatest-chances-movers-after-first-rounds-of-world-cup
https://articleinsportsbetting.blogspot.com/2024/03/Winwinning-strategies-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/monitoring-west-virginia-s-games-wagering-plans-and-send-off
https://bestsportsbettingarticles.blogspot.com/2024/03/art-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/oregon-lottery-preparing-portable-application-with-versatile-games-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/level-up-betting-game.html
https://daddys-lucky000.mystrikingly.com/blog/rhode-island-makes-stride-nearer-to-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/03/excel-sports-wagering-like-pro.html
https://lucky-razi-777.mystrikingly.com/blog/seriously-fighting-yet-no-democratic-in-new-york-state
https://gameswageringarticles.blogspot.com/2024/03/psychological-tricks-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/choctaw-intend-to-be-first-in-quite-a-while-to-offer-games-wagering
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/murphy-makes-sports-wagering-lawful-in-new-jersey
https://sportsbettingindustry.blogspot.com/2024/03/key-success-sports-betting.html
https://casino999.mystrikingly.com/blog/stage-set-for-decision-on-legitimate-new-york-sports-wagering
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/why-is-murphy-delayed-to-make-nj-sports-wagering-regulation
https://bestsportsbettingarticles.blogspot.com/2024/03/mental-aspect-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/at-any-rate-so-what-in-blazes-is-uprightness-checking
https://bettingstratigicarticle.blogspot.com/2024/03/power-mind-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/lead-representative-wagers-on-phillies-to-start-up-lawful-delaware-sports
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-mental-mastery.html
https://lucky-razi-777.mystrikingly.com/blog/mailbag-mythbusters-uigea-and-sports-wagering-made-sense
https://gameswageringarticles.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/massachusetts-preparing-to-make-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/03/strategies-success-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/nfl-divisional-round-weekend-sportsbooks-say-victories-are-coming
https://sportsbettingindustry.blogspot.com/2024/03/successful-sports-betting.html
https://casino999.mystrikingly.com/blog/pennsylvania-closures-notable-year-with-record-month-to-month-sports-betting
https://articleinsportsbetting.blogspot.com/2024/03/mind-game-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/somewhere-around-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bestsportsbettingarticles.blogspot.com/2024/03/Comprehensiveuide-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting-success.html
https://daddys-lucky000.mystrikingly.com/blog/versatile-games-betting-authoritatively-dispatches-in-vermont
https://sportswageringinfo.blogspot.com/2024/03/strategy-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/georgia-administrator-once-again-introduces-sports-wagering-bill
https://gameswageringarticles.blogspot.com/2024/03/mastering-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-sharps-activity-giving-prime-games-looked-for-in-ohio
https://onlinesportsbettingstory.blogspot.com/2024/03/world-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maryland-legislators-might-put-destiny-of-sports-wagering-in-citizens-grasp
https://sportsbettingindustry.blogspot.com/2024/03/blog-post_18.html
https://casino999.mystrikingly.com/blog/draftkings-perspectives-sports-wagering-as-tremendous-learning-experience
https://articleinsportsbetting.blogspot.com/2024/03/mental-side-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/kansas-legislators-get-educated-on-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-mind-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-seeking-after-different-roads-to-sanction-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-mindset-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/could-face-sports-book-become-a-thing
https://sportswageringinfo.blogspot.com/2024/03/mastering-mind-game-betting.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-lead-representative-expresses-associations-a-desire-for-peace
https://gameswageringarticles.blogspot.com/2024/03/mastering-psychology-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-bill-russell-denounces-any-kind-of-authority-on-miami
https://onlinesportsbettingstory.blogspot.com/2024/03/mind-game-sports-betting_01715168387.html
https://site-6828921-2394-9309.mystrikingly.com/blog/high-court-strikes-down-government-sports-wagering-boycott-opening-entryway
https://sportsbettingindustry.blogspot.com/2024/03/strategies-betting-success.html
https://casino999.mystrikingly.com/blog/ancestral-worries-supposedly-easing-back-michigan-on-sports-wagering-regulation
https://articleinsportsbetting.blogspot.com/2024/03/mastering-mental-game-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-fiend-examines-strife-recuperation
https://bestsportsbettingarticles.blogspot.com/2024/03/secrets-strategic-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-tangle
https://bettingstratigicarticle.blogspot.com/2024/03/expert-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/the-most-effective-method-to-explore-the-games-wagering-data-and-promote
https://sportswageringinfo.blogspot.com/2024/03/key-success-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/connecticut-bill-escapes-council-yet-clans-in-conflict-with-state
https://gameswageringarticles.blogspot.com/2024/03/winning-mindset-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/a-terrible-bet-slighting-sanctioned-betting-s-expected-new-clients
https://onlinesportsbettingstory.blogspot.com/2024/03/mastering-mind-game-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/monetary-examination-the-amount-will-connecticut-advantage-from-sports
https://sportsbettingindustry.blogspot.com/2024/03/mastery-sports-betting-success.html
https://casino999.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://articleinsportsbetting.blogspot.com/2024/03/sports-bet-wins-redefine-strategy.html
https://site-4763676-9520-5980.mystrikingly.com/blog/what-precisely-is-a-games-wagering-promote
https://bestsportsbettingarticles.blogspot.com/2024/03/ultimate-guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/possibilities-obscure-for-entry-of-connecticut-sports-wagering-bill
https://bettingstratigicarticle.blogspot.com/2024/03/mastering-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/how-a-sex-embarrassment-is-easing-back-missouri-s-capacity-to-legitimize
https://sportswageringinfo.blogspot.com/2024/03/dark-side-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/players-associations-stake-guarantee-for-cut-of-sports-wagering-pie-now-as-well
https://onlinesportsbettingstory.blogspot.com/2024/03/winning-sports-bets-every-time.html
https://site-6828921-2394-9309.mystrikingly.com/blog/massachusetts-deliveries-extensive-survey-of-state-s-games-wagering-an
https://sportsbettingindustry.blogspot.com/2024/03/psychology-behind-successful-sports-betting.html
https://casino999.mystrikingly.com/blog/the-wire-demonstration-of-1961-when-the-web-met-the-bedrock-government
https://articleinsportsbetting.blogspot.com/2024/03/advanced-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-not-keen-on-paying-associations-a-games-wagering-sovereignty
https://bestsportsbettingarticles.blogspot.com/2024/03/beginners-guide-making-money-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/laissez-les-bons-temps-rouler-louisiana-opens-sports-wagering-discussion
https://bettingstratigicarticle.blogspot.com/2024/03/revolutionizing-world-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/connecticut-dems-squeezing-for-legitimized-sports-wagering
https://sportswageringinfo.blogspot.com/2024/03/sports-betting-strategies-work.html
https://lucky-razi-777.mystrikingly.com/blog/oklahoma-ancestral-games-wagering-bill-clears-board-heads-to-full-house
https://gameswageringarticles.blogspot.com/2024/03/sports-outcomes-beat-odds.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-jersey-tolerating-sports-wagering-applications-chief-affirms
https://onlinesportsbettingstory.blogspot.com/2024/03/tips-tricks-insider-knowledge-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-prepares-crazy-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/03/art-sports-betting-like-champ.html
https://casino999.mystrikingly.com/blog/west-virginia-official-examines-state-s-games-wagering-bill-and-future-of
https://articleinsportsbetting.blogspot.com/2024/03/biggest-sports-bet-wins.html
https://site-4763676-9520-5980.mystrikingly.com/blog/iowa-bill-to-legitimize-sports-wagering-advances-subtleties-need-pressing
https://bestsportsbettingarticles.blogspot.com/2024/03/mastering-art-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/charge-presented-in-missouri-pointed-toward-sanctioning-games-wagering-in-state
https://bettingstratigicarticle.blogspot.com/2024/03/blog-post_25.html
https://daddys-lucky000.mystrikingly.com/blog/ball-gets-going-on-iowa-authorization-of-sports-wagering-with-hsb-592
https://sportswageringinfo.blogspot.com/2024/03/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/west-virginia-sports-wagering-bill-would-allow-betting-at-five-club-and-on
https://gameswageringarticles.blogspot.com/2024/03/enhance-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-legitimate-games-wagering-regulation-temporary-fad
https://onlinesportsbettingstory.blogspot.com/2024/03/sharpening-your-skills-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-junkie-talks-about-disturbance-recuperation
https://sportsbettingindustry.blogspot.com/2024/03/level-up-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/illinois-sports-wagering-regulation-hits-obstacle
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-4763676-9520-5980.mystrikingly.com/blog/instructions-to-explore-the-games-wagering-data-and-promote-industry
https://bestsportsbettingarticles.blogspot.com/2024/04/advanced-sports-betting-triumph.html
https://site-6861241-1631-6047.mystrikingly.com/blog/in-the-event-that-sports-wagering-made-lawful-mississippi-intends-to
https://bettingstratigicarticle.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/a-terrible-bet-disregarding-legitimized-betting-s-expected-new-clients
https://sportswageringinfo.blogspot.com/2024/04/improving-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/monetary-investigation-the-amount-will-connecticut-advantage-from-sports
https://gameswageringarticles.blogspot.com/2024/04/refining-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/status-of-sports-wagering-bills-around-the-country-as-meetings-wind-down
https://onlinesportsbettingstory.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/maine-senate-supersedes-sports-wagering-blackball-sets-state-on-way-for
https://sportsbettingindustry.blogspot.com/2024/04/supercharge-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/wading-into-controversy-dialing-back-kentucky-games-wagering-vote
https://articleinsportsbetting.blogspot.com/2024/04/improving-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nonattendances-defer-maine-blackball-supersede-decision-on-legitimate-games
https://bestsportsbettingarticles.blogspot.com/2024/04/enhance-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/colorado-inches-nearer-to-may-sports-wagering-send-off-notwithstanding
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/in-the-midst-of-epic-u-s-sports-wagering-turf-war-penn-public-plants
https://sportswageringinfo.blogspot.com/2024/04/secrets-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/sports-betting-principles-among-plan-things-for-illinois-gaming-executive
https://gameswageringarticles.blogspot.com/2024/04/elevate-skills-rookie-pro.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maine-lead-representative-denials-sports-wagering-regulation
https://onlinesportsbettingstory.blogspot.com/2024/04/essential-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/report-ncaa-has-found-175-games-betting-infringement-beginning-around-2018
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-skills-today.html
https://casino999.mystrikingly.com/blog/new-york-crosses-25-billion-in-all-time-sports-betting-handle
https://articleinsportsbetting.blogspot.com/2024/04/elevate-sports-betting-skills.html
https://site-4763676-9520-5980.mystrikingly.com/blog/mlb-rule-changes-lead-to-additional-wagering-on-taken-bases
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sportsbetting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/concentrate-on-shows-huge-flood-in-wagering-among-ladies-on-ladies-games
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-skills-unveiled.html
https://daddys-lucky000.mystrikingly.com/blog/crisis-kentucky-games-wagering-guidelines-preclude-deluding-publicizing
https://sportswageringinfo.blogspot.com/2024/04/sharpen-sports-betting-skills.html
https://lucky-razi-777.mystrikingly.com/blog/three-occasion-betting-licenses-destined-to-be-available-for-anyone-in-arizona
https://gameswageringarticles.blogspot.com/2024/04/strategies-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/seminoles-will-not-have-the-option-to-send-off-sports-betting-in-florida
https://onlinesportsbettingstory.blogspot.com/2024/04/improve-sports-betting-skills.html
https://site-6828921-2394-9309.mystrikingly.com/blog/hold-rate-for-new-york-sports-betting-remaining-parts-stuck-on-high
https://sportsbettingindustry.blogspot.com/2024/04/essential-sports-betting-skills.html
https://casino999.mystrikingly.com/blog/most-recent-surveying-recommends-backing-for-california-betting-drives
https://articleinsportsbetting.blogspot.com/2024/04/enhance-sports-betting-skills-now.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-gaming-commission-sitting-tight-for-public-remarks
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861241-1631-6047.mystrikingly.com/blog/elys-game-innovation-uses-d-c-as-retail-sports-betting-model
https://bettingstratigicarticle.blogspot.com/2024/04/polishing-sports-betting-skills.html
https://daddys-lucky000.mystrikingly.com/blog/capitalizing-on-changing-out-bookmaker-dishes-on-money-out-apparatus
https://sportswageringinfo.blogspot.com/2024/04/mastering-sports-betting-pro.html
https://lucky-razi-777.mystrikingly.com/blog/maryland-lottery-chief-offers-versatile-send-off-course-of-events-subtleties
https://gameswageringarticles.blogspot.com/2024/04/master-sports-betting-5-steps.html
https://site-6861405-2997-4499.mystrikingly.com/blog/best-wagering-scenes-in-possessing-mahowny-the-activity-is-the-juice
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-insider-tips.html
https://site-6828921-2394-9309.mystrikingly.com/blog/oregon-lottery-carries-out-application-sports-wagering-coming-the-following
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://casino999.mystrikingly.com/blog/sports-wagering-and-wounds-in-school-football-isolating-great-data-from-clamor
https://articleinsportsbetting.blogspot.com/2024/04/mastering-art-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/elite-athletics-associations-proceed-with-full-court-push-on-west-virginia
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-wins.html
https://site-6861241-1631-6047.mystrikingly.com/blog/where-the-midwest-is-at-on-sports-wagering-regulation
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/aga-to-schumer-sports-wagering-doesn-t-need-government-oversight
https://sportswageringinfo.blogspot.com/2024/04/mastering-science-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/arkansas-gaming-sports-wagering-polling-form-drive-enduring-an-onslaught
https://gameswageringarticles.blogspot.com/2024/04/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-expert-will-congress-engage-with-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/mind-master-sports-bettor.html
https://site-6828921-2394-9309.mystrikingly.com/blog/intelligent-bettors-ought-to-pay-attention-to-the-buzz-prior-to-wagering
https://sportsbettingindustry.blogspot.com/2024/04/sports-betting-management-techniques.html
https://casino999.mystrikingly.com/blog/maine-officials-move-toward-legitimate-games-wagering
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-analytics.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-s-conflict-over-betting-gets-more-sizzling
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-success.html
https://site-6861241-1631-6047.mystrikingly.com/blog/tennessee-posts-370-million-games-wagering-handle-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://daddys-lucky000.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://sportswageringinfo.blogspot.com/2024/04/master-sports-betting-intelligence.html
https://lucky-razi-777.mystrikingly.com/blog/american-gaming-affiliation-sports-bettors-need-to-cooperate
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-strategies.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-bill-bites-the-dust-in-panel-on-conclusive-day-of-kentucky
https://onlinesportsbettingstory.blogspot.com/2024/04/master-sports-betting-like-pro.html
https://site-6828921-2394-9309.mystrikingly.com/blog/kentucky-crisis-sports-wagering-regs-could-make-worry-among-administrators
https://sportsbettingindustry.blogspot.com/2024/04/mastering-art-sports-betting.html
https://casino999.mystrikingly.com/blog/maryland-report-records-conceivable-dependable-betting-upgrades
https://articleinsportsbetting.blogspot.com/2024/04/journey-sports-betting-succes.html
https://site-4763676-9520-5980.mystrikingly.com/blog/connecticut-draws-nearer-to-inking-manage-new-games-betting-accomplice
https://bestsportsbettingarticles.blogspot.com/2024/04/mastering-sports-betting-techniques.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-controller-endorses-most-recent-games-betting-methods
https://bettingstratigicarticle.blogspot.com/2024/04/become-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-scarcely-misses-10-hold-for-june-sports-betting
https://sportswageringinfo.blogspot.com/2024/04/tips-sports-betting-success.html
https://lucky-razi-777.mystrikingly.com/blog/concentrate-on-shows-critical-flood-in-wagering-among-ladies-on-ladies-games
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-maximum-returns.html
https://site-6861405-2997-4499.mystrikingly.com/blog/meet-north-carolina-s-games-wagering-administrative-commission
https://onlinesportsbettingstory.blogspot.com/2024/04/mastering-sports-betting-trends.html
https://site-6828921-2394-9309.mystrikingly.com/blog/is-vermont-getting-ready-to-consider-sanctioning-games-wagering
https://sportsbettingindustry.blogspot.com/2024/04/mastering-sports-betting-psychology.html
https://casino999.mystrikingly.com/blog/espn-refreshing-growing-focusing-on-sports-betting-substance
https://articleinsportsbetting.blogspot.com/2024/04/mastering-sports-betting-strategies_01879442689.html
https://site-4763676-9520-5980.mystrikingly.com/blog/survey-says-most-ohioans-don-t-want-to-bet-on-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/guide-mastering-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pointsbet-plans-to-handle-wagers-in-less-than-a-second-as-shift-to-in-game
https://bettingstratigicarticle.blogspot.com/2024/04/strategies-master-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/online-sportsbooks-are-playing-get-up-to-speed-with-regards-to-ada-consistence
https://sportswageringinfo.blogspot.com/2024/04/becoming-master-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/bally-s-applies-to-offer-versatile-games-betting-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/mastering-sports-betting-skills.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesotans-aren-t-in-with-no-reservations-on-sports-wagering
https://onlinesportsbettingstory.blogspot.com/2024/04/future-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/yasiel-puig-pulls-out-blameworthy-request-in-unlawful-games-wagering-case
https://sportsbettingindustry.blogspot.com/2024/04/revolutionizing-sports-betting.html
https://casino999.mystrikingly.com/blog/espn-affirms-postpone-in-sports-wagering-send-off-as-iger-behaviors-key-audit
https://articleinsportsbetting.blogspot.com/2024/04/top-sports-betting-trends-2024.html
https://site-4763676-9520-5980.mystrikingly.com/blog/will-sports-wagering-twitter-endure-musk-s-fart-mode-second
https://bestsportsbettingarticles.blogspot.com/2024/04/inside-world-in-play-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/massachusetts-gaming-commission-consents-to-acknowledge-mgm-s-late-application
https://bettingstratigicarticle.blogspot.com/2024/04/rise-mobile-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/massachusetts-computerized-betting-authorizing-contest-not-really-solid
https://sportswageringinfo.blogspot.com/2024/04/data-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-times-fiercely-comes-up-short-on-sports-wagering-story
https://gameswageringarticles.blogspot.com/2024/04/blog-post_12.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-might-ftx-s-crash-affect-crypto-in-sports-wagering-5cbefb65-9344-4f22-bb25-1dcf520d525a
https://onlinesportsbettingstory.blogspot.com/2024/04/AI-transforming-sports-betting-strategies.html
https://site-6828921-2394-9309.mystrikingly.com/blog/versatile-sportsbooks-will-go-live-in-maryland-on-wednesday-lottery-reports
https://sportsbettingindustry.blogspot.com/2024/04/traditional-sports-betting.html
https://casino999.mystrikingly.com/blog/california-betting-drives-made-the-most-awful-sort-of-history
https://articleinsportsbetting.blogspot.com/2024/04/deep-dive-prop-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://bestsportsbettingarticles.blogspot.com/2024/04/popularity-virtual-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-wrongfully-on-sports
https://bettingstratigicarticle.blogspot.com/2024/04/sports-betting-beginners.html
https://daddys-lucky000.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://sportswageringinfo.blogspot.com/2024/04/blog-post.html
https://lucky-razi-777.mystrikingly.com/blog/california-betting-drives-made-the-most-obviously-awful-sort-of-history
https://gameswageringarticles.blogspot.com/2024/04/blockchain-sports-betting-security.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-york-outclasses-own-u-s-month-to-month-income-record-for-october
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-responsible-gaming.html
https://site-6828921-2394-9309.mystrikingly.com/blog/could-yasiel-puig-play-in-mlb-again-subsequent-to-wagering-unlawfully-on-sports
https://sportsbettingindustry.blogspot.com/2024/04/trends-live-betting.html
https://casino999.mystrikingly.com/blog/one-bet-costs-illinois-month-to-month-state-sports-betting-income-record
https://articleinsportsbetting.blogspot.com/2024/04/dynamic-world-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/maryland-portable-send-off-unavoidable-as-betting-commission-grants-10-licenses
https://bestsportsbettingarticles.blogspot.com/2024/04/cross-section-sports-betting-social-media.html
https://site-6861241-1631-6047.mystrikingly.com/blog/caesars-offering-100-in-credits-in-front-of-maryland-sports-wagering-send-off
https://bettingstratigicarticle.blogspot.com/2024/04/latest-mobile-betting-apps.html
https://daddys-lucky000.mystrikingly.com/blog/texas-representative-acquaints-goal-with-permit-sports-betting-club-betting
https://sportswageringinfo.blogspot.com/2024/04/predictive-analytics-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/holey-strokes-little-golf-betting-endorsed-in-colorado-wyoming
https://gameswageringarticles.blogspot.com/2024/04/sports-betting-regulations.html
https://site-6861405-2997-4499.mystrikingly.com/blog/one-day-on-one-free-day-plan-for-maryland-send-off-certain-to-befuddle-bettors
https://onlinesportsbettingstory.blogspot.com/2024/04/sports-betting-markets-rise.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sportsbook-celebrity-projects-ain-t-what-they-used-to-be-bettors-say
https://sportsbettingindustry.blogspot.com/2024/04/leverage-data-smart-betting-decisions.html
https://casino999.mystrikingly.com/blog/month-to-month-stock-watch-sports-wagering-stocks-keep-afloat-in-front-of
https://articleinsportsbetting.blogspot.com/2024/04/popularity-niche-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/not-dead-yet-sports-wagering-restored-in-missouri
https://bestsportsbettingarticles.blogspot.com/2024/04/influence-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/ohio-sports-betting-application-window-opens-june-15
https://bettingstratigicarticle.blogspot.com/2024/04/live-streaming-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/the-usfl-needs-to-embrace-sports-bettors-and-dfs-players-right-away
https://sportswageringinfo.blogspot.com/2024/04/sports-betting-trends-social-media.html
https://lucky-razi-777.mystrikingly.com/blog/best-games-wagering-scenes-riding-the-borail-with-mine-that-bird-in-50-to-1
https://gameswageringarticles.blogspot.com/2024/04/tips-sports-betting-beginners.html
https://site-6861405-2997-4499.mystrikingly.com/blog/arizona-sports-wagering-handle-falls-barely-short-of-500m-for-december
https://onlinesportsbettingstory.blogspot.com/2024/04/evolution-parlay-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pennsylvania-posts-800-million-games-wagering-handle-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/mind-betting-rofessional.html
https://casino999.mystrikingly.com/blog/nba-hands-lifetime-boycott-to-jontay-watchman-for-conspicuous-betting
https://articleinsportsbetting.blogspot.com/2024/04/think-like-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/draftkings-and-enthusiasts-go-head-to-head-in-court-over-leader-abandonment
https://bestsportsbettingarticles.blogspot.com/2024/04/journey-becoming-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-changing-game.html
https://daddys-lucky000.mystrikingly.com/blog/espn-unlv-declare-beneficiaries-of-first-dependable-betting-partnerships
https://sportswageringinfo.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://gameswageringarticles.blogspot.com/2024/04/from-hobbyist-betting-professional.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-decrees-bettors-need-to-follow-for-capable-parlay-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/secrets-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-sports-wagering-handle-aggregates-1-07-billion-for-february
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-playbook.html
https://casino999.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-sanctioning-endeavors-fizzled
https://articleinsportsbetting.blogspot.com/2024/04/insights-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-bettors-bound-to-knock-back-the-firewater-than-different-players
https://bestsportsbettingarticles.blogspot.com/2024/04/evolution-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/iowa-south-carolina-establishes-ladies-games-wagering-standards
https://bettingstratigicarticle.blogspot.com/2024/04/true-betting-professional.html
https://daddys-lucky000.mystrikingly.com/blog/caitlin-clark-previously-carrying-gigantic-wagering-interest-to-the-wnba
https://sportswageringinfo.blogspot.com/2024/04/world-inside-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/sports-wagering-bill-spotlights-on-issue-betting-third-minnesota
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-vs-casual-bettors.html
https://site-6861405-2997-4499.mystrikingly.com/blog/minnesota-sports-wagering-sanctioning-discussion-warms-up-in-house-advisory
https://onlinesportsbettingstory.blogspot.com/2024/04/psychology-behind-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/draftkings-recruits-lori-kalani-as-first-boss-dependable-gaming-official
https://sportsbettingindustry.blogspot.com/2024/04/winning-like-betting-professional.html
https://casino999.mystrikingly.com/blog/more-secure-betting-conversation-addresses-late-embarrassments
https://articleinsportsbetting.blogspot.com/2024/04/consistent-success-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ontario-sportsbooks-not-permitted-to-take-wagers-on-wba-boxing
https://bestsportsbettingarticles.blogspot.com/2024/04/truth-behind-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/missouri-sports-wagering-polling-form-drive-outperforms-300-000-marks
https://bettingstratigicarticle.blogspot.com/2024/04/blog-post.html
https://daddys-lucky000.mystrikingly.com/blog/washington-d-c-sports-wagering-handle-under-16-million-for-spring
https://sportswageringinfo.blogspot.com/2024/04/life-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-sports-wagering-handle-tops-497-million-for-spring
https://onlinesportsbettingstory.blogspot.com/2024/04/tools-resources-betting-professionals.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-jersey-sports-wagering-handle-tops-1-3-billion-for-spring
https://sportsbettingindustry.blogspot.com/2024/04/transitioning-betting-professional.html
https://casino999.mystrikingly.com/blog/north-carolina-wows-with-659-million-games-wagering-handle
https://articleinsportsbetting.blogspot.com/2024/04/financial-realities-professional-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/the-precepts-bettors-need-to-follow-for-dependable-parlay-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/betting-professionals-trends-make-decisions.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-guide.html
https://daddys-lucky000.mystrikingly.com/blog/2024-nba-season-finisher-wagering-review
https://sportswageringinfo.blogspot.com/2024/04/betting-professionals-stay-top.html
https://lucky-razi-777.mystrikingly.com/blog/sources-jontay-doorman-kept-a-celebrity-sports-wagering-record-in-colorado
https://gameswageringarticles.blogspot.com/2024/04/betting-professionals-future.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-becomes-essential-games-wagering-application-in-washington-d-c
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post.html
https://site-6828921-2394-9309.mystrikingly.com/blog/crusade-for-more-pleasant-betting-billions-bet-illicitly-on-ncaa-competitions
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-balancing-luck-skill.html
https://casino999.mystrikingly.com/blog/bally-bet-plans-for-june-sports-wagering-send-off-in-massachusetts
https://articleinsportsbetting.blogspot.com/2024/04/takes-betting-professional.html
https://site-4763676-9520-5980.mystrikingly.com/blog/tennessee-games-wagering-handle-hits-472-million-for-spring
https://bestsportsbettingarticles.blogspot.com/2024/04/ethical-dilemmas-betting-professional.html
https://site-6861241-1631-6047.mystrikingly.com/blog/here-s-to-you-mr-robinson-nate-robinson-dishes-on-the-knicks-sports
https://bettingstratigicarticle.blogspot.com/2024/04/betting-like-pro.html
https://daddys-lucky000.mystrikingly.com/blog/three-reasons-georgia-sports-wagering-authorization-endeavors-fizzled
https://sportswageringinfo.blogspot.com/2024/04/secrets-behind-betting-professional.html
https://lucky-razi-777.mystrikingly.com/blog/sports-bettors-bound-to-hit-the-bottle-hard-than-different-speculators
https://gameswageringarticles.blogspot.com/2024/04/psychology-betting-professionals.html
https://site-6861405-2997-4499.mystrikingly.com/blog/caitlin-clark-previously-carrying-monstrous-wagering-interest-to-the-wnba
https://onlinesportsbettingstory.blogspot.com/2024/04/blog-post_25.html
https://site-6828921-2394-9309.mystrikingly.com/blog/more-modest-games-associations-hustling-for-legitimate-games-wagering-open-doors
https://sportsbettingindustry.blogspot.com/2024/05/betting-change-perspective.html
https://casino999.mystrikingly.com/blog/football-is-top-dog-ms-september-sports-wagering-handle-ranges-31-77-million
https://articleinsportsbetting.blogspot.com/2024/04/betting-blunders-brilliance.html
https://site-4763676-9520-5980.mystrikingly.com/blog/after-lively-hearing-indiana-legislators-will-keep-on-investigating-sports
https://bestsportsbettingarticles.blogspot.com/2024/04/lessons-learned-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://bettingstratigicarticle.blogspot.com/2024/04/lessons-from-world-betting.html
https://daddys-lucky000.mystrikingly.com/blog/study-mlb-nba-to-yield-joined-1-7-billion-from-lawful-games-wagering
https://sportswageringinfo.blogspot.com/2024/04/essential-life-lessons-learned-betting.html
https://lucky-razi-777.mystrikingly.com/blog/hearing-uncovers-illinois-legislators-advancing-toward-legitimizing-sports
https://onlinesportsbettingstory.blogspot.com/2024/04/Career-betting-professional.html
https://site-6828921-2394-9309.mystrikingly.com/blog/the-arrangements-keep-comin-new-york-planes-mgm-figure-out-advertising
https://sportsbettingindustry.blogspot.com/2024/04/betting-professionals-top-predictions.html
https://casino999.mystrikingly.com/blog/looking-at-replies-to-enter-sports-wagering-inquiries-in-illinois
https://articleinsportsbetting.blogspot.com/2024/04/mysteries-betting-professionals.html
https://site-4763676-9520-5980.mystrikingly.com/blog/pgcb-to-hold-hearings-decision-on-three-additional-games-betting
https://bestsportsbettingarticles.blogspot.com/2024/04/highly-effective-betting-professionals.html
https://site-6861241-1631-6047.mystrikingly.com/blog/minnesota-administrator-hopeful-but-still-guarded-about-sports-wagering
https://bettingstratigicarticle.blogspot.com/2024/04/betting-professionals-exposed.html
https://daddys-lucky000.mystrikingly.com/blog/mgm-resorts-turns-into-nhl-s-true-games-wagering-accomplice-in-memorable-new
https://sportswageringinfo.blogspot.com/2024/04/lessons-learned-betting-professionals.html
https://lucky-razi-777.mystrikingly.com/blog/with-significant-declaration-impending-nhl-supposedly-betting-everything-on
https://gameswageringarticles.blogspot.com/2024/04/betting-will-blow-your-mind.html
https://site-6861405-2997-4499.mystrikingly.com/blog/ncaa-declares-foundation-of-new-advisory-group-to-look-at-sports-betting
https://onlinesportsbettingstory.blogspot.com/2024/04/unconventional-lessons-learned-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/louisiana-officials-looking-at-sports-wagering-legitimization
https://sportsbettingindustry.blogspot.com/2024/04/lessons-learned-betting-strategies.html
https://casino999.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://articleinsportsbetting.blogspot.com/2024/04/unveiling-betting-wisdom.html
https://site-4763676-9520-5980.mystrikingly.com/blog/more-modest-games-associations-dashing-for-legitimate-games-wagering-open-doors
https://bestsportsbettingarticles.blogspot.com/2024/04/essential-lessons-every-risk-taker.html
https://site-6861241-1631-6047.mystrikingly.com/blog/football-is-above-all-else-ms-september-sports-wagering-handle-ranges-31-77
https://bettingstratigicarticle.blogspot.com/2024/04/betting-strategies-success.html
https://daddys-lucky000.mystrikingly.com/blog/after-energetic-hearing-indiana-officials-will-keep-on-investigating-sports
https://sportswageringinfo.blogspot.com/2024/04/blog-post_29.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-sheds-some-alarm-strategies-in-sports-wagering-articulation-in-illinois
https://gameswageringarticles.blogspot.com/2024/04/blog-post_26.html
https://site-6861405-2997-4499.mystrikingly.com/blog/how-states-are-spending-their-games-wagering-duty-income
https://onlinesportsbettingstory.blogspot.com/2024/05/practical-lessons-smarter-decision-making.html
https://site-6828921-2394-9309.mystrikingly.com/blog/legislators-pushing-for-legitimate-games-wagering-in-washington-d-c
https://sportsbettingindustry.blogspot.com/2024/05/betting-lessons-kickstart-journey.html
https://casino999.mystrikingly.com/blog/sports-wagering-dispatches-in-new-mexico-we-anticipate-a-major-weekend
https://articleinsportsbetting.blogspot.com/2024/05/betting-lessons-learned-strategic-living.html
https://site-4763676-9520-5980.mystrikingly.com/blog/where-do-gubernatorial-competitors-stand-west-version
https://bestsportsbettingarticles.blogspot.com/2024/05/essential-lessons-every-gambler.html
https://site-6861241-1631-6047.mystrikingly.com/blog/the-examiner-in-game-betting-one-of-smartest-choices-to-make
https://bettingstratigicarticle.blogspot.com/2024/05/lessons-learned-betting-psychology.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-on-plan-this-week-in-indiana-illinois-and-washington-d-c
https://sportswageringinfo.blogspot.com/2024/05/betting-secrets-unveiled.html
https://lucky-razi-777.mystrikingly.com/blog/september-new-jersey-sports-wagering-handle-leaps-to-184m-income-24m
https://gameswageringarticles.blogspot.com/2024/05/lessons-learned-high-stakes-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/where-do-gubernatorial-up-and-comers-stand-on-sports-wagering-east-version
아시안커넥트 먹튀검증 https://spo337.com/
머니라인247 먹튀검증 https://spo337.com/
황룡카지노 먹튀검증 https://spo337.com/
안전 온라인카지노 추천 https://spo337.com/
안전 카지노사이트 추천 https://spo337.com/
실시간 라이브배팅 https://spo337.com/
해외배팅사이트 추천 https://spo337.com/
해외스포츠배팅사이트 추천 https://spo337.com/
해외배팅사이트에이전시 추천 https://spo337.com/
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://spo337.com/
www.joeshouse.org/booking?link=https://spo337.com/&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=https://spo337.com/
swra.backagent.net/ext/rdr/?https://spo337.com/
namiotle.pl/?wptouch_switch=mobile&redirect=https://spo337.com/
golfpark.jp/banner/counter.aspx?url=https://spo337.com/
www.mexicolore.co.uk/click.php?url=https://spo337.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=https://spo337.com/
www.invisalign-doctor.in/api/redirect?url=https://spo337.com/
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=https://spo337.com/
www.immunallergo.ru/bitrix/redirect.php?goto=https://www.https://spo337.com/
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=https://spo337.com/
trackingapp4.embluejet.com/Mod_Campaigns/tracking.asp?idem=31069343&em=larauz@untref.edu.ar&ca=73143&ci=0&me=72706&of=581028&adirecta=0&url=https://spo337.com/
smils.ru/bitrix/redirect.php?goto=https://spo337.com/
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=https://spo337.com/
cipresso.ru/bitrix/redirect.php?goto=https://spo337.com/
www.vacacionartravel.com/DTCSpot/public/banner_redirect.aspx?idca=286&ids=75665176&cp=167&idcli=0&ci=2&p=https://spo337.com/
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=https://spo337.com/
www.malles-bertault.com/?change_langue=en&url=http%253a%252f%252fhttps://spo337.com/
www.beeicons.com/redirect.php?site=https://spo337.com/
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=https://spo337.com/
www.cccowe.org/lang.php?lang=en&url=https://spo337.com/
www.mytokachi.jp/index.php?type=click&mode=sbm&code=2981&url=https://spo337.com/
www.wqketang.com/logout?goto=https://spo337.com/
access.bridges.com/externalRedirector.do?url=https://spo337.com/
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://spo337.com/
bot.buymeapie.com/recipe?url=https://spo337.com/
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://spo337.com/
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=https://spo337.com/
quartiernetz-friesenberg.ch/links-go.php?to=https://spo337.com/
www.maultalk.com/url.php?to=https://spo337.com/
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=https://spo337.com/
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=https://spo337.com/
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=https://spo337.com/
http://getdatasheet.com/url.php?url=https://spo337.com/
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://spo337.com/
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=https://spo337.com/
www.aldolarcher.com/tools/esstat/esdown.asp?File=https://spo337.com/
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&nochat=1&domain=https://spo337.com/
http://blackwhitepleasure.com/cgi-bin/atx/out.cgi?trade=https://spo337.com/
azlan.techdata.com/InTouch/GUIBnrT3/BnrTrackerPublic.aspx?CountryCode=18&BannerLangCulture=nl-nl&URL=https://spo337.com/&Target=2&BannerId=41919&Zoneid=281&Parameters=&cos=Azlan
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44zoneid=1cb=7743e8d201oadest=https://spo337.com/
https://www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=https://spo337.com/
www.lzmfjj.com/Go.asp?URL=https://spo337.com/
http://alexmovs.com/cgi-bin/atx/out.cgi?id=148&tag=topatx&trade=https://spo337.com/
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2bannerid=40zoneid=16cb=fc1d72225coadest=https://spo337.com/
psylive.ru/success.aspx?id=0&goto=https://spo337.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=https://spo337.com/
www.mybunnies.net/te3/out.php?u=https://spo337.com/
lubaczowskie.pl/rdir/?l=https://spo337.com/&lid=1315
www.kowaisite.com/bin/out.cgi?id=kyouhuna&url=https://spo337.com/
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=https://spo337.com/
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=https://spo337.com/
https://asstomouth.guru/out.php?url=https://spo337.com/
advtest.exibart.com/adv/adv.php?id_banner=7201&link=https://spo337.com/
https://thairesidents.com/l.php?b=85&p=2,5&l=https://spo337.com/
www.latestnigeriannews.com/link_channel.php?channel=https://spo337.com/
www.haogaoyao.com/proad/default.aspx?url=https://spo337.com/
globalmedia51.ru/bitrix/redirect.php?goto=https://spo337.com/
citysafari.nl/Home/setCulture?language=en&returnUrl=https://spo337.com/
es.catholic.net/ligas/ligasframe.phtml?liga=https://spo337.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://spo337.com/
www.webshoptrustmark.fr/Change/en?returnUrl=https://spo337.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=https://spo337.com/
e.ourger.com/?c=scene&a=link&id=47154371&url=https://spo337.com/
zh-hk.guitarians.com/home/redirect/ubid/1015?r=https://spo337.com/
www.mastercleaningsupply.com/trigger.php?r_link=https://spo337.com/
lrnews.ru/xgo.php?url=https://spo337.com/
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://https://spo337.com/
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=https://spo337.com/
spb-medcom.ru/redirect.php?https://spo337.com/
forest.ru/links.php?go=https://spo337.com/
reefcentral.ru/bitrix/rk.php?goto=https://spo337.com/
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=https://spo337.com/
https://trackdaytoday.com/redirect-out?url=https://spo337.com/
https://bigjobslittlejobs.com/jobclick/?RedirectURL=https://spo337.com/&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2bannerid=4zoneid=4cb=68dbdae1d1oadest=https://spo337.com/
www.obertaeva.com/include/get.php?go=https://spo337.com/
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=https://spo337.com/
https://zueeen123.blogspot.com/2024/01/for-its-2023-games-wagering-industry.html
https://site-6828921-2394-9309.mystrikingly.com/blog/to-be-a-really-successful-season-in-sports-wagering-sanctioning-don-t-expect
https://leejk777.blogspot.com/2024/01/ohios-most-memorable-year-was-news.html
https://casino999.mystrikingly.com/blog/terrible-beats-of-2023-in-the-most-obviously-awful-games-wagering
https://parkjk777.blogspot.com/2024/01/states-revision-3-certainly.html
https://site-4763676-9520-5980.mystrikingly.com/blog/top-500-million-handle-for-fifth-time-in-new-york-portable-sportsbooks
https://emiko1231.blogspot.com/2024/01/wizards-leaving-dc-could-fundamentally.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-in-golden-state-bettors-not-seeing-green
https://yoo-777.blogspot.com/2024/01/will-wagering-be-lawful-in-california.html
https://daddys-lucky000.mystrikingly.com/blog/arizona-puts-bow-on-unrivaled-october-of-sports-wagering-in-u-s
https://jay-777.blogspot.com/2024/01/which-sports-wagering-administrators.html
https://lucky-razi-777.mystrikingly.com/blog/statistical-surveying-shows-betting-minimal-impacted-notwithstanding-expansion
https://jody222000.blogspot.com/2024/01/ex-jags-worker-having-to-carry-out.html
https://site-6861405-2997-4499.mystrikingly.com/blog/massachusetts-draftkings-overstepped-state-regulation-in-tolerating
https://zueeen123.blogspot.com/2024/01/sports-wagering-rules-in-north-carolina.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-zeroing-in-additional-on-the-most-proficient-method-to-address-badgering
https://leejk777.blogspot.com/2024/01/renunciation-of-entain-chief-might.html
https://casino999.mystrikingly.com/blog/has-the-opportunity-come-to-boost-card-sharks-to-utilize-mindful-betting
https://parkjk777.blogspot.com/2024/01/kentucky-breaks-from-entryways-with-340.html
https://site-4763676-9520-5980.mystrikingly.com/blog/vermont-lottery-chooses-draftkings-aficionados-and-fanduel-for-jan-11-send
https://emiko1231.blogspot.com/2024/01/sportsbook-administrators-answer-ohio.html
https://site-6861241-1631-6047.mystrikingly.com/blog/could-hard-rock-wager-florida-outperform-all-of-new-york-in-month-to-month
https://yoo-777.blogspot.com/2024/01/ideal-time-overs-monday-night.html
https://daddys-lucky000.mystrikingly.com/blog/nfl-week-14-sports-wagering-men-not-engaging-in-sexual-relations-real
https://jay-777.blogspot.com/2024/01/cuban-adelson-exchange-could-hold.html
https://lucky-razi-777.mystrikingly.com/blog/report-unlv-new-mexico-examined-for-dubious-betting-action
https://jody222000.blogspot.com/2024/01/fanduel-and-draftkings-took-on-virginia.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-rests-on-pga-visit-for-2024-north-carolina-market-access
https://zueeen123.blogspot.com/2024/01/7-of-greatest-possible-advantages-of.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pot-in-las-vegas-club-it-s-up-for-conversation
https://leejk777.blogspot.com/2024/01/sports-wagering-bankroll-executives.html
https://casino999.mystrikingly.com/blog/the-primary-post-this-is-sportshandle
https://parkjk777.blogspot.com/2024/01/where-significant-games-associations.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-101-nfl-lines-chances-point-spreads-and-that-s-only-the
https://emiko1231.blogspot.com/2024/01/nj-representative-leonard-spear-on.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sportshandle-s-brett-smiley-talks-sports-wagering-with-chris-moore-of-cbs
https://yoo-777.blogspot.com/2024/01/a-breakdown-of-draftkings-2023-tycoon.html
https://daddys-lucky000.mystrikingly.com/blog/sportshandle-talks-sports-wagering-authorization-with-cbs-sports-radio-s-bill
https://jay-777.blogspot.com/2024/01/new-jerseys-briefs-in-high-court-sports.html
https://lucky-razi-777.mystrikingly.com/blog/ron-paul-pens-section-tearing-charlie-imprint-s-web-based-gaming-boycott
https://jody222000.blogspot.com/2024/01/matthew-and-mikey-of-espn-in-lexington.html
https://site-6861405-2997-4499.mystrikingly.com/blog/supercontest-legend-james-salinas-standards-for-sports-wagering-achievement
https://zueeen123.blogspot.com/2024/01/supercontest-legend-james-salinas.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-jersey-senator-pallone-submits-amicus-brief-in-sports-wagering-case-in
https://leejk777.blogspot.com/2024/01/20-states-join-new-jersey-to-help.html
https://casino999.mystrikingly.com/blog/michigan-discussions-bill-that-would-legitimize-internet-gaming
https://parkjk777.blogspot.com/2024/01/sports-regulation-teacher-on-wagering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-would-be-simple-expansion-for-atlantic-city-gambling-clubs
https://emiko1231.blogspot.com/2024/01/logical-games-gaining-nyx-gaming-in.html
https://site-6861241-1631-6047.mystrikingly.com/blog/richard-sherman-says-nfl-injury-reports-are-explicitly-for-players
https://yoo-777.blogspot.com/2024/01/the-total-imbeciles-manual-for-sports.html
https://daddys-lucky000.mystrikingly.com/blog/there-s-zero-amazing-sections-staying-in-westgate-las-vegas-supercontest
https://jay-777.blogspot.com/2024/01/new-jersey-delegate-straightforward.html
https://lucky-razi-777.mystrikingly.com/blog/new-survey-larger-part-of-americans-backing-legitimizing-sports-wagering-for
https://jody222000.blogspot.com/2024/01/this-wagering-resembles-in-london-for.html
https://site-6861405-2997-4499.mystrikingly.com/blog/longshot-covers-produce-down-week-in-westgate-las-vegas-supercontest
https://zueeen123.blogspot.com/2024/01/nba-legend-wizardry-johnson-predicts.html
https://site-6828921-2394-9309.mystrikingly.com/blog/the-ascent-of-esports-and-wagering-opportunity-and-entanglements
https://leejk777.blogspot.com/2024/01/crazy-bosses-redskins-last-play-flipped.html
https://casino999.mystrikingly.com/blog/the-south-oaks-betting-screen-take-the-issue-betting-test
https://parkjk777.blogspot.com/2024/01/sports-regulation-teacher-on-wagering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/the-chernin-gathering-pushes-all-in-on-sports-wagering-and-dream-with
https://emiko1231.blogspot.com/2024/01/nfl-week-5-wagering-recap-street-groups.html
https://site-6861241-1631-6047.mystrikingly.com/blog/how-three-bettors-benefitted-from-one-wagering-ticket-thanks-to-giancarlo
https://yoo-777.blogspot.com/2024/01/new-jersey-representatives-urge-house.html
https://daddys-lucky000.mystrikingly.com/blog/gaming-regulation-master-predicts-new-jersey-triumph-in-high-court-sports
https://jay-777.blogspot.com/2024/01/legitimate-master-assesses-high-court.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-passes-bill-that-would-sanction-sports-wagering-yet-with-a
https://jody222000.blogspot.com/2024/01/previous-wr-mike-pritchard-talks-dark.html
https://site-6861405-2997-4499.mystrikingly.com/blog/long-term-redskins-te-chris-cooley-gives-player-s-perspective-on-sports-wagering
https://zueeen123.blogspot.com/2024/01/maryland-legislator-and-paper-needs-to.html
https://site-6828921-2394-9309.mystrikingly.com/blog/andrew-brandt-on-the-nfl-and-sports-wagering-dream-helps-overcome-any-barrier
https://leejk777.blogspot.com/2024/01/today-is-only-seventeenth-games.html
https://casino999.mystrikingly.com/blog/previous-wr-mike-pritchard-talks-dark-horses-and-sports-wagering-future-for
https://parkjk777.blogspot.com/2024/01/on-sports-wagering-long-term-redskins.html
https://site-4763676-9520-5980.mystrikingly.com/blog/supporting-games-wagering-boycott-trump-named-lawyer-documents-high-court-brief
https://emiko1231.blogspot.com/2024/01/celtics-attorney-explains-adam-silvers.html
https://site-6861241-1631-6047.mystrikingly.com/blog/7-nfl-wagering-notes-at-the-season-s-close-halfway-point
https://yoo-777.blogspot.com/2024/01/why-nfl-really-needs-to-lose-high-court.html
https://daddys-lucky000.mystrikingly.com/blog/dodgers-versus-astros-worldwide-championship-wagering-lines-and-props
https://jay-777.blogspot.com/2024/01/new-jersey-flames-back-at-associations.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-authorizes-sports-wagering-forthcoming-change-in-government
https://jody222000.blogspot.com/2024/01/sports-wagering-usa-important-points.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-gathering-action-items-money-might-be-above-all-else-however
https://zueeen123.blogspot.com/2024/01/unlv-meeting-audit-ncaa-actually.html
https://site-6828921-2394-9309.mystrikingly.com/blog/antitrust-tripwires-lawful-master-makes-sense-of-sports-wagering-information
https://leejk777.blogspot.com/2024/01/a.html
https://casino999.mystrikingly.com/blog/james-holzhauer-resists-pattern-gets-peril-rewards-before-country-learns-of
https://parkjk777.blogspot.com/2024/01/imperfections-and-everything-il-senate.html
https://site-4763676-9520-5980.mystrikingly.com/blog/illinois-sports-wagering-heads-to-senate-for-conclusive-endorsement
https://emiko1231.blogspot.com/2024/01/illinois-meeting-expanded-and-sports.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nba-finals-the-same-old-thing-for-vegas-books-regardless-of-critical
https://yoo-777.blogspot.com/2024/01/where-to-play-online-sportsbooks-and.html
https://daddys-lucky000.mystrikingly.com/blog/the-street-ahead-for-the-rotogrinders-organization
https://jay-777.blogspot.com/2024/01/us-open-wagering-review-streams-koepka.html
https://lucky-razi-777.mystrikingly.com/blog/montana-administrators-neglect-to-supersede-lead-representative-on-sports
https://jody222000.blogspot.com/2024/01/legitimate-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/oregon-representatives-attempting-to-kill-sports-wagering
https://zueeen123.blogspot.com/2024/01/westgate-supercontest-players-score.html
https://site-6828921-2394-9309.mystrikingly.com/blog/celtics-attorney-explains-adam-silver-s-backing-for-authorized-sports-wagering
https://leejk777.blogspot.com/2024/01/7-nfl-wagering-notes-at-seasons-close.html
https://casino999.mystrikingly.com/blog/worldwide-championship-wagering-lines-and-props-of-dodgers-versus-astros
https://parkjk777.blogspot.com/2024/01/sports-wagering-usa-action-items-part.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-gathering-focal-points-money-might-be-top-dog-yet
https://emiko1231.blogspot.com/2024/01/a-background-marked-by-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/just-around-the-corner-the-debut-sports-wagering-usa-meeting
https://yoo-777.blogspot.com/2024/01/tps-report-nfl-week-10-picks.html
https://daddys-lucky000.mystrikingly.com/blog/house-legal-executive-director-and-sports-wagering-rival-weave-goodlatte-to
https://jay-777.blogspot.com/2024/01/might-draftkings-at-some-point-truly.html
https://lucky-razi-777.mystrikingly.com/blog/sportshandle-talks-sports-wagering-with-jody-mcdonald-on-cbs-sports-radio
https://jody222000.blogspot.com/2024/01/new-jersey-congressperson-beam-lesniak.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nj-senator-sports-wagering-defender-candid-lobiondo-to-resign
https://zueeen123.blogspot.com/2024/01/michigan-could-prepared-itself-for.html
https://site-6828921-2394-9309.mystrikingly.com/blog/longshots-packers-power-nice-week-in-westgate-las-vegas-supercontest
https://leejk777.blogspot.com/2024/01/sitting-tight-for-sports-wagering-at-us.html
https://casino999.mystrikingly.com/blog/here-s-sound-for-oral-contention-in-new-jersey-s-high-court-sports-wagering-case
https://parkjk777.blogspot.com/2024/01/what-global-soccer-can-instruct-us.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-peering-toward-sports-wagering-in-wake-of-high-legal-dispute
https://emiko1231.blogspot.com/2024/01/senator-pallone-acquaints-bill-with.html
https://site-6861241-1631-6047.mystrikingly.com/blog/las-vegas-supercontest-players-score-enormous-in-week-13-as-canines-return
https://jay-777.blogspot.com/2024/01/mississippi-locked-and-stacked-for.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-administrators-hope-to-authorize-sports-wagering-put-arrangement-on
https://jody222000.blogspot.com/2024/01/somewhat-less-discussion-somewhat-more.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-ascent-and-energy-of-in-play-wagering-made-sense-of-by-master
https://zueeen123.blogspot.com/2024/01/indiana-sports-wagering-bill-contains.html
https://site-6828921-2394-9309.mystrikingly.com/blog/congress-a-gem-ball-and-the-debate-over-sports-wagering
https://leejk777.blogspot.com/2024/01/nfl-trump-card-end-of-week-wagering.html
https://casino999.mystrikingly.com/blog/vegas-legend-dave-cokin-joins-the-support-why-i-bet-with-jimmy-shapiro
https://parkjk777.blogspot.com/2024/01/how-might-jeff-meetings-enemy-of-weed.html
https://site-4763676-9520-5980.mystrikingly.com/blog/georgia-legislators-will-push-for-gambling-clubs-in-18-regardless-of-resistance
https://emiko1231.blogspot.com/2024/01/nfl-2017-normal-season-wagering-recap.html
https://site-6861241-1631-6047.mystrikingly.com/blog/indiana-legislator-wanting-to-support-bill-sanctioning-games-wagering-in-state
https://yoo-777.blogspot.com/2024/01/west-virginia-officials-reestablish.html
https://daddys-lucky000.mystrikingly.com/blog/nfl-divisional-round-wagering-recap-lookahead-to-titles
https://jay-777.blogspot.com/2024/01/effect-of-1-duty-on-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-senate-to-hold-hearing-on-capability-of-sports-wagering-in-the-state
https://jody222000.blogspot.com/2024/01/should-indianas-proposed-1-games.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nba-pushes-for-legitimate-games-wagering-in-new-york-yet-needs-slice-of-the-pie
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-legalized.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-enters-legitimate-games-wagering-bill-fight-as-congressperson
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-strategy.html
https://casino999.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-journey.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-sports-wagering-bill-would-allow-betting-at-five-club-and-on
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-mastery.html
https://site-6828921-2394-9309.mystrikingly.com/blog/fanduel-closures-2023-with-top-games-wagering-portion-of-the-overall-industry
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-skill.html
https://casino999.mystrikingly.com/blog/no-less-than-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-guide.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-wagering-bills-acquire-from-ohio-maryland-regulation
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-articles-wins.html
https://site-6861241-1631-6047.mystrikingly.com/blog/a-hawks-fan-stands-up-to-the-uncommon-blended-profound-support-bet
https://bettingstratigicarticle.blogspot.com/2024/01/betting-stratigic-articles-decision.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-ultimate.html
https://lucky-razi-777.mystrikingly.com/blog/florida-gaming-magistrate-sports-bettors-show-enthusiasm-in-first-month
https://gameswageringarticles.blogspot.com/2024/01/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/versatile-games-betting-authoritatively-dispatches-in-vermont
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-guide.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-an-idea-for-gubernatorial-up-and-comers
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-successful.html
https://casino999.mystrikingly.com/blog/colorado-clears-50-million-in-september-sports-betting-income
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-long-term-success.html
https://site-4763676-9520-5980.mystrikingly.com/blog/tennessee-observes-two-years-of-lawful-portable-games-betting
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-articles.html
https://site-6861241-1631-6047.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-versatile-betting-send-off
https://bettingstratigicarticle.blogspot.com/2024/01/betting-tratigic-article-basic-bet.html
https://daddys-lucky000.mystrikingly.com/blog/new-york-wagering-handle-arrives-at-most-elevated-point-since-college-basketball
https://sportswageringinfo.blogspot.com/2024/01/live-sports-wagaring-info.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-controller-nixes-draftkings-solicitation-for-widespread-day-for
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-defying-crowd.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maryland-bettors-draw-one-stage-nearer-to-lawful-versatile-betting
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-underdog.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-an-idea-for-gubernatorial-competitors
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-multiple-bets.html
https://casino999.mystrikingly.com/blog/ontario-dispatches-face-to-face-sports-wagering-at-retail-club-in-area
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-point-spread.html
https://site-4763676-9520-5980.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-portable-betting-send-off
https://bestsportsbettingarticles.blogspot.com/2024/01/best%20sports%20betting%20articles.html
https://site-6861241-1631-6047.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bettingstratigicarticle.blogspot.com/2024/01/betting-strategic-articles-disciplined-mindset.html
https://daddys-lucky000.mystrikingly.com/blog/betr-acquires-sports-betting-business-sector-access-in-three-additional-states
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-profits.html
https://lucky-razi-777.mystrikingly.com/blog/nascar-prepared-to-exploit-north-carolina-sports-wagering
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-specialized.html
https://site-6861405-2997-4499.mystrikingly.com/blog/somewhere-around-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-proposition-bets.html
https://site-6828921-2394-9309.mystrikingly.com/blog/north-carolina-online-games-betting-to-send-off-on-walk-11
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-strategy_01802557671.html
https://casino999.mystrikingly.com/blog/reports-iowa-specialist-blamed-for-warrantless-sports-wagering-search
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-analysis.html
https://site-4763676-9520-5980.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-portable-betting-send-off
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-markets.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pennsylvania-finishes-memorable-year-with-record-month-to-month-sports
https://bettingstratigicarticle.blogspot.com/2024/01/betting-strategic-articles-influences.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-mobile-tips.html
https://lucky-razi-777.mystrikingly.com/blog/rocha-california-drive-will-encourage-harm-the-brand-of-versatile-games
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-supercharge.html
https://site-6861405-2997-4499.mystrikingly.com/blog/is-america-terrible-with-awful-games-bettors
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-overview.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-legislator-needs-to-school-himself-partners-on-sports-wagering
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-beginner.html
https://casino999.mystrikingly.com/blog/lawful-games-wagering-in-the-u-s-the-english-are-coming-the-english-are
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-modern-data.html
https://site-4763676-9520-5980.mystrikingly.com/blog/hollywood-club-set-to-offer-west-virginia-sports-wagering-before-sept-1
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-different-types.html
https://site-6861241-1631-6047.mystrikingly.com/blog/delaware-gambling-clubs-state-to-partake-in-450-000-after-second-month-of
https://bettingstratigicarticle.blogspot.com/2024/01/sustanable-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/connecticut-lead-representative-gives-officials-cutoff-time-to-follow-up-on
https://sportswageringinfo.blogspot.com/2024/01/sports-real-time-wagering-info-.html
https://lucky-razi-777.mystrikingly.com/blog/what-s-in-store-at-the-book-sportsbook-coming-soon-to-the-horseshoe-tunica
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-article-players.html
https://site-6861405-2997-4499.mystrikingly.com/blog/pennsylvania-s-parx-gambling-club-and-gan-report-sports-wagering-organization
https://onlinesportsbettingstory.blogspot.com/2024/01/mobile-online-sports-beting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/hawaii-s-statewide-versatile-bill-is-feeling-the-loss-of-a-few-critical-parts
https://sportsbettingindustry.blogspot.com/2024/01/common-mistake-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/what-s-the-games-wagering-worth-of-a-mentor
https://articleinsportsbetting.blogspot.com/2024/01/legal-landscape-article-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/report-barstool-sports-to-go-into-advertising-concurrence-with-draftkings
https://bestsportsbettingarticles.blogspot.com/2024/01/impact-psychology-best-sport-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/fanduel-starts-offering-monetary-proficiency-administrations-to-bettors
https://bettingstratigicarticle.blogspot.com/2024/01/opportunities-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/little-north-carolina-universities-excited-for-wagering-duty-bonus
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-odds.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-finishes-notable-year-with-record-month-to-month-sports-betting
https://gameswageringarticles.blogspot.com/2024/01/dynamic-games-wagering-articles.html
https://site-6861405-2997-4499.mystrikingly.com/blog/virginia-bill-would-legitimize-wagering-in-state-school-groups
https://onlinesportsbettingstory.blogspot.com/2024/01/asian-handicap-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-creates-absurd-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/01/fantasy-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/connecticut-considering-new-advertisement-club-and-sports-wagering-bill
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-profit.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-official-talks-about-state-s-games-wagering-bill-and-future-of
https://bestsportsbettingarticles.blogspot.com/2024/01/consideration-sports-betting-best-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/why-sports-expectation-markets-are-like-onions-and-film-industry-film-receipts
https://bettingstratigicarticle.blogspot.com/2024/01/technology-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/iowa-bill-to-authorize-sports-wagering-advances-subtleties-need-pressing
https://sportswageringinfo.blogspot.com/2024/01/future-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://gameswageringarticles.blogspot.com/2024/01/blog-post_31.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-lawful-games-wagering-regulation-trend
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-pro-level.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-bankroll-the-executives-benefits-victors-and-discipline-1623a8ef-e6da-4dd6-bedb-5596e78ec1af
https://sportsbettingindustry.blogspot.com/2024/01/game-changing-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/sports-wagering-nfl-lines-chances-point-spreads-and-then-some
https://articleinsportsbetting.blogspot.com/2024/01/optimal-wins-sports-betting-.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-jersey-representative-pallone-submits-amicus-brief-in-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/best-odds-earnings-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/american-gaming-affiliation-records-brief-in-sports-wagering-case-in-high-court
https://bettingstratigicarticle.blogspot.com/2024/02/essential-tips-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/n-j-representative-leonard-spear-on-sports-wagering-jersey-merits
https://sportswageringinfo.blogspot.com/2024/02/sports-wagering-triumph.html
https://lucky-razi-777.mystrikingly.com/blog/7-of-the-greatest-possible-advantages-of-lawful-managed-sports-wagering
https://gameswageringarticles.blogspot.com/2024/02/games-wagering-victory-tips.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettor-at-focus-of-alabama-baseball-embarrassment-has-to-deal-with-upwards-of
https://onlinesportsbettingstory.blogspot.com/2024/02/mastering-online-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-sports-wagering-bill-starts-development-through-senate
https://sportsbettingindustry.blogspot.com/2024/02/successful-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/looking-for-bettors-and-administrators-for-wide-arriving-at-industry-reviews
https://articleinsportsbetting.blogspot.com/2024/02/savvy-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bestsportsbettingarticles.blogspot.com/2024/02/winning-streak-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/something-like-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups-f8de9e02-c6a6-495d-9ec9-14a31daf9d51
https://onlinesportsbettingstory.blogspot.com/2024/02/changing-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportsbettingindustry.blogspot.com/2024/02/mastering-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/rocha-california-drive-will-assist-harm-the-brand-of-portable-games-wagering
https://articleinsportsbetting.blogspot.com/2024/02/mastering-revolutionary-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/is-america-crummy-with-awful-games-bettors
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tip-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-s-three-portable-sportsbooks-all-set-with-send-off-advancements
https://bettingstratigicarticle.blogspot.com/2024/02/expert-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/media-note-pad-where-might-the-telecom-companies-be-without-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/successful-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/yearlings-texans-same-game-parlay-expects-pass-cheerful-undertaking
https://gameswageringarticles.blogspot.com/2024/02/secret-success-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maturing-superteams-among-wagering-top-choices-to-bring-home-nba-championship
https://onlinesportsbettingstory.blogspot.com/2024/02/proven-winnings-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/climate-abbreviated-pga-visit-occasion-sparkles-sports-wagering-discussion
https://sportsbettingindustry.blogspot.com/2024/02/mastering%20strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/an-hour-endeavors-to-handle-sports-wagering-thrashes-about-all-things-being
https://articleinsportsbetting.blogspot.com/2024/02/art-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ncaa-solicitations-restriction-on-school-player-prop-wagers-in-ohio
https://bestsportsbettingarticles.blogspot.com/2024/02/unbeatable-sports%20betting-tips.html
https://site-6861241-1631-6047.mystrikingly.com/blog/liv-golf-wagering-markets-presented-in-additional-states-for-2024-season
https://bettingstratigicarticle.blogspot.com/2024/02/profit-expert-betting-stratigic.html
https://daddys-lucky000.mystrikingly.com/blog/mississippi-online-games-wagering-bill-clears-house-missouri-bill-escapes-panel
https://sportswageringinfo.blogspot.com/2024/02/Winning-Sports-Wagering.html
https://lucky-razi-777.mystrikingly.com/blog/swift-at-the-super-bowl-can-t-wager-on-it
https://gameswageringarticles.blogspot.com/2024/02/strategies-excel-ports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-betr-getting-ready-to-leave-massachusetts-market
https://zueeen123.blogspot.com/2023/11/cavalier-of-california-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/from-activity-organization-nfl-smartest-options-for-week-8
https://leejk777.blogspot.com/2023/11/for-wagering-on-golf-pga-suspends-two.html
https://casino999.mystrikingly.com/blog/21-rule-in-massachusetts-a-potential-problem-for-espn-devotees
https://parkjk777.blogspot.com/2023/11/betway-pulls-out-application-for-online.html
https://site-4763676-9520-5980.mystrikingly.com/blog/first-nhl-player-to-get-betting-suspension-shane-pinto-supposedly-suspended
https://emiko1231.blogspot.com/2023/11/liv-golf-needs-major-wagering-interest.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-again-legitimate-yet-not-live-in-florida-as-legal-disputes
https://yoo-777.blogspot.com/2023/11/heres-reason-media-review-gave-edge-to.html
https://daddys-lucky000.mystrikingly.com/blog/does-house-cash-or-expertise-make-sports-bettors-want-more-and-more
https://jay-777.blogspot.com/2023/11/versatile-wagering-authorization.html
https://lucky-razi-777.mystrikingly.com/blog/early-inquiries-emerge-around-tennessee-s-wagering-expense-design
https://jody222000.blogspot.com/2023/11/this-year-with-astros-wagers-sleeping.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nba-prospects-wagers-we-love-for-2023-2024
https://zueeen123.blogspot.com/2023/12/florida-high-court-stretches-out.html
https://site-6828921-2394-9309.mystrikingly.com/blog/at-arizona-pga-visit-objective-draftkings-opens-retail-sportsbook
https://leejk777.blogspot.com/2023/12/driving-investor-thinks-espn-bet-will.html
https://casino999.mystrikingly.com/blog/g2e-consents-to-installment-settlement-for-israel-based-lsports-in-the-midst
https://parkjk777.blogspot.com/2023/12/american-gaming-affiliation-proceeds.html
https://site-4763676-9520-5980.mystrikingly.com/blog/desantis-solicitations-expansion-on-the-off-chance-that-that-could-direct
https://emiko1231.blogspot.com/2023/12/following-up-high-court-administering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/espn-bet-divulges-official-logo-in-front-of-november-send-off
https://yoo-777.blogspot.com/2023/12/record-promotion-offers-aided-lift.html
https://daddys-lucky000.mystrikingly.com/blog/draftkings-crawls-toward-maryland-retail-sportsbook-opening
https://jay-777.blogspot.com/2023/12/massachusetts-gaming-commission-hopes.html
https://lucky-razi-777.mystrikingly.com/blog/north-carolina-lottery-pushes-ahead-with-fundamental-games-wagering-rules
https://jody222000.blogspot.com/2023/12/media-note-pad-ascent-of-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-jersey-sports-betting-handle-floods-to-1-3-billion-in-september
https://zueeen123.blogspot.com/2023/12/sportradar-virtuoso-games-hit-with.html
https://site-6828921-2394-9309.mystrikingly.com/blog/25-billion-in-post-paspa-sports-betting-handle-in-illinois-outperforms
https://leejk777.blogspot.com/2023/12/there-are-2-key-business-sectors-it.html
https://casino999.mystrikingly.com/blog/more-youthful-bettors-have-more-assorted-betting-propensities-than-more
https://parkjk777.blogspot.com/2023/12/from-sending-off-hard-rock-bet-high.html
https://site-4763676-9520-5980.mystrikingly.com/blog/kansas-sets-new-high-for-sports-betting-handle-at-219-3-million
https://emiko1231.blogspot.com/2023/12/portable-handle-record-with-1692.html
https://site-6861241-1631-6047.mystrikingly.com/blog/houston-is-preferred-over-its-division-adversary-in-an-all-texas-al-series
https://yoo-777.blogspot.com/2023/12/ncaas-disposition-toward-sports.html
https://daddys-lucky000.mystrikingly.com/blog/which-live-characters-will-advance-betting-at-the-point-when-espn-bet-shows-up
https://jay-777.blogspot.com/2023/12/sports-wagering-controllers-acclimate.html
https://lucky-razi-777.mystrikingly.com/blog/nhl-abounding-with-incredible-connors-to-wager-on-as-season-starts
https://jody222000.blogspot.com/2023/12/new-longshot-dream-asset-will-support.html
https://site-6861405-2997-4499.mystrikingly.com/blog/greatest-administrators-astounded-to-find-out-about-potential-california
https://zueeen123.blogspot.com/2023/12/mindful-betting-development-new.html
https://site-6828921-2394-9309.mystrikingly.com/blog/astonished-to-catch-wind-of-potential-california-sports-wagering-drive
https://leejk777.blogspot.com/2023/12/espn-bet-is-lock-to-send-off-by-this.html
https://casino999.mystrikingly.com/blog/what-s-on-draft-nfl-week-6-nhl-season-opener-and-2023-worldwide-gaming
https://parkjk777.blogspot.com/2023/12/an-early-groundwork-school-football.html
https://site-4763676-9520-5980.mystrikingly.com/blog/activity-organization-s-nfl-smartest-options-for-week-5
https://emiko1231.blogspot.com/2023/12/overcomes-and-dodgers-are-greatest-mlb.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nevada-rep-commends-nfl-for-patched-up-sports-wagering-strategy-however
https://yoo-777.blogspot.com/2023/12/around-sports-formally-opens-second.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-remains-school-football-wagering-number-one-after-unsteady-september
https://jay-777.blogspot.com/2023/12/ncaa-hopes-to-return-to-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/maine-controller-going-for-the-gold-computerized-send-off
https://jody222000.blogspot.com/2023/12/finally-around-is-prepared-to-play-in.html
https://site-6861405-2997-4499.mystrikingly.com/blog/potential-chances-to-hop-on-line-development-in-bills-jags-falcons-rams
https://zueeen123.blogspot.com/2023/12/whats-in-store-at-meadowlands-fanduel.html
https://site-6828921-2394-9309.mystrikingly.com/blog/timetable-of-state-and-sportsbook-improvements-u-s-sports-wagering-in-2018
https://leejk777.blogspot.com/2023/12/the-week-in-sports-wagering-and-sports.html
https://casino999.mystrikingly.com/blog/to-restrict-portable-wagering-to-on-premises-pga-visit-concurs-it-d-be-absurd
https://parkjk777.blogspot.com/2023/12/fanduel-scores-arrangement-to-oversee.html
https://site-4763676-9520-5980.mystrikingly.com/blog/mailbag-mythbusting-the-kitchen-sink-rules
https://emiko1231.blogspot.com/2023/12/hammerin-hank-goldberg-offers-accounts.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pittsburgh-privateers-take-sports-wagering-respectability-expense-to
https://yoo-777.blogspot.com/2023/12/monitoring-west-virginias-games.html
https://daddys-lucky000.mystrikingly.com/blog/the-unlawful-betting-organizations-act-and-sports-wagering-mailbag-mythbusting
https://jay-777.blogspot.com/2023/12/new-jersey-sports-wagering-dispatches.html
https://lucky-razi-777.mystrikingly.com/blog/pounding-hank-goldberg-joins-the-support-why-i-bet-with-jimmy-shapiro
https://jody222000.blogspot.com/2023/12/in-any-case-so-what-in-blazes-is.html
https://site-6861405-2997-4499.mystrikingly.com/blog/mailbag-mythbusters-uigea-and-sports-wagering-made-sense-of
https://zueeen123.blogspot.com/2023/12/furthermore-pickem-dream-is-restricted.html
https://site-6828921-2394-9309.mystrikingly.com/blog/to-rule-virginia-sports-betting-scene-fanduel-proceeds
https://leejk777.blogspot.com/2023/12/income-on-ascent-again-in-ohio-wagering.html
https://casino999.mystrikingly.com/blog/dodgers-and-every-other-person-as-nl-special-case-week-starts-wagering
https://parkjk777.blogspot.com/2023/12/activity-organizations-games-bet-nfl.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-rolls-out-enormous-improvements-to-betting-arrangement
https://emiko1231.blogspot.com/2023/12/its-ideal-time-again-as-sanders.html
https://site-6861241-1631-6047.mystrikingly.com/blog/retail-book-in-illinois-around-sports-dispatches-versatile-application
https://yoo-777.blogspot.com/2023/12/virginia-sportsbooks-beat-bettors-up.html
https://daddys-lucky000.mystrikingly.com/blog/court-choice-clears-way-for-hard-rock-bet-to-send-off-in-florida-however
https://jay-777.blogspot.com/2023/12/caesars-approaches-send-off-of-firebets.html
https://lucky-razi-777.mystrikingly.com/blog/in-maryland-draftkings-draws-nearer-to-opening-retail-sportsbook
https://jody222000.blogspot.com/2023/12/live-blog-whats-going-on-kentucky.html
https://site-6861405-2997-4499.mystrikingly.com/blog/europe-a-thin-ryder-cup-wagering-number-one
https://zueeen123.blogspot.com/2023/12/hard-rock-bet-might-be-fastest-and.html
https://site-6828921-2394-9309.mystrikingly.com/blog/north-carolina-commission-sure-of-portable-betting-send-off-by-june-cutoff-time
https://leejk777.blogspot.com/2023/12/canelo-means-to-join-boxings-uncommon.html
https://casino999.mystrikingly.com/blog/florida-parimutuels-train-in-on-seminole-minimal-in-state-court
https://parkjk777.blogspot.com/2023/12/the-arizona-division-of-gaming-detailed.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-versatile-betting-authorizing-change-draws-in-industry
https://emiko1231.blogspot.com/2023/12/ontario-partners-need-clearness-on-most.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nfl-virtuoso-games-reveal-stream-and-bet-stage-through-betvision
https://yoo-777.blogspot.com/2023/12/in-most-recent-documenting-doi-advises.html
https://daddys-lucky000.mystrikingly.com/blog/two-of-seven-stages-in-kentucky-will-permit-wagering-at-age-18-beginning
https://jay-777.blogspot.com/2023/12/schuetz-substantial-mistakes-in-sports.html
https://lucky-razi-777.mystrikingly.com/blog/school-brotherhood-accomplices-with-psychological-wellness-supplier-to-check
https://jody222000.blogspot.com/2023/12/activity-organizations-nfl-smartest.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nfl-week-3-alexander-mattison-murmurs-and-whenever-score-wagers
https://zueeen123.blogspot.com/2023/12/i-need-you-to-quant-me-super-advanced.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-sumsub-stage-permits-sportsbooks-to-handle-mindful-betting
https://leejk777.blogspot.com/2023/12/for-versatile-betting-send-off-north.html
https://casino999.mystrikingly.com/blog/for-web-based-betting-in-kentucky-sportsbooks-carrying-out-pre-send-off-offers
https://parkjk777.blogspot.com/2023/12/with-same-game-parlay-potential-week-3.html
https://site-4763676-9520-5980.mystrikingly.com/blog/report-d-c-lottery-cutoff-points-retail-bets-after-cafe-bettor-wins-huge
https://emiko1231.blogspot.com/2023/12/also-bettors-improved-pennsylvania.html
https://site-6861241-1631-6047.mystrikingly.com/blog/furthermore-presumably-substantially-more-portable-games-wagering-in
https://yoo-777.blogspot.com/2023/12/about-his-betting-compulsion-phil.html
https://daddys-lucky000.mystrikingly.com/blog/for-sports-wagering-and-igaming-asset-roundhill-to-change-fundamental-file
https://jay-777.blogspot.com/2023/12/north-carolina-financial-plan.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-sports-betting-scene-draftkings-keeps-on-overwhelming
https://jody222000.blogspot.com/2023/12/gross-income-for-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-sets-single-week-portable-income-record-in-new-york
https://zueeen123.blogspot.com/2023/12/will-prohibet-give-one-quit-shopping-to.html
https://site-6828921-2394-9309.mystrikingly.com/blog/how-do-stodgy-noses-need-to-manage-capable-betting
https://leejk777.blogspot.com/2023/12/five-significant-administrators-submit.html
https://casino999.mystrikingly.com/blog/sports-wagering-authorizing-development-in-maryland-legislator-looks-for-retail
https://parkjk777.blogspot.com/2023/12/prime-sportsbook-set-to-start-up-in.html
https://site-4763676-9520-5980.mystrikingly.com/blog/geocomply-reports-242-million-nfl-week-1-wagering-exchanges-up-56-over-a
https://emiko1231.blogspot.com/2023/12/with-around-90-of-2023-mlb-season.html
https://site-6861241-1631-6047.mystrikingly.com/blog/looks-for-retail-sports-wagering-permitting-development-in-maryland-official
https://yoo-777.blogspot.com/2023/12/sports-betting-invites-any-and-all.html
https://daddys-lucky000.mystrikingly.com/blog/superteams-supercharge-wnba-wagering-interest-as-end-of-the-season-games-start
https://jay-777.blogspot.com/2023/12/colorado-becomes-6th-state-to.html
https://lucky-razi-777.mystrikingly.com/blog/in-any-case-most-don-t-utilize-apparatuses-bettors-have-a-lot-of-experience
https://jody222000.blogspot.com/2023/12/chances-development-mirrors-colorados.html
https://site-6861405-2997-4499.mystrikingly.com/blog/rodgers-possibly-season-finishing-injury-rocks-planes-prospects-chances
https://zueeen123.blogspot.com/2023/12/maryland-bettors-at-last-thump-hold.html
https://site-6828921-2394-9309.mystrikingly.com/blog/media-scratch-pad-fanduel-television-s-new-cousin-sal-shows-join-swarmed
https://leejk777.blogspot.com/2023/12/draftkings-posts-then-at-that-point.html
https://casino999.mystrikingly.com/blog/north-carolina-lottery-looks-for-sports-betting-authorizing-chief
https://parkjk777.blogspot.com/2023/12/in-sports-betting-income-for-2023-new.html
https://site-4763676-9520-5980.mystrikingly.com/blog/activity-organization-s-nfl-smartest-choices-in-sports-wagering
https://emiko1231.blogspot.com/2023/12/allowed-to-play-supercontest-now.html
https://site-6861241-1631-6047.mystrikingly.com/blog/devotees-sportsbook-promotion-code-arrangement-awards-official-group-jersey
https://yoo-777.blogspot.com/2023/12/kansas-sees-ups-and-downs-in-initial.html
https://daddys-lucky000.mystrikingly.com/blog/missouri-polling-form-proposition-documented-would-send-choice-to-authorize
https://jay-777.blogspot.com/2023/12/friday-live-blog-following-games.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-week-1-notes-from-a-non-wagering-master-and-a-ton-of-alexander-mattison
https://jody222000.blogspot.com/2023/12/iowa-controller-proposes-refreshed.html
https://site-6861405-2997-4499.mystrikingly.com/blog/thursday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable
https://zueeen123.blogspot.com/2023/12/kansas-sees-ups-and-downs-in-initial.html
https://site-6828921-2394-9309.mystrikingly.com/blog/friday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable-week
https://leejk777.blogspot.com/2023/12/refreshed-wagering-rules-after-school.html
https://casino999.mystrikingly.com/blog/thursday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable
https://parkjk777.blogspot.com/2023/12/what-around-stress-little-overlay.html
https://site-4763676-9520-5980.mystrikingly.com/blog/draftkings-offering-prop-on-absolute-nfl-focuses-for-2023-season
https://emiko1231.blogspot.com/2023/12/heres-where-youll-have-option-to-place.html
https://site-6861241-1631-6047.mystrikingly.com/blog/some-iowa-iowa-state-competitors-acknowledge-betting-supplication-arrangements
https://yoo-777.blogspot.com/2023/12/schuetz-football-is-back-fail-to.html
https://daddys-lucky000.mystrikingly.com/blog/betr-dispatches-its-genuine-cash-portable-sportsbook-in-virginia
https://jay-777.blogspot.com/2023/12/wednesday-live-blog-following-games.html
https://lucky-razi-777.mystrikingly.com/blog/approved-for-departure-sports-handle-staff-picks-for-2023-nfl-season
https://jody222000.blogspot.com/2023/12/for-week-1-worth-street-canine-titans.html
https://site-6861405-2997-4499.mystrikingly.com/blog/acuna-or-betts-for-mvp-you-choose-on-the-grounds-that-we-can-t
https://zueeen123.blogspot.com/2023/12/espn-moves-sports-wagering-project.html
https://site-6828921-2394-9309.mystrikingly.com/blog/prospects-payouts-put-drag-on-july-sportsbook-income-in-nevada
https://leejk777.blogspot.com/2023/12/north-carolina-lottery-making-progress.html
https://casino999.mystrikingly.com/blog/ontario-carries-out-more-severe-betting-publicizing-rules
https://parkjk777.blogspot.com/2023/12/schuetz-business-passed-up-great.html
https://site-4763676-9520-5980.mystrikingly.com/blog/arizona-awards-sports-wagering-permit-to-bet365-as-seventeenth-administrator
https://emiko1231.blogspot.com/2023/12/nascar-re-ups-with-sportradar-signs.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sporttrade-dispatches-in-colorado-as-upstart-novig-seeks-after-permit
https://yoo-777.blogspot.com/2023/12/huge-ten-football-to-give-game-day.html
https://daddys-lucky000.mystrikingly.com/blog/there-s-no-need-to-focus-on-everyone-beginning-a-similar-page-it-s-about
https://jay-777.blogspot.com/2023/12/wagering-markets-give-djokovic-alcaraz.html
https://lucky-razi-777.mystrikingly.com/blog/the-bettors-annus-horribilis-a-year-of-betting-across-the-u-s
https://jody222000.blogspot.com/2023/12/maryland-lottery-fines-draftkings-94400.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sporttrade-gets-second-administrator-permit-this-one-in-colorado
https://zueeen123.blogspot.com/2023/12/south-dakota-lead-representative-signs.html
https://site-6828921-2394-9309.mystrikingly.com/blog/connecticut-lead-representative-arrives-at-sports-wagering-manage-clans
https://leejk777.blogspot.com/2023/12/north-dakota-official-looks-for-two.html
https://casino999.mystrikingly.com/blog/maryland-sports-wagering-online-md-sportsbooks-and-join-offers
https://parkjk777.blogspot.com/2023/12/wyoming-officials-turn-around-course-as.html
https://site-4763676-9520-5980.mystrikingly.com/blog/wyoming-house-votes-against-lawful-games-wagering
https://emiko1231.blogspot.com/2023/12/texas-electors-need-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/connecticut-sports-wagering-injured-last-week-yet-wagering-in-time-for-nfl
https://yoo-777.blogspot.com/2023/12/georgia-senate-supports-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/take-a-few-to-get-back-some-composure-the-week-in-sports-wagering
https://jay-777.blogspot.com/2023/12/arizona-house-sends-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/south-dakota-administrators-send-sports-wagering-bill-to-lead-representative
https://jody222000.blogspot.com/2023/12/things-turn-severe-in-battle-for.html
https://site-6861405-2997-4499.mystrikingly.com/blog/these-states-have-sports-wagering-on-house-senate-floors-yet-will-any-move
https://zueeen123.blogspot.com/2023/12/fanduel-currently-taking-pickleball.html
https://site-6828921-2394-9309.mystrikingly.com/blog/yippee-sportsbook-opens-up-at-the-venetian-hotel-las-vegas
https://leejk777.blogspot.com/2023/12/schuetz-meeting-hello-wagering-slamming.html
https://casino999.mystrikingly.com/blog/usa-s-true-j-v-group-expected-to-win-fiba-world-cup
https://parkjk777.blogspot.com/2023/12/college-of-iowa-competitors-learn.html
https://site-4763676-9520-5980.mystrikingly.com/blog/u-s-business-sports-betting-handle-outperforms-250-billion-post-paspa
https://emiko1231.blogspot.com/2023/12/superbook-bar-and-cafe-at-camden-yards.html
https://site-6861241-1631-6047.mystrikingly.com/blog/enthusiasts-sportsbook-cleared-for-send-off-soon-in-pennsylvania
https://yoo-777.blogspot.com/2023/12/industry-insiders-dave-portnoy-perhaps.html
https://daddys-lucky000.mystrikingly.com/blog/contention-carries-same-game-parlays-to-esports-wagering
https://jay-777.blogspot.com/2023/12/media-note-pad-distrust-has-large.html
https://lucky-razi-777.mystrikingly.com/blog/fan-sportsbook-dispatches-official-application-in-four-states
https://jody222000.blogspot.com/2023/12/whether-overs-or-unders-there-are-ncaa.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-leave-means-licenses-will-be-accessible-in-arizona-and-virginia
https://zueeen123.blogspot.com/2023/12/caesars-probably-will-not-have-retail.html
https://site-6828921-2394-9309.mystrikingly.com/blog/devotees-starts-taking-retail-wagers-in-ohio-rapidly-after-send-off-of
https://leejk777.blogspot.com/2023/12/arizonas-june-sports-betting-handle-up.html
https://casino999.mystrikingly.com/blog/parlays-power-new-jersey-sportsbooks-to-third-consecutive-10-month-to-month
https://parkjk777.blogspot.com/2023/12/media-journal-wariness-has-large.html
https://site-4763676-9520-5980.mystrikingly.com/blog/most-recent-court-recording-focuses-to-no-florida-sports-wagering-before-nfl
https://emiko1231.blogspot.com/2023/12/most-recent-court-documenting-focuses.html
https://site-6861241-1631-6047.mystrikingly.com/blog/massachusetts-sportsbooks-guarantee-30-1-million-in-income-for-july
https://yoo-777.blogspot.com/2023/12/expected-to-send-off-in-three-states-by.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-s-privileged-hugs-state-of-the-art-innovation
https://jay-777.blogspot.com/2023/12/schools-stress-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/illinois-sportsbooks-proceed-solid-2023-with-54-7-million-income-for-junethe
https://jody222000.blogspot.com/2023/12/wynn-resorts-intends-to-close-wynnbet.html
https://site-6861405-2997-4499.mystrikingly.com/blog/draftkings-almost-overwhelms-fanduel-for-best-position-in-new-york-income
https://zueeen123.blogspot.com/2023/12/us-sports-betting-handle-outperforms.html
https://site-6828921-2394-9309.mystrikingly.com/blog/has-the-opportunity-come-to-boost-players-to-utilize-capable-betting-devices
https://leejk777.blogspot.com/2023/12/the-most-horrendously-awful-games.html
https://casino999.mystrikingly.com/blog/north-carolina-sports-wagering-regulation-course-of-events-and-most
https://parkjk777.blogspot.com/2023/12/seven-significant-administrators-have.html
https://site-4763676-9520-5980.mystrikingly.com/blog/vacillate-expects-fierce-opposition-from-espn-bet-is-on-target-for-u-s-posting
https://emiko1231.blogspot.com/2023/12/sports-wagering-or-dream-sports.html
https://site-6861241-1631-6047.mystrikingly.com/blog/will-espn-bet-shake-up-the-highest-point-of-the-sportsbook-rankings
https://yoo-777.blogspot.com/2023/12/all-in-one-resource-for-betting-media.html
https://daddys-lucky000.mystrikingly.com/blog/barstool-sportsbook-to-be-rebranded-as-espn-bet
https://jay-777.blogspot.com/2023/12/the-delight-of-line-shopping-on-nfl-win.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-ohio-state-enormous-ten-wagering-top-choices-entering-2023
https://jody222000.blogspot.com/2023/12/robins-satisfied-with-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/as-nfl-season-approaches-enthusiasts-draws-nearer-to-new-york-sports
https://zueeen123.blogspot.com/2023/12/raise-ruckus-around-town-for-its-2023.html
https://site-6828921-2394-9309.mystrikingly.com/blog/versatile-games-wagering-goes-live-in-delaware-by-means-of-betrivers
https://leejk777.blogspot.com/2023/12/wounds-select-outs-make-florida-state.html
https://casino999.mystrikingly.com/blog/north-carolina-portable-wagering-send-off-conceivable-before-college-basketball
https://parkjk777.blogspot.com/2023/12/nevada-sports-betting-handle-basically.html
https://site-4763676-9520-5980.mystrikingly.com/blog/try-not-to-expect-2024-to-be-a-really-successful-season-in-sports-wagering
https://emiko1231.blogspot.com/2023/12/wounds-select-outs-make-florida-state.html
https://site-6861241-1631-6047.mystrikingly.com/blog/as-sports-wagering-introductions-go-ohio-s-most-memorable-year-was-a
https://yoo-777.blogspot.com/2023/12/the-most-horrendously-awful-games.html
https://daddys-lucky000.mystrikingly.com/blog/ask-a-bookmaker-glancing-back-at-2023-and-toward-2024
https://jay-777.blogspot.com/2023/12/florida-parimutuels-states-change-3.html
https://lucky-razi-777.mystrikingly.com/blog/powers-of-fate-marked-and-lined-up-in-l-a-have-pushed-dodgers-chances-to
https://jody222000.blogspot.com/2023/12/sports-wagering-in-2023-hi-to-espn-bet.html
https://site-6861405-2997-4499.mystrikingly.com/blog/send-off-of-espn-bet-aficionados-tops-rundown-of-2023-games-wagering
https://adricewines.wine/blogs/news/first-wine-contest-and-already-winning?comment=133853741333#comments
https://wildcraftbrewery.co.uk/blogs/recipes/wild-eye-pa-fruit-cake-recipe?comment=127693226166#comments
https://adricewines.wine/blogs/news/first-wine-contest-and-already-winning?comment=133853806869#comments
https://marysmallwood.com/blogs/news/summers-in-jackson-hole-aint-it-grand?comment=129574174817#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130551087341#comments
https://greenfoods.com/blogs/news/7-benefits-of-barley-juice-powder?comment=129952022691#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/5-ways-to-brew-coffee-at-home?comment=131708649560#comments
https://www.truselforganics.com/blogs/news/skin-purging-or-breaking-out?comment=130744615086#comments
https://www.truselforganics.com/blogs/news/skin-purging-or-breaking-out?comment=130744647854#comments
https://lajashley.com/blogs/the-chemist-speaks/panthenol?comment=127174574261#comments
https://burnbear.com/blogs/journal/a-new-direction?comment=133983174937#comments
https://www.scubatoo.net/blogs/news/114188100-cleaning-your-new-mask?comment=124843262102#comments
https://danabronfman.com/blogs/blog/met-gala-2017-dedicated-to-comme-des-garcons-s-rei-kawakubo?comment=124050669604#comments
https://swannies.co/blogs/digest/top-5-greatest-golf-movies?comment=131045097745#comments
https://xavierfoley.com/blogs/xavier-foleys-blog/how-to-get-more-ticket-sales?comment=128977698915#comments
https://www.riverwoodtradingco.com/blogs/news/2021-shows?comment=130887352488#comments
https://toadhollownj.com/blogs/news/making-christmas-cards?comment=135359234342#comments
https://houstonskateboards.com/blogs/news/missouri-city-skatepark-grand-opening-with-houston-skateboards?comment=127782223938#comments
https://freckbeauty.com/blogs/ooze/color-correcting-w-%F0%9D%98%8A%F0%9D%98%8F%F0%9D%98%8C%F0%9D%98%8C%F0%9D%98%92%F0%9D%98%9A%F0%9D%98%93%F0%9D%98%90%F0%9D%98%94%F0%9D%98%8C?comment=129471774906#comments
https://amyssecretkitchen.com/blogs/news/fathers-day-creation?comment=131106209890#comments
https://informaltea.co.nz/blogs/news/this-week-in-tea-1?comment=129355612318#Comments
https://sigiskin.com/blogs/news/why-do-you-need-dream-capsule-in-your-night-routine?comment=133623841077#comments
https://raw-me.com/ar/blogs/news/7-tips-to-start-living-a-plant-based-lifestyle?comment=131615457369#comments
https://physiclo.com/blogs/news/get-fit-in-2017-giveaway?comment=134112477261#comments
https://milohshop.com/blogs/news/the-story-of-miloh?comment=130344255726#comments
https://deadonpaper.com/blogs/news/11312645-calaveras-shipping-yay?comment=130114453615#Comments
https://teamfarang.com/blogs/news/luca-playlist?comment=133723062588#comments
https://shopfantasyguides.com/blogs/articles/buffalo-bills?comment=125376528468#comments
https://wheretonext.ph/blogs/stories/iceland?comment=130476540146#comments
https://knitonecrochettoo.com/blogs/news/peony-blues-tee?comment=126648811707#comments
https://stinkydog.com/blogs/news/stinky-and-i-have-been-working-like-crazy?comment=128821756063#comments
https://www.reagansanai.com/blogs/news/new-product-alert?comment=130779152597#comments
https://www.tomsonelectronics.com/blogs/news/how-to-set-up-raspberry-pi-pico-rp2040-microcontroller?comment=130344288494#comments
https://covenandco.com/blogs/news/flower-doughnuts?comment=122026131626#comments
https://www.ossboardsupply.com/blogs/news/palace-normcore?comment=131106242658#Comments
https://smoodies.in/blogs/news/smoodies-in-mumbai?comment=129791492179#comments
https://radiateportablecampfire.com/blogs/beyond-the-campfire/camping-essentials-the-best-products-for-your-next-camping-trip?comment=131078193399#comments
https://www.jenni-smith.co.uk/blogs/news/happy-international-womens-day?comment=130501148743#comments
https://www.bellawren.ca/blogs/bellas-blogs/how-sustainable-is-linen?comment=130084962516#comments
https://pseudolabs.com/blogs/in-pseudo/seen-in-pseudo-vol-83?comment=130476572914#comments
https://www.arenlace.com/blogs/blog/tips-to-help-newlyweds-manage-the-holidays?comment=130770960463#comments
https://ferriswheelpress.com/blogs/fan-art/october-22-fan-art-rollup?comment=130659713118#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134115098906#comments
https://www.gussloan.com/blogs/gus-sloan-the-blog/not-your-average-summer-style-guide?comment=130696183923#comments
https://ahwgeorgiaezra.com/blogs/build/our-home-renovation-2-0-part-3?comment=132803297331#comments
https://groundedfactory.com/blogs/news/how-to-find-and-prepare-for-a-yoga-teacher-training?comment=134372852056#comments
https://www.nikkiwilliams.com/blogs/news/interview-with-hawkins-co?comment=134219268395#comments
https://www.spicyelement.com/blogs/recipe/grilled-salmon-head?comment=125775642820#comments
https://www.katiesplates.com/blogs/sherry-greeks-blog-katies-plates-official-registered-dietitian/gluten-free?comment=131377004801#Comments
https://formula2skincare.com/blogs/news/beauty-hacks-with-formula-2-skin-care-cream?comment=130696249459#comments
https://kryptoneconsultingltd.weebly.com/articles/procedure-of-buying-land-in-kenya-a-presentation
https://www.dohertyphotography.com/blogs/a-year-underwater/la-paz-and-cabo?comment=133915082886#comments
https://www.ghanzibrand.com/blogs/news-1/offshoot-project-with-remi-fargier?comment=135684325711#comments
https://glitzandspursboutique.com/blogs/news/tiered-lace-babydoll-top?comment=129451556917#comments
https://www.skinnymetea.com.au/blogs/smtblog/the-best-supplements-to-take-for-your-health?comment=134112510029#comments
https://elkshape.com/blogs/articles/archery-shoulder-exercises?comment=133508235579#comments
https://www.sportstakeoff.com/blogs/look-at-this/dirty-but-yet-smartest-cheap-shot-ive-ever-seen?comment=135767785811#comments
https://www.babybento.com.au/blogs/recipes/sushi?comment=128658866258#comments
https://absolutemerch.com/blogs/news/good-cause?comment=135030309175#comments
https://www.homadic.com/blogs/news/delegates-of-german-federal-ministry-of-environment-visit?comment=135887520076#comments
https://hourloop.com/blogs/news/i-want-much-more-than-this-provincial-life-beauty-and-the-beast-1740-to-2017?comment=128067305530#comments
https://www.tonewoodmaple.com/blogs/news/5885643-tonewood-maple-sugarmaker-david-hartshorn?comment=131207888963#comments
https://tinyrabbithole.com/blogs/news-1/make-naiise-handmade-plushie-at-naiise?comment=130695037147#comments
https://aandscovers.com/blogs/news/is-it-just-me-or-are-magnets-really-attractive?comment=134690865460#comments
https://smartygirlbrand.com/blogs/news/why-does-smartygirl-use-so-much-pink-and-purple?comment=124907159598#comments
https://www.utzmarket.com/blogs/musings/utz-market-n-c-leather?comment=126693114028#comments
https://hardnightgoodmorning.com/blogs/hard-night-good-morning-blog/8863659-the-secret-behind-barley?comment=130006155334#comments
https://jinmee.co.uk/blogs/news/aha-vs-bha-what-s-the-difference?comment=132803493939#comments
https://hovdenwear.com/blogs/news/hovden-art?comment=126938448056#comments
https://shop.nucoconut.com/blogs/recipes/moroccan-cigars-aip-paleo-keto?comment=131106275426#comments
https://retreatyourself.com/blogs/news/mystery-box-brands-october-2022-edition?comment=131615588441#comments
http://www.justindoran.ie/blog/new-tutorials-added
https://cincyshirts.com/blogs/news/free-fc-cincinnati-opening-match-commemorative-tee?comment=127723667610#comments
https://gardenersorchardandbakery.com/blogs/news/farm-fresh-tomatoes-and-missouri-peaches-are-here?comment=122830258221#comments
https://bohochicfiberco.com/blogs/news/87446145-handmade-wardrobe?comment=127782322242#comments
https://www.nortonhousequilting.com/blogs/news/februarys-quilting-your-legacy-monthly-challenge-is-u-f-o-projects?comment=131337683160#comments
https://mehperu.com/en/blogs/blog-meh/no-todos-los-lunes-son-buenos-1?comment=133047550247#comments
https://lagom142.com/blogs/news/plastic-free?comment=134156910893#comments
https://playmobi.com/blogs/blog/kids-and-pets?comment=129258586155#comments
https://www.head-lites.com/blogs/the-sbark/45834241-guiding-lites?comment=125475848381#comments
https://www.emergencylights.net/blogs/blog/title-24-light-switches?comment=131387064556#comments
https://naattumarundhukadai.com/blogs/news/tummy-trim-gel-visible-sliming-on-7th-day-onwords?comment=131254976770#comments
https://yatesprecision.com/blogs/from-the-breakroom/machinist-survey-with-ben-jenkinson-of-jimko-machine?comment=127723700378#comments
https://sofragrance.com/blogs/news/so-sorry-not-sorry-girls-night-in?comment=130695069915#comments
https://www.kassausa.com/blogs/inspiration/how-to-make-your-own-chalkboard-wall-calendar?comment=131377070337#comments
https://shitthatiknit.com/blogs/news/beneath-the-beanie-ashleigh-stone?comment=130344321262#comments
https://saltypaloma.com/blogs/salty-talk/salty-paloma-holiday-party-2018?comment=133742428451#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128658931794#comments
https://misajewelry.com/blogs/blog/18894159-oscar-faves?comment=122026164394#comments
https://www.vbcityfc.com/blogs/news/match-preview-vbvfrd?comment=122026197162#comments
https://luangisa.com/blogs/news/for-wholesalers-interested-in-african-art-african-home-decor-jewelry-and-more-contact-luangisa-art-gallery?comment=130863366399#comments
https://www.theartdream.com/blog/black-panther-pop-up
https://woodleon.com/blogs/blog/what-swag-employees-want?comment=130452259036#comments
https://vincita.cc/blogs/news/introducing-vincita-north-point?comment=130744811694#comments
https://www.plantnmore.com/blogs/news/square-foot-gardening?comment=132484530410#comments
https://lizzie-loves.com/blogs/blog/baked-oat-berry-bars?comment=129952219299#comments
https://flowersofjoy.biz/blog/Tulips
https://www.simplysavorybyrachel.com/blogs/news/breakfast-dirty-rice?comment=128658964562#comments
http://www.yadvindermalhi.org/blog/the-ecomodernism-debate-with-mark-lynas
https://balandlaw.com/blog/guest-blog-post-by-attorney-paul-birnberg-on-security-deposits-was-mungall-v-garry-correctly-decided
https://www.believeiam.com/blogs/biablog/7319402-yoga-for-runners-the-yin-for-the-yang?comment=129433829552#comments
https://www.oddpears.com/blogs/blog/the-meditative-minimal-compositions-of-bobby-clark?comment=130148237504#comments
https://trubeautyshop.com/blogs/default-blog/what-is-a-jade-roller-beauty-benefits-and-tips?comment=125775675588#comments
https://iccwbo.uk/blogs/news/it-s-time-for-a-more-intelligent-approach-to-tackling-covid-19?comment=131003515132#comments
https://www.littlegreenpouch.com/blogs/recipes/6380464-tricked-out-bananameal?comment=124050735140#comments
https://www.savvysleepers.com/blogs/the-benefits-of-satin/savvy-sleepers-testimonials-around-the-web?comment=124907192366#comments
https://stylealertsa.com/blogs/news/thuli-mola-on-common-fashion-mistakes-women-make?comment=127814828113#comments
https://www.staranisefoods.com/blogs/recipes/brown-rice-noodles-with-broccoli-in-almond-butter-sauce?comment=121481494572#comments
https://russellroadracing.co.uk/blogs/news/obd-bench-boot-tuning-explained?comment=127145410634#comments
https://www.somata.ca/blog/rebooting-with-nutrient-therapy
https://shevoke.com/blogs/news/a-day-with-abbey-stockwell?comment=132840816802#comments
https://themodushop.com/blogs/news/thanksgiving-week-sale?comment=134196396369#comments
https://oliofood.com/blogs/olio-blog/article-1?comment=130265350389#comments
https://twowheelsbiglife.com/blogs/you-tube/hunger-pains?comment=129433895088#comments
https://doddleandco.com/blogs/parenting/bobbi-browns-self-care-advice-for-mamas?comment=125100097559#comments
https://neofilmshop.com/blogs/news/film-review-the-mule-2018-usa?comment=133675155754#comments
https://www.gardensundials.com/blogs/news/black-friday-is-on?comment=131615719513#comments
https://thefloralfixx.ca/blogs/news/valentines-day-flowers-and-gift-ideas?comment=131208052803#comments
https://topmusicarts.com/blogs/news/tips-for-new-music-producers?comment=131022586087#comments
https://www.532yoga.com/blog/born-tough
https://judgementyard.org/blogs/news/sizzla-kalonji-happy-earthday-caveman-studio?comment=128731840598#comments
https://makely.shop/blogs/inspiration/everything-you-need-to-know-about-adhesive-styrene?comment=134098223417#comments
https://tagzfoods.com/blogs/news/tagz-why?comment=131721658608#comments
https://evxocosmetics.com/blogs/news/makeup-tips-for-mature-skin?comment=133742461219#comments
https://bespokebinny.com/blogs/a-place-to-call-home/our-founder-natalie-manima-shares-how-she-has-balanced-her-phd-motherhood-practicing-as-a-therapist-and-entrepreneurship?comment=131337781464#comments
https://denishajulea.com/blogs/fashion-blog/more-than-a-bride-secrets-for-a-wonderful-union?comment=130696282227#comments
https://www.pureboardshop.com/blogs/pure-boardshop/2022-masters-game-of-skate?comment=132803821619#comments
https://www.zwaveoutlet.com/blogs/news/email-question-of-the-week-z-wave-and-zigbee-the-same-thing-or-different?comment=134115295514#comments
https://www.operationofhope.org/blogs/news/dr-kevin-healy-anesthesiologist-on-his-recent-medical-mission-to-malawi?comment=127814860881#comments
https://www.superfood-world.com/blogs/news/what-can-ceylon-cinnamon-do-for-your-health?comment=129433960624#comments
https://worldofdive.com/blogs/news/fit-kids-fit-you?comment=128369295511#comments
https://sajawedding.com/blogs/news/jennas-wedding-part-i?comment=125775741124#comments
https://inkboxartistry.com/blogs/inkbox-artistry-blog/15-tips-to-beat-the-instagram-algorithm?comment=135030604087#comments
https://olddirty.boston/blogs/news/jamaica-plain?comment=137655910463#comments
https://miracase.com/blogs/news/omg-should-i-get-the-iphone-case-for-the-xr?comment=129232142449#comments
http://www.themacroexperiment.com/blog/enjoying-food-protein-pumpkin-bread
https://measinasamoa.com.au/blogs/news/fai-mai-tyla-hina-lei-floral-design?comment=129791754323#comments
https://tournaments.spikeball.com/blogs/the-rally/will-origin-impact-finally-beat-cisek_showalter-at-midwest-regionals?comment=129433993392#comments
https://www.swordandplough.com/blogs/news/interview-with-a-brand-champion-the-proud-parent?comment=121188155531#comments
https://www.melodylanedesigns.com/blogs/news/starting-to-think-about-halloween?comment=125582737455#comments
https://palmettowinesellers.com/blogs/news/party-time?comment=133733810461#comments
https://www.vintagegrooming.com/blogs/grooming-articles-and-tutorials/does-size-matter-a-guide-to-beard-length?comment=134115393818#comments
https://www.thetroutlook.com/latest-updates/could-barriers-benefit-brook-trout
https://www.onevillagecoffee.com/blogs/ask-steve/how-to-choose-coffee-for-cold-brew?comment=131254911209#comments
https://www.pamhurst.com/blogs/news/changes-local-national-and-personal?comment=126468128886#comments
https://oceanzenbikini.com/blogs/press/founder-steph-gabriel-finalist-young-entrepreneur-year-award?comment=129232371825#comments
https://mulberrytreeathome.com/blogs/news/how-to-style-your-hallway?comment=128370573463#comments
https://www.vomfassusa.com/blogs/store-news/creamy-pesto-shrimp-with-lemon-pepper-linguine?comment=129574633569#comments
https://rockstreet-art.com/blogs/whats-new/i-live-a-life-of-colours-do-you-artist-alina-prodan-talking-about-colours-of-childhood-and-their-impact-on-her-art?comment=130501279815#comments
https://adorit.ca/blogs/news/10-years-old-what-can-we-say?comment=127782780994#comments
https://cafeliegeois.ca/blogs/actualite-nouvelles-cafe-liegeois/by-the-way-what-are-ese-pods?comment=131615916121#comments
https://www.vehiclebodyfittings.co.uk/blogs/news/50948165-vehicle-body-fittings-ambulance-fleetcare-services-ltd?comment=131321888846#comments
https://www.apollonnutrition.com/blogs/news/breaking-news-apollon-nutrition-founder-and-ceo-robert-samborsky-announces-upcoming-fight-in-the-warriors-cup?comment=130265678069#comments
https://viensavecmoi.ca/blogs/news/so-long-sweet-summer?comment=134098813241#comments
https://www.luckydipltd.co.uk/blogs/lucky-dip-blog/roald-to-rowling-the-wonka-legacy-of-literary-candy?comment=127724748954#comments
https://www.theflowernook.com.au/blogs/cut-blooms/cut-blooms?comment=125888594000#comments
https://www.petfon.com/blogs/love-letters/love-letter-from-marquis?comment=132117135606#comments
https://balanceandolavida.com/blogs/snacks-colaciones-y-postres/crema-de-almendra-casera?comment=129466597555#comments
https://www.inthemeadow.com.au/blogs/news/113068547-oh-hey-mazza?comment=137098166359#comments
https://socksoho.com/blogs/news/the-sock-tradition?comment=133966725413#comments
https://rebournebodyandhome.com/blogs/news/coping-with-trauma-tips-to-getting-through-it?comment=126938972344#comments
https://merkaba.nz/blogs/hemp-blog/origin-and-evolution-of-hemp-foods?comment=137657778239#comments
https://www.undracelesteny.com/blogs/ucnynews/what-a-time-to-be-alive-the-great-things-that-happened-in-2020?comment=129996161190#comments
https://givehersix.com/blogs/blog/60362501-welcome-to-give-her-six?comment=129452539957#comments
https://mihamade.com/blogs/stories/meet-the-maker-ana-maria?comment=130515730650#comments
https://sigiskin.com/blogs/news/why-do-you-need-dream-capsule-in-your-night-routine?comment=133624037685#comments
https://offonatangentshop.com/blogs/news/97445825-lets-get-this-thing-going?comment=129900970149#comments
https://www.garmentprinterink.com/blogs/sprint/anajet-sprint-resetting-the-service-counter?comment=130660761694#comments
https://www.creativeacademic.uk/blog/creativity-in-the-making
https://isabellacoutureshop.com/blogs/news/82533443-pre-introduction?comment=127174803637#Comments
https://covenandco.com/blogs/news/spring-strawberry-vanilla-cupcakes?comment=122026623146#comments
https://liondiamonds.nyc/blogs/blog/ultra-precision-machining-sysytem?comment=126770610249#comments
https://www.jeeperspeepers.co.uk/blogs/news/introducing-miranda?comment=128711950529#comments
https://www.aristelle.com/blogs/news/108343302-how-to-buy-lingerie-for-your-someone-special?comment=131395911980#comments
https://www.undracelesteny.com/blogs/ucnynews/singing-its-a-new-dawn-its-a-new-day?comment=129998749862#comments
https://www.truselforganics.com/blogs/news/what-exactly-is-a-face-serum?comment=130747072686#comments
https://mrstone.com/blogs/articles/safety-first-types-of-safety-equipment-stone-fabricators-and-installers-use?comment=127146360906#Comments
https://mrstone.com/blogs/articles/safety-first-types-of-safety-equipment-stone-fabricators-and-installers-use?comment=127146393674#Comments
https://www.kstuned.com/blogs/knowledge/to-spray-or-not-to-spray?comment=133686526232#comments
https://www.aprilcoffeeroasters.com/blogs/coffee-with-april-podcast/kapo-chiu?comment=135866188122#comments
https://oskerstore.com/blogs/news/5-australian-fashion-trends-to-keep-you-ahead-of-the-curve-this-summer-2018?comment=135360381222#comments
https://www.thekindstoreonline.co.uk/blogs/blog/diy-liquid-hand-soap?comment=132119429366#comments
https://www.sculpeyproducts.com/blogs/news/is-free-shipping-really-free?comment=130660827230#comments
https://greenfoods.com/blogs/news/choco-coco-martini-with-vibrant-collagen-creamer?comment=129956151459#comments
https://www.undracelesteny.com/blogs/ucnynews/spring-home-refresh?comment=129998782630#comments
https://us.gozney.com/blogs/news/roccbox-tops-the-lot-named-a-which-best-buy?comment=121279185033#comments
https://glamtheory.store/blogs/glam-school/how-to-avoid-the-makeup-devil-flashback?comment=125038460987#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134115229773#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135133331721#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133326536978#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127694995638#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135832961371#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684489551#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709468760#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128508526752#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624365365#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133629870364#comments
https://www.evogennutrition.com/blogs/fst-7/train-with-the-pro-creator-hadi-choopan-fst-7-quads-one-week-out?comment=121483558956#comments
https://gosciencecrazy.com/blogs/blog-science-crazy/how-ready-are-we-for-ready-player-one?comment=131112370404#comments
https://mevrouandco.com/blogs/news/milla-peertuin?comment=128925827224#comments
https://waterlemonkids.com/blogs/news/happy-anniversary?comment=128953516087#comments
https://vani-t.com/blogs/news/farewell-ghostly-paleness-hello-bronze-beauty?comment=130599846061#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/harmony-week?comment=131819405511#comments
https://shop.wattsfarms.co.uk/blogs/recipes/vegan-fresh-pesto-sauce?comment=121483591724#comments
https://micapeet.com/blogs/blog/ultimate-monstera-lovers-gift-guide-10-products-from-indie-brands-every-cheese-plant-enthusiast-need-in-their-lives?comment=129356136606#comments
https://www.ezscreenprint.com/blogs/diy-screen-printing-at-home/diy-t-shirt-screen-printing-video?comment=133975638121#comments
https://www.baristaspace.com/blogs/baristaspace/stephen-hawking-the-scientific-superstar-leave-the-world?comment=130007400518#comments
https://pulpprintshop.com/blogs/pulp-blog/pulp-artists?comment=131819733159#comments
https://www.onevillagecoffee.com/blogs/ask-steve/5-reasons-to-start-a-one-village-coffee-subscription-today?comment=131256123625#comments
https://www.bigbrattboutique.com/blogs/news/the-momma?comment=133754454332#comments
https://sofragrance.com/blogs/news/the-so-christmas-gift-set-guide-you-ve-been-waiting-for?comment=130695659739#comments
https://www.thelooploft.com/blogs/ryans-corner/29331073-drum-direktor-tips-tricks?comment=126744297652#comments
https://swannies.co/blogs/digest/brawl-on-the-back-9?comment=131045228817#comments
https://www.getgiddy.com/blogs/giddy-blog/not-just-your-average-hand-balm?comment=131681681634#comments
https://www.iridescentglassdesign.co.uk/blogs/news/tiz-the-season?comment=129956806819#comments
https://lastu.co/blogs/lastu-blog-and-vlog/minimalist-style-from-the-forests-walnut?comment=134935249239#comments
https://plushpawsproducts.com/blogs/news/whats-the-best-remedy-for-bad-breath-in-dogs?comment=132757750003#comments
https://banudesigns.com/blogs/ethnicfashion/bridal-accessories-2021?comment=133975736425#comments
https://mrstone.com/blogs/articles/30223425-a-buyer-s-guide-to-choosing-the-perfect-sink?comment=127146983498#Comments
https://www.pawzroad.com/blogs/news/amt0089-installation-video?comment=126469406838#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134116900941#comments
https://www.swishfashion.com.au/blogs/latest-blog-1/swish-armidale-interview?comment=131108733026#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130553086189#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135134445833#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133326864658#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135833878875#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684620623#comments
https://www.maxihome.com.sg/blogs/news/9-benefits-that-come-with-buying-new-furniture-for-your-home-and-living-room?comment=127727141018#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709730904#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128508952736#comments
https://gemkart.com/blogs/news/the-role-of-luck-in-stock-market?comment=130747990190#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624398133#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133629935900#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127695782070#comments
https://tailgatengo.com/blogs/press/now-they-re-cooking-inventive-family-develops-portable-outdoor-kitchens?comment=132757815539#comments
https://shelbykregel.com/blogs/the-green-bus-us/learning-to-receive?comment=130135359597#comments
https://accesslawonlineltd.com/blogs/case-law-brought-to-life/carlill-v-carbolic-smoke-ball-co-1893?comment=119910760583#comments
https://physiclo.com/blogs/news/get-fit-in-2017-giveaway?comment=134116966477#comments
https://historyonashirt.com/blogs/knights-without-parachutes/boarding-ship-from-iwo-jima?comment=129233584241#comments
https://www.operationofhope.org/blogs/news/burn-mission-cho-ray-hospital?comment=127815450705#comments
https://brandonsteiner.com/blogs/what-else/video-when-you-put-in-the-work-people-notice-1?comment=126694686892#comments
https://jophiel.com/blogs/style-news/132385283-how-does-a-fashion-trend-start?comment=132490330346#comments
https://dinamackney.com/blogs/trends/dina-mackney-trend-rainbows-and-flowers?comment=132163338461#comments
https://melapteh.com/blogs/news/a-feature-on-elle-cote-divoire?comment=131504537755#comments
https://amourducake.com/en/blogs/english-blog/swirls-crepes-or-spirals-crepes-with-video?comment=136178237768#comments
https://terrathelabel.com/blogs/news/ethicalclothing?comment=129952874599#comments
https://enjoytheriderecords.com/blogs/what-were-listening-to/what-were-listening-to-and-more-1?comment=130347925742#comments
https://harrisandfrank.com/blogs/news/how-to-wear-a-suit-in-the-summer-1?comment=131256353026#comments
https://www.spicyelement.com/blogs/recipe/grilled-salmon-head?comment=125776494788#comments
https://www.bysashatattooing.com/blogs/news/exclusive-interview-with-amazing-noraink?comment=127783600194#comments
https://orr.is/blogs/orrbyorr/orr-by-orr-blog-blog?comment=129575387233#comments
https://www.truselforganics.com/blogs/news/alexisjadekaiser?comment=130748481710#comments
https://jordandene.com/blogs/blog/coffee-break-monstress-volume-one?comment=130553217261#comments
https://nicolezizistudio.com/blogs/news/sweet-sneak-studios-photo-series-focuses-on-microplastics-in-the-food-chain?comment=119378477187#comments
https://sebastianmccall.com/blogs/pocketplacement/camo-chic?comment=130020901059#comments
https://cupofte.com/blogs/news/how-much-caffeine-is-in-a-cup-of-tea?comment=130780004565#comments
https://www.compawssion.com/blogs/blog/8361706-polo-proofs?comment=130600173741#comments
https://sudzbox.com/blogs/news/how-to-correctly-wash-your-detailing-towels?comment=133042405627#comments
https://www.dollymoo.com/blogs/news/6606909-fresh-batch-of-lavender?comment=127885180970#comments
https://milohshop.com/blogs/news/the-story-of-miloh?comment=130347958510#comments
https://thegentlepit.com/blogs/the-gentle-pit/why-are-pit-bulls-the-most-euthanized-breed-in-shelters?comment=133629968668#comments
https://www.sablebeauty.com/blogs/news/why-looking-after-your-most-initimate-parts-is-the-ultimate-form-of-selfcare?comment=137661677631#comments
https://www.melissaallenmoodessentials.com/blogs/news/positive-vibes-to-dance-love-and-laugh-more-in-2021?comment=127280742577#comments
https://flysupplyclothing.com/blogs/news/check-out-who-is-wearing-fly-supply-clothing?comment=132163600605#comments
https://vincita.cc/blogs/news/118603012-come-and-meet-us-at-vincita-lifestore-booth-at-central-festival-eastville?comment=130748547246#comments
https://jamhuriwear.com/blogs/jw-blog/yes-we-cannes?comment=126976426159#comments
https://kryptoneconsultingltd.weebly.com/articles/solid-waste-management-operations-process
https://www.wyldsmoke.com.au/blogs/news/113701062-the-shank-brothers-rib-athon?comment=130007466054#comments
https://alchemizefightwear.com/blogs/news/3-keys-for-survival-in-a-male-dominated-gym?comment=127695814838#comments
https://alchemizefightwear.com/blogs/news/3-keys-for-survival-in-a-male-dominated-gym?comment=127695847606#comments
https://raw-me.com/blogs/news/the-benefits-of-juice-cleansing?comment=131617587289#comments
https://www.oddpears.com/blogs/blog/113582275-minimal-considerate-slow-fashion-from-melbourne-label-scott-benedictine?comment=130149155008#comments
https://freckbeauty.com/blogs/ooze/shape-your-cheekslime?comment=129482260666#comments
https://www.sportstakeoff.com/blogs/look-at-this/football-hacks-missing-visor-clips-dont-worry-we-have-a-hack-for-that?comment=135768277331#comments
https://mamabanna.com/blogs/stories/beauty-treatments-from-mama?comment=125584015407#comments
https://cedarburgthreads.com/blogs/news/storytime-part-2-i-me-my-and-mine?comment=122027016362#comments
https://www.arenlace.com/blogs/blog/birthday-bae-gifts-tshirts?comment=130774597711#comments
https://www.skinnymetea.com.au/blogs/smtblog/7-green-diy-beauty-recipes?comment=134117523533#comments
https://bootstees.com/blogs/boots-tees-t-shirt-blog/40046401-believe-in-yourself-shirt-available-on-shirt-woot?comment=130748612782#comments
https://www.caulicure.com/blogs/lend-me-your-ear/the-reality-of-brazilian-jiu-jitsu-and-cauliflower-ear?comment=133686788376#comments
https://www.watsonandlou.com/blogs/news/a-nice-place-to-be?comment=137661743167#comments
https://globaltrendz.ca/blogs/blog/fishnet-knee-high-socks?comment=127783665730#comments
https://localpetmarket.com/blogs/news/the-truth-about-prescription-pet-food?comment=128735412310#comments
https://adilsons.org/blogs/anime/top-10-strongest-black-clover-characters?comment=133734170909#comments
https://gratefulgnome.com/blogs/news/riddle-time-50?comment=133676204330#comments
https://yatesprecision.com/blogs/from-the-breakroom/machinist-interview-003-jt-belknap-of-dfm-tool-works?comment=127727665306#comments
https://kizildenizgroup.com/ar/comment/37
https://merkaba.nz/blogs/hemp-blog/hemp-tofu-a-great-choice-for-vegans?comment=137662660671#comments
https://oskerstore.com/blogs/news/osker-bringing-australian-fashion-to-the-world?comment=135361233190#comments
https://jaderollerbeauty.com/blogs/jade-lifestyle-blog/elevating-esthetics-the-jade-roller-beauty%C2%AE-mission?comment=131507650789#comments
https://www.head-lites.com/blogs/the-sbark/45834241-guiding-lites?comment=125476372669#comments
https://backyardsafarico.com/blogs/news/introducing-our-new-pollinator-garden-the-honey-bee-habitat?comment=125403398315#comments
https://slipins.com/blogs/news/we-are-partnering-with-pow-people-of-the-water-a-wonderful-non-profit?comment=130781905049#comments
https://www.extensions-plus.com/blogs/hair-blog/how-to-achieve-bouncy-curls?comment=133687050520#comments
https://tex.com.tw/blogs/manufacture/about-jis-layout-design?comment=131504865435#comments
https://susuaccessories.com/blogs/the-susu-gal-blog/welcome-to-the-guild?comment=131504898203#comments
https://maisondemings.com/blogs/news/shop-small-with-living-soul-pottery?comment=134536462619#comments
https://www.grunt.com/blogs/news/marine-of-the-week-9?comment=125101899799#comments
https://dalmanjumpco.com/blogs/news/inside-vasco-flores-tack-locker?comment=126744428724#comments
https://bodsupport.com/blogs/news/causes-of-leg-pain-and-foot-pain?comment=128509313184#comments
https://provinggroundstraining.com/blogs/crossfit-motivation/how-to-get-good-at-crossfit-by-not-doing-crossfit?comment=128509345952#comments
https://www.cctung.com/it/challenge
https://kenyarn.com/blogs/kenyarn-blog/camp-kenyarn-2021-collaborative-shop-update?comment=133734203677#comments
https://www.radiancestl.com/blogs/news/iv-nutrient-therapy-boosting-health-drop-by-drop?comment=130781937817#comments
https://www.stockandbarrelco.com/blogs/s-b-blog/making-a-western-leather-laptop-bag?comment=130007498822#comments
https://saintphilippe.com/blogs/news/the-collection-2-new-collection-release?comment=131210149955#comments
https://www.springbreakwatches.com/blogs/dreamers/76238085-how-netflix-is-changing-the-world?comment=131163554024#comments
https://www.nundlenaturalskin.com.au/blogs/news/restock-and-save?comment=128071630906#Comments
https://viensavecmoi.ca/blogs/news/viens-avec-moi-inside-the-boutique?comment=134099829049#comments
https://www.chiclifestyle.com/blogs/news/once-upon-a-time?comment=129773830229#comments
https://tagzfoods.com/blogs/news/tagz-why?comment=131723821296#comments
https://s8tattoo.com/blogs/stencil-science/stencil-science-the-s8-stencil-printer?comment=131819700423#comments
https://rebournebodyandhome.com/blogs/news/5-reasons-to-go-vegan?comment=126940479672#comments
https://www.superfood-world.com/blogs/news/what-can-ceylon-cinnamon-do-for-your-health?comment=129434648752#comments
https://www.creativeacademic.uk/blog/creativity-in-the-making
https://www.theflyingkids.com/blogs/bloggers/an-ordinary-family-of-6?comment=128823034015#comments
https://www.sculpeyproducts.com/blogs/news/is-free-shipping-really-free?comment=130662236254#comments
https://greenfoods.com/blogs/news/choco-coco-martini-with-vibrant-collagen-creamer?comment=129956905123#comments
https://www.undracelesteny.com/blogs/ucnynews/spring-home-refresh?comment=129999208614#comments
https://us.gozney.com/blogs/news/roccbox-tops-the-lot-named-a-which-best-buy?comment=121279840393#comments
https://glamtheory.store/blogs/glam-school/how-to-avoid-the-makeup-devil-flashback?comment=125039509563#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134118015053#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130553774317#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135135396105#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133327356178#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127695945910#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135834173787#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684882767#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709861976#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128509378720#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624430901#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133630001436#comments
https://gosciencecrazy.com/blogs/blog-science-crazy/how-ready-are-we-for-ready-player-one?comment=131112796388#comments
https://mevrouandco.com/blogs/news/milla-peertuin?comment=128926089368#comments
https://vani-t.com/blogs/news/farewell-ghostly-paleness-hello-bronze-beauty?comment=130601222317#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/harmony-week?comment=131820617927#comments
https://brandonsteiner.com/blogs/what-else/122123523-customer-service-how-do-you-know-what-you-dont-know?comment=126694949036#comments
https://www.iceandaslice.co.uk/blogs/news/a-juniper-berry-is-a-female-seed-cone?comment=128660013138#comments
https://crawford-denim.com/blogs/blog-posts/47452933-gunne-sax-dresses?comment=128953876535#Comments
https://enjoytheriderecords.com/blogs/what-were-listening-to/what-were-listening-to-april-2018?comment=130348318958#comments
https://ruthshouseinc.com/blogs/rh-blog/14069453-lowcountry-local-first-2014-chefs-potluck-middleton-place?comment=130007531590#comments
https://www.grunt.com/blogs/news/fear-of-marines-1?comment=125102063639#comments
https://cityfarmhouseantiques.com/blogs/news/collectible-folk-art-makes-for-great-conversation-pieces-to-display-in-the-home?comment=130007564358#comments
https://shitthatiknit.com/blogs/news/how-to-care-for-your-sh-t?comment=130349072622#comments
https://www.beckystallowtreasures.com/blogs/news/shredded-luffa-ground-coffee-creative-skincare-options?comment=131618242649#Comments
https://braveamerican.com/blogs/news/3-creative-ways-to-change-your-morning-cup-of-coffee
https://www.jackofalltradesclothing.com/blogs/news/8504347-who-doesnt-put-on-a-graphic-tee-at-some-point-during-the-week-nobody?comment=129901920421#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/east-end-flower-market-wins-s-as-best-florist-2020?comment=131821371591#comments
https://www.superchargeyourgut.com/blogs/news/meet-and-greet-with-lee-holmes-in-london?comment=133688164640#comments
https://theunchartedstudio.com/blogs/uncharted-goods/surfing-magazine-help-save-puerto-ricos-waves?comment=133630066972#comments
https://lisatibalditerramia.com/en/blogs/notizie-e-curiosita/scuola-al-via-da-oggi-nuove-regole-per-la-sicurezza-di-tutti?comment=134198231377#comments
https://elkshape.com/blogs/articles/building-a-garage-gym?comment=133508628795#comments
https://www.dotandlil.com/blogs/blog/86324804-new-things-and-places-to-be?comment=133641011479#comments
https://thetemplewolf.com/blogs/news/the-temple-wolf-gives-back?comment=135888273740#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128660406354#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134119031066#comments
https://paz-creations.com/blogs/blog/mothers-day-memorial-day-may-madness?comment=131005055228#comments
https://helloring.net/blogs/news/everything-you-need-to-know-about-sapphire-engagement-rings?comment=131074130144#comments
https://vickibrowndesigns.com/blogs/blog/gloucestershire-etsy-craft-party?comment=134025412895#comments
https://sofragrance.com/blogs/news/so-top-10-vegan-bath-body-products-all-under-10?comment=130696020187#comments
https://greenfoods.com/blogs/news/should-you-be-supplementing-with-collagen?comment=129957101731#comments
https://sneakerbinge.com/blogs/news/37523969-nick-young-2-chainz-shop-for-25k-jordans-most-expensivest-shit?comment=132169564381#comments
https://www.goldrushnuggetbucket.com/blogs/gold-panning-news/48004485-famous-folks-the-california-gold-rush?comment=126629085378#comments
https://www.jenni-smith.co.uk/blogs/news/90121153-my-very-first-trade-show-stitches-2016?comment=130501673031#comments
https://www.dropbottle.com/blogs/recipes/mint-cucumber-lemon-detox-water?comment=131110305890#comments
https://bradoria.com/blogs/blog/fashion-tricks-on-how-to-hide-your-fat?comment=131682238690#comments
https://www.baristaspace.com/blogs/baristaspace/stephen-hawking-the-scientific-superstar-leave-the-world?comment=130008023110#comments
https://www.macshop.com.sg/blogs/news/happy-2021?comment=130501705799#comments
https://trenaryhomebakery.com/blogs/history-series/stand-out-this-holiday-season-with-trenary-home-bakery?comment=126977147055#comments
https://vinoparaprincipiantes.com/blogs/podcast/episodio-04-sauvignon-blanc?comment=134719242342#comments
https://waywardgourmet.com/blogs/wayward-gourmet-blog/76557573-slow-cooker-buttery-indian-chicken-stew?comment=128926351512#comments
https://www.accessoryconcierge.com/blogs/news/14483101-nighttime-denim?comment=133670306099#comments
https://www.ultratribe.com/nl/blogs/news/the-mind-s-self-defense?comment=130864972031#comments
https://www.prevenbus.com/blog/2021/11/02/nouvelle-interview-france-3—-19-20-du-2-novembre-2021.html#commentaire
https://www.smallscalesseafood.com/blogs/recipes/halibut-with-morels-and-asparagus?comment=127175196853#comments
https://timelyandtimeless.com/blogs/blog/cuckoo-clocks-for-young-and-old?comment=135104037175#comments
https://blackmagicalchemy.com/blogs/blog/how-to-create-a-vegan-chaga-cheesecake?comment=130554790125#comments
https://dianeericson.com/blogs/journal/calling-in-2021-on-a-wing-n-a-prayer?comment=133755371836#comments
https://nikaukai.com/blogs/news/first-impressions-gofoil-gl140-vs-gl180?comment=137664921663#comments
https://vidavegana.mx/blogs/vida-vegana/torta-burrera-de-pan-vegana?comment=134119358746#comments
https://earthsideecobums.com.au/blogs/top-tips/does-size-matter?comment=130604007597#comments
https://monroehouseboutique.com/blogs/daily-life-of-a-retired-rn/our-kitchen-reno?comment=132015587558#comments
https://heavy-vinyl.com/blogs/live-gig-coverage/carolina-rebellion-friday-may-5-2017?comment=124844245142#comments
https://maisondemings.com/blogs/news/diy-carriage-doors?comment=134539608347#comments
https://physiclo.com/blogs/news/116063173-6-ways-to-get-creative-with-physiclo-instagram-contest?comment=134119751757#comments
https://www.lettingref.co.uk/blog/welcome-to-our-blog
https://www.bysashatattooing.com/blogs/news/top-tattoos-back-in-stock?comment=127785730114#comments
https://deltasurvivalist.com/blogs/news/12878885-how-to-survive-a-tornado?comment=125476569277#comments
https://www.chiclifestyle.com/blogs/news/how-to-prep-your-hair-and-use-a-wig-band?comment=129774157909#comments
https://www.hazaelgaray.com/blogs/blog/como-lidiar-con-el-rechazo?comment=130424242399#comments
https://anyonefortennis.sg/blogs/all-about-tennis/15735789-tip-of-the-week-coach-dave-why-should-my-child-use-coloured-tennis-balls-surely-the-sooner-they-get-to-full-pressure-yellow-balls-the-better-right-wrong?comment=124914237486#comments
https://www.yokentheam.com/blogs/yoke-theam/one-woman-show-alia-bastamam?comment=134933610782#comments
https://www.getyournailsdid.com/blogs/the-nail-files/d?comment=134021513530#comments
https://www.blackroll.com.au/blogs/exercises/workout-mobility-exercises?comment=118992961600#comments
https://www.noreciperequired.com/technique/10-steps-perfect-pork-chop?page=19#comment-343370
https://wildernessculture.com/blogs/adventures/5-adventure-spots-you-may-not-know-about?comment=133734498589#comments
https://bespokebinny.com/de/blogs/a-place-to-call-home/passport-to-africa?comment=131339616472#comments
https://jinmee.co.uk/blogs/news/skincare-products-sizing-guide-how-much-product-do-you-really-need?comment=132812341299#comments
https://www.tejariandco.com/blogs/blog/kale-apple-banana-smoothie?comment=133733941567#comments
https://www.babybento.com.au/blogs/product-review/bento-boxes-kids-will-love?comment=128661127250#comments
https://fikacoffee.com/blogs/fikablog/122639681-the-story-behind-the-red-winged-blackbird?comment=129473904819#comments
https://www.juntadeandalucia.es/averroes/centros-tic/41009937/helvia/bitacora/index.cgi
https://www.superfood-world.com/blogs/news/benefits-baobab-pre-post-workout-endurance?comment=129435599024#comments
https://www.moirabeauty.com/blogs/news/moira-prime-plus-primer-water?comment=127149834314#comments
http://www.tpso.moc.go.th/th/node/275?page=675#comment-3624685
https://tinysparkboutique.com/blogs/news/boundaries-and-balance?comment=129490878525#comments
https://punisherspb.com/blogs/news/the-gi-stealth-paintball-marker-a-better-gtek-160r?comment=126471078006#comments
https://www.wilkinswalk.com/blogs/blogger/nike-air-max-90-total-orange-photo-blue?comment=130362278052#comments
https://www.camelhug.com/blogs/camel-hair-clothing-fabric/74318595-100-sustainable-fibre?comment=125377249364#comments
https://www.kassausa.com/blogs/inspiration/diy-whiteboard-pantry-door?comment=131380052225#comments
http://fromscotland.org.uk/news-and-events/thank-you-from-malama-feeding-centre-trust
https://www.wunderkinco.com/blogs/news/alice-and-ames?comment=126744592564#comments
https://www.chatostudio.com/blogs/news/white-sand-dune-vietnam?comment=128015302817#comments
https://www.ezscreenprint.com/blogs/diy-screen-printing-at-home/christmas-silk-screen-stencils?comment=133987532905#comments
https://gardenersorchardandbakery.com/blogs/news/save-on-your-holiday-orders?comment=122830487597#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-shipping-update-8?comment=127696863414#comments
https://johnwind.com/blogs/gotta-have-it/102816513-gifts-for-dad?comment=132173922525#comments
https://margospatterns.com/blogs/news/the-big-project?comment=131323723854#comments
https://brandonsteiner.com/blogs/what-else/122123523-customer-service-how-do-you-know-what-you-dont-know?comment=126695932076#comments
https://www.shopthebyb.com/blogs/blog-the-byb-atom/outfit-inspo-casually-cropped?comment=133733974335#comments
https://johnwind.com/blogs/gotta-have-it/102816513-gifts-for-dad?comment=132173955293#comments
https://margospatterns.com/blogs/news/the-big-project?comment=131323756622#comments
https://devilmountaincoffee.com/blogs/blogs/cold-brew-recipe?comment=130555347181#comments
https://slipins.com/blogs/news/14868935-welcome-to-our-new-website-its-opening-day?comment=130783051929#comments
https://www.missrosesisterviolet.com/blogs/all/monday-motivation?comment=128739147862#comments
https://www.thekindstoreonline.co.uk/blogs/blog/the-period-product-tax-has-finally-been-scrapped-what-now?comment=132132831478#comments
https://www.iceandaslice.co.uk/blogs/news/a-juniper-berry-is-a-female-seed-cone?comment=128661160018#comments
https://petitpeony.com/blogs/news/69803395-petit-spook-out?comment=129794211923#comments
https://crawford-denim.com/blogs/blog-posts/47452933-gunne-sax-dresses?comment=128955613239#Comments
https://ruthshouseinc.com/blogs/rh-blog/14069453-lowcountry-local-first-2014-chefs-potluck-middleton-place?comment=130008350790#comments
https://merkaba.nz/blogs/hemp-blog/how-to-make-hemp-burgers-with-hemp-seeds?comment=137670099007#comments
https://houseofsarah14.com/blogs/health-business/join-longrich?comment=131084026103#comments
https://maivino.com/blogs/maivinopours/vogue-printed-this-crazy-wine-diet-in-the-70s?comment=130352742638#comments
https://www.dotandlil.com/blogs/blog/86324804-new-things-and-places-to-be?comment=133642617111#comments
https://lisatibalditerramia.com/en/blogs/notizie-e-curiosita/scuola-al-via-da-oggi-nuove-regole-per-la-sicurezza-di-tutti?comment=134199509329#comments
https://bohochicfiberco.com/blogs/news/87446145-handmade-wardrobe?comment=127786745922#comments
https://thetemplewolf.com/blogs/news/the-temple-wolf-gives-back?comment=135889813836#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128662241362#comments
https://paz-creations.com/blogs/blog/mothers-day-memorial-day-may-madness?comment=131006169340#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134121160986#comments
https://helloring.net/blogs/news/everything-you-need-to-know-about-sapphire-engagement-rings?comment=131076292832#comments
https://vickibrowndesigns.com/blogs/blog/gloucestershire-etsy-craft-party?comment=134027378975#comments
https://savannahhayes.com/blogs/blog/artemis-design-co?comment=128413958313#comments
https://sneakerbinge.com/blogs/news/37523969-nick-young-2-chainz-shop-for-25k-jordans-most-expensivest-shit?comment=132181983453#comments
https://www.goldrushnuggetbucket.com/blogs/gold-panning-news/48004485-famous-folks-the-california-gold-rush?comment=126630953154#comments
https://trenaryhomebakery.com/blogs/history-series/stand-out-this-holiday-season-with-trenary-home-bakery?comment=126978326703#comments
https://zazu.cl/en/blogs/mundo-zazu/cuarentena-si-pero-con-estilo?comment=128194576453#comments
https://www.accessoryconcierge.com/blogs/news/14483101-nighttime-denim?comment=133670666547#comments
https://www.ultratribe.com/fr/blogs/news/the-mind-s-self-defense?comment=130866151679#comments
https://www.bysashatattooing.com/blogs/news/sashatattooing-giveaway?comment=127786844226#comments
https://naattumarundhukadai.com/blogs/news/%E0%AE%87%E0%AE%AF%E0%AE%B1%E0%AF%8D%E0%AE%95%E0%AF%88%E0%AE%AF%E0%AE%BF%E0%AE%A9%E0%AF%8D-%E0%AE%B5%E0%AE%AF%E0%AE%BE%E0%AE%95%E0%AE%B0%E0%AE%BE-%E0%AE%AE%E0%AF%81%E0%AE%B0%E0%AF%81%E0%AE%99%E0%AF%8D%E0%AE%95%E0%AF%88?comment=131257270530#comments
https://chicletzipline.com/blogs/news/los-suenos-marriott?comment=133727584553#comments
https://www.jakemax.com/blogs/news/test-blog?comment=125652041916#comments
https://sigiskin.com/blogs/news/de-puffing-your-eyes-with-gentle-gaze?comment=133625282869#comments
https://onechewthree.com.au/blogs/news/mindful-and-simplicity-gifting?comment=135285670192#comments
https://pseudolabs.com/blogs/in-pseudo/seen-in-pseudo-vol-58?comment=130479390962#comments
https://www.austinkeen.com/blogs/news/skimming-in-the-street?comment=126072291525#comments
https://nanasbabywear.co.za/blogs/behind-the-scenes/tea-party?comment=126771953737#comments
https://goteamstripes.com/blogs/the-ref-tribune/starting-with-a-handshake-the-perspective-of-a-youth-official?comment=129235812465#comments
https://www.kissedbythesunspiceco.com/blogs/blog/101370753-what-is-seasoned-sea-salt?comment=134157140269#comments
https://www.onevillagecoffee.com/blogs/ask-steve/brewing-a-chemex?comment=131262415081#comments
https://www.united-tees.com/blogs/united-tees-blog/8307948-american-legends?comment=129493794877#Comments
https://petitpeony.com/blogs/news/69803395-petit-spook-out?comment=129795588179#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/why-you-should-treat-yourself-to-an-indoor-plant?comment=131824517319#comments
https://duluthlabs.com/blogs/weeklymolecule/styrene-and-polystyrene?comment=131113746530#comments
https://www.luckydipltd.co.uk/blogs/lucky-dip-blog/knives-and-fawkes-a-bonfire-feast?comment=127733596314#comments
https://pekesafety.com/blogs/news/when-to-change-your-filters-in-the-work-place?comment=133972951333#comments
https://www.spicyelement.com/blogs/recipe/bamboo-shoots-salad?comment=125778034884#comments
https://www.utzmarket.com/blogs/musings/guatemala-is-our-muse?comment=126697701548#comments
https://cherokeesoap.com/blogs/news/hallmark-holidays?comment=127816302673#comments
https://www.shopsocialthreads.com/blogs/news/maximum-impact-minimum-effort-our-best-dresses-for-summer?comment=128662274130#comments
https://shelbykregel.com/blogs/the-green-bus-us/whimsy?comment=130141126765#comments
https://www.oddpears.com/blogs/blog/119848451-amber-day?comment=130150629568#comments
https://wendigotea.com/blogs/news/16217141-its-eyes-have-opened?comment=134936658206#comments
https://www.pinkfreshstudio.com/blogs/card-making-challenges/april-2022-challenge?comment=130766307502#comments
https://www.smallscalesseafood.com/blogs/recipes/alaska-sablefish-marinated-with-acacia-honey?comment=127175655605#comments
https://renewskinsolutions.com/blogs/news/hair-loss-the-many-causes?comment=129357447326#comments
https://birdling.com/blogs/notes-from-the-nest/diaper-duty?comment=133898993942#comments
https://www.nundlenaturalskin.com.au/blogs/news/exceptional-value-family-skin-care-pack?comment=128079790138#Comments
https://goalkicksoccer.com/blogs/news/the-new-nemeziz-cleats-and-the-tango-shoe?comment=134704922944#comments
https://starpapermoon.com/blogs/news/7-tips-to-make-your-home-beautiful-for-ramadan-and-eid?comment=129477771443#comments
https://www.sportstakeoff.com/blogs/look-at-this/vlog-2-i-am-not-a-model-deante-battle?comment=135770702163#comments
https://hourloop.com/blogs/news/40945537-disney-villains-part-5-sing-sweet-nightingale?comment=128082837562#comments
https://hardtimesclothing.co.uk/blogs/news/black-friday-2016?comment=131300982999#comments
https://www.kstuned.com/blogs/knowledge/all-h-f-series-engines-have-the-same-deck-height?comment=133687869720#comments
https://www.fortnegrita.com/blogs/fort-negrita-blog/we-understood-the-assignment?comment=128095813707#comments
https://jophiel.com/blogs/style-news/132385283-how-does-a-fashion-trend-start?comment=132505567466#comments
https://battlebars.com/blogs/news/commanders-reading-list-pumpkin-flowers-by-matti-friedman?comment=131683385570#comments
https://jamhuriwear.com/blogs/jw-blog/africa-top-5-music-videos-week-43-nov-2018?comment=126978883759#comments
https://www.plantnmore.com/blogs/news/article?comment=132505600234#comments
https://jinmee.co.uk/blogs/news/the-3-second-rule-the-korean-skincare-trend-that-improves-your-hydration?comment=132818108467#comments
https://www.wildlandorganics.com/blogs/livelight/a-very-tiny-christmas?comment=130866577663#comments
https://www.gallantoro.com/blogs/gallantoro/why-men-want-war?comment=131264807145#comments
https://sectsshop.com/blogs/sects-blog/sects-showcase-hubert?comment=131264839913#Comments
https://www.skinnymetea.com.au/blogs/smtblog/how-to-keep-active-when-you-have-a-desk-job?comment=134126108749#comments
https://www.donnasrecipe.com/blogs/donnas-blog/difference-between-leave-in-conditioner-deep-conditioner?comment=126978949295#comments
https://www.sigeeka.com/blogs/articles/shakha-pola-loha-bangles?comment=133678563626#comments
https://easternoutfitter.com/blogs/blog/six-old-duck-hunting-tips-that-still-work?comment=127787794498#comments
https://maivino.com/blogs/maivinopours/vogue-printed-this-crazy-wine-diet-in-the-70s?comment=130354446574#comments
https://imageskincare.com.au/blogs/blog/how-to-turn-your-skincare-masks-into-halloween-masks?comment=130667610206#comments
http://seminar.eventnn.ru/redir.php?link=https://spo337.com/
http://svadba.eventnn.ru/redir.php?link=https://spo337.com/
http://www.eventnn.ru/catalog/actions/?a=redirect&link=https://spo337.com/
http://www.evrika41.ru/redirect?url=https://spo337.com/
http://expomodel.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.extrimdrive.ru/bitrix/rk.php?goto=https://spo337.com/
https://facto.ru/bitrix/rk.php?goto=https://spo337.com/
http://fallout3.ru/utils/ref.php?url=https://spo337.com/
http://fbsearch.ru/away.php?url=https://spo337.com/
https://fc-zenit.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.finanalis.ru/out.php?linkout=https://spo337.com/
https://flyd.ru/away.php?to=https://spo337.com/
http://forum-region.ru/forum/away.php?s=https://spo337.com/
http://forumdate.ru/redirect-to/?redirect=https://spo337.com/
https://fr-gtr.ru/go?https://spo337.com/
https://freediving.ru/bitrix/redirect.php?goto=https://spo337.com/
http://sevensport.ru/go.php?to=https://spo337.com/
https://www.sgvavia.ru/go?https://spo337.com/
http://shckp.ru/ext_link?url=https://spo337.com/
https://sherif-karter.ru/bitrix/redirect.php?goto=https://spo337.com/
https://shinglas.ru/bitrix/redirect.php?goto=https://spo337.com/
https://shtilniisad.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.shtrih-m.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.sibpsa.ru/bitrix/redirect.php?goto=https://spo337.com/
https://sibran.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.sibsiu.ru/bitrix/rk.php?goto=https://spo337.com/
http://simvol-veri.ru/xp/?goto=https://spo337.com/
https://www.sinetic.ru/bitrix/redirect.php?goto=https://spo337.com/
http://sintez-oka.ru/bitrix/redirect.php?goto=https://spo337.com/
https://lk.sistemagorod.ru/lk/away?to=https://spo337.com/
https://lk.sistemagorod.ru/lk/away?to=https://spo337.com/
https://skamata.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.skamata.ru/bitrix/redirect.php?event1=cafesreda&event2=&event3=&goto=https://spo337.com/
http://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
https://skibaza.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=https://spo337.com/
http://mias.skoda-avto.ru/bitrix/redirect.php?event1=news_out&event2=&goto=https://spo337.com/
http://slotex.ru/bitrix/redirect.php?goto=https://spo337.com/
https://smartservices.ru/bitrix/rk.php?goto=https://spo337.com/
http://smartsourcing.ru/link?https://spo337.com/
https://smolkassa.ru/?b=1&r=https://spo337.com/
http://rexband.top4cats.ru/scripts/redirect.php?url=https://spo337.com/
http://zvezda-kuril.top4cats.ru/scripts/redirect.php?url=https://spo337.com/
http://www.topkam.ru/gtu/?url=https://spo337.com/
https://torggrad.ru/bitrix/rk.php?goto=https://spo337.com/
http://torgi-rybinsk.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.totzyv.ru/cgi-bin/clicks/redirect.pl?ei=rgb_thai_tour&goto=https://spo337.com/
http://tpprt.ru/bitrix/rk.php?goto=https://spo337.com/
https://www.tral.ru/images/get.php?go=https://spo337.com/
http://www.trucktown.ru/go.php?https://spo337.com/
http://tsm.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.tsm.ru/bitrix/rk.php?goto=https://spo337.com/
https://www.tu-bryansk.ru/bitrix/redirect.php?goto=https://spo337.com/
https://turbazar.ru/url/index?url=https://spo337.com/
https://www.tutanetam.ru/redirect?url=https://spo337.com/
http://stanko.tw1.ru/redirect.php?url=https://spo337.com/
https://twilightrussia.ru/go?https://spo337.com/
https://u74.ru/away?to=https://spo337.com/
https://go.ucrazy.ru/?to=aHR0cHM6Ly93d3cuc3BpbnN1cGVyc2xvdC5jb20v
https://ulfishing.ru/forum/go.php?https://spo337.com/
https://underwood.ru/away.html?url=https://spo337.com/
https://unicom.ru/links.php?go=https://spo337.com/
http://www.unifin.ru/bitrix/redirect.php?goto=https://spo337.com/
https://uogorod.ru/feed/520?redirect=https://spo337.com/
https://www.urso.ru/action.redirect/url/aHR0cHM6Ly93d3cuc3BpbnN1cGVyc2xvdC5jb20v/dG9wX2lkPTQxNDk=
https://utmagazine.ru/r?url=https://spo337.com/
https://utmagazine.ru/r?url=https://spo337.com/
http://econom.uu.ru/?a[]=<a+href=https://spo337.com/
http://econom.uu.ru/?a[]=<a+href=https://spo337.com/
http://uvbnb.ru/go?https://spo337.com/
https://uziolog.ru/redirect?url=https://spo337.com/
https://www.businessinsider.com.au/?s=https://spo337.com/
https://www.ehow.com/search?q=https://spo337.com/
https://www.codeproject.com/search.aspx?q=https://spo337.com/
https://www.javaworld.com/search?query=https://spo337.com/
https://www.meetup.com/find/?keywords=https://spo337.com/
https://www.familylife.com/?s=https://spo337.com/
https://thefamilydinnerproject.org/?s=https://spo337.com/
https://www.ufs.ac.za/Search-Results?indexCatalogue=All-Sites-Search-Index&searchQuery=https://https://spo337.com/
https://www.glamour.com/search?q=HTTPS%3A%2F%2Fhttps://spo337.com/
https://www.bloomberg.com/search?query=https://https://spo337.com/
https://www.chalmers.se/en/search/Pages/default.aspx?q=https://spo337.com/
https://en-urban.tau.ac.il/tau/search?keys=https://https://spo337.com/
https://www.eaie.org/search.html?queryStr=https://spo337.com/
https://www.co.monterey.ca.us/how-do-i/search?q=https://spo337.com/
https://www.handbook.fca.org.uk/handbook?site-search=https://spo337.com/
https://iconic.ftn.uns.ac.rs/?s=https://spo337.com/
https://mixxmix.com/product/search.html?banner_action=&keyword=https://spo337.com/
https://link.springer.com/search?query=https://spo337.com/
https://www.sciencedirect.com/search?qs=https://spo337.com/
https://www.nature.com/search?q=https://spo337.com/
https://www.sapo.pt/pesquisa/web/tudo?q=https://spo337.com/
https://videos.sapo.pt/search.html?word=https://spo337.com/
https://www.nbcnews.com/search/?q=https://spo337.com/
https://discover.hubpages.com/search?query=https://spo337.com/
https://search.sheffield.ac.uk/s/search.html?query=https://spo337.com/
https://news.abs-cbn.com/special-pages/search?q=https://spo337.com/#gsc.tab=0&gsc.q=https://spo337.com/
https://www.microsoft.com/nl-nl/search/explore?q=https://spo337.com/
https://en.wikipedia.org/w/index.php?search=https://spo337.com/
https://www.istockphoto.com/nl/search/2/image?
family=creative&phrase=https://spo337.com/
https://github.com/search?q=https://spo337.com/
https://www.youtube.com/results?search_query=https://spo337.com/
https://play.google.com/store/search?q=https://spo337.com/
https://www.globo.com/busca/?q=https://spo337.com/
https://www.hugedomains.com/domain_search.cfm?domain_name=https://spo337.com/
https://www.reuters.com/site-search/?query=https://spo337.com/
https://www.brandbucket.com/search?q=https://spo337.com/
https://www.weebly.com/domains?search=https://spo337.com/
https://www.gmanetwork.com/news/#/search;query=https://spo337.com/
https://edex.adobe.com/search?q=https://spo337.com/
https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=www.healthit.gov&query=https://spo337.com/
https://www.tumblr.com/search/https:https://www.businessinsider.com.au/?s=https://spo337.com/
https://www.ehow.com/search?q=https://spo337.com/
https://www.codeproject.com/search.aspx?q=https://spo337.com/
https://www.javaworld.com/search?query=https://spo337.com/
https://www.meetup.com/find/?keywords=https://spo337.com///spo337.com/
https://www.deviantart.com/search?q=https://spo337.com/
https://domains.lycos.com/search/?search=https://spo337.com/
https://www.instructables.com/howto/https://spo337.com/
https://discover.hubpages.com/search?query=https://spo337.com/
https://www.soup.io/?s=https://spo337.com/
https://startupxplore.com/en/search?q=https://spo337.com/
https://www.mywot.com/scorecard/https://spo337.com/
https://www.designspiration.com/search/saves/?q=https://spo337.com/
https://www.isixsigma.com/qsearch/?sphrase=https://spo337.com/
https://www.bloglovin.com/search?q=https://spo337.com/&search_term=https://spo337.com/
https://casa.abril.com.br/?s=https://spo337.com/
https://blogs.ubc.ca/mannatcheema/?s=https://spo337.com/&submit=Search
https://trove.nla.gov.au/search/category/websites?keyword=https://spo337.com/
https://edukite.org/?s=https://spo337.com/&post_type=course
https://www.australiantraveller.com/search/?search_term=https://spo337.com/
https://www.coupondunia.in/blog/?s=https://spo337.com/
https://jbf4093j.videomarketingplatform.co/search/perform?search=https://spo337.com/
https://ga.videomarketingplatform.co/search/perform?search=https://spo337.com/
https://www.apple.com/nz/search/https%3A-https://spo337.com/
https://www.reddit.com/search/?q=https://spo337.com/
https://observer.com/?s=https://spo337.com/
https://gektor-nsk.ru/bitrix/redirect.php?goto=https://spo337.com/
https://getfaster.ru/bitrix/rk.php?goto=https://spo337.com/
http://gfaq.ru/go?https://spo337.com/
http://ggeek.ru/bitrix/redirect.php?goto=https://spo337.com/
http://azt.ggeek.ru/azt-zbt.php?p=ggeek&backurl=https://spo337.com/
http://www.gigatran.ru/go?url=https://spo337.com/
https://gituha.ru/root/redirect.php?https://spo337.com/
https://glavkniga.ru/away.php?to=https://spo337.com/
https://glazev.ru/redirect?url=https://spo337.com/
https://globalmedia51.ru/bitrix/redirect.php?goto=https://spo337.com/
http://www.go2golf.ru/link/redirect?link=https://spo337.com/
https://godsvoice.ru/redirect?url=https://spo337.com/
https://golden-resort.ru/out.php?out=https://spo337.com/
https://goldfish.ru/bitrix/rk.php?goto=https://spo337.com/
http://avalon.gondor.ru/away.php?link=https://spo337.com/
https://good-surf.ru/r.php?g=https://spo337.com/
https://www.gooddoor.ru/bitrix/rk.php?goto=https://spo337.com/
https://clients1.google.ad/url?q=https://spo337.com/
https://cse.google.ad/url?q=https://spo337.com/
https://images.google.ad/url?q=https://spo337.com/
https://maps.google.ad/url?q=https://spo337.com/
https://www.google.ad/url?q=https://spo337.com/
https://emaratyah.ae/new-redirect.php?w=https://spo337.com/
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=https://spo337.com/
http://rafco.ae/container.asp?url=https://spo337.com/
http://for-css.ucoz.ae/go?https://spo337.com/
https://clients1.google.com.af/url?q=https://spo337.com/
https://cse.google.com.af/url?q=https://spo337.com/
https://images.google.com.af/url?q=https://spo337.com/
http://toolbarqueries.google.com.af/url?sa=t&url=https://spo337.com/
https://www.google.com.af/url?q=https://spo337.com/
https://www.snek.ai/redirect?url=https://spo337.com/
http://www.torrent.ai/lt/redirect.php?url=https://spo337.com/
http://avto.al/az/home/redirect?carId=1639612&url=https://spo337.com/
https://clients1.google.al/url?q=https://spo337.com/
https://cse.google.al/url?q=https://spo337.com/
https://images.google.al/url?q=https://spo337.com/
https://images.google.al/url?q=https://spo337.com/
http://toolbarqueries.google.al/url?q=https://spo337.com/
https://www.google.al/url?q=https://spo337.com/
http://tido.al/vazhdo.php?url=https://spo337.com/
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=https://spo337.com/
https://oxleys.app/friends.php?q=https://spo337.com/
http://www.ain.com.ar/openpop.php?url=https://spo337.com/
http://webfeeds.brookings.edu/%7E/t/0/0/%7Ewww.https://spo337.com/
https://www.weebly.com/domains?search=spo337.com
https://www.gmanetwork.com/news/#/search;query=spo337.com
https://edex.adobe.com/search?q=spo337.com
https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=www.healthit.gov&query=spo337.com
https://www.tumblr.com/search/spo337.com/
https://www.deviantart.com/search?q=spo337.com/
https://domains.lycos.com/search/?search=spo337.com/
https://www.instructables.com/howto/spo337.com/
https://discover.hubpages.com/search?query=spo337.com/
https://www.imp.mx/salto.php?va=https://spo337.com/
https://splash.hume.vic.gov.a u/analytics/outbound?url=https://spo337.com/
http://www.tupassi.pr.gov.br/enquete/enquete.php?id_cliente=1027&url=https://spo337.com/
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=https://spo337.com/
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2012011201&return=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2014062701&return=https://spo337.com/
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2014062701&return=https://spo337.com/
https://splash.hume.vic.gov.au/analytics/outbound?url=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2012011201&return=https://spo337.com/
http://www.tupassi.pr.gov.br/enquete/enquete.php?id_cliente=1027&url=https://spo337.com/
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://spo337.com/
https://clubs.london.edu/click?uid=24ce7c96-76b4-11e6-8631-0050569d64d5&r=https://spo337.com/
https://www.nbc.edu/videoplayer.php?u=https://spo337.com/
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=https://spo337.com/
http://rs.rikkyo.ac.jp/rs/error/ApplicationError.aspx?TopURL=https://spo337.com/
https://publishing.brookes.ac.uk/?URL=https://spo337.com/
https://toolbarqueries.google.co.vi/url?q=https://spo337.com/
http://jclick.koreadaily.com/JAgent1.dll?3*16*https://spo337.com/
http://my.51edu.cc/link.php?url=https://spo337.com/
https://mybrawlstats.com/redirect.php?lang=german&urlReceived=https://spo337.com/
http://www.peterblum.com/DES/DateAndTime.aspx?Returnurl=https://spo337.com/
http://m.17ll.com/apply/tourl/?url=https://spo337.com/
https://arxip.com/bitrix/rk.php?goto=https://spo337.com/
https://fpess.scu.ac.ir/en/web/cow/display/-/asset_publisher/6j7CwiyKWPfS/document/id/1307867?redirect=https://spo337.com/
https://www.autocar.co.nz/Redirect.aspx?destination=https://spo337.com/
https://track.effiliation.com/servlet/effi.redir?id_compteur=13215059&url=https://spo337.com/
http://ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=https://spo337.com/
https://www.usjournal.com/go.php?campusID=190&url=https://spo337.com/
https://www.winnipegfreepress.com/s?action=doLogout&rurl=https://spo337.com/
https://www.kyoto-good.jp/ad.php?url=https://spo337.com/
https://analytics.bluekai.com/site/16231?phint=event=click&phint=campaign=BRAND-TAB&phint=platform=search&done=https://spo337.com/
https://temptationsaga.com/buy.php?url=https://spo337.com/&store=Books-A-Million
https://socialmart.ru/redirect.php?url=https://spo337.com/
https://www.interpals.net/url_redirect.php?href=https://spo337.com/
https://toolbarqueries.google.co.zw/url?q=https://spo337.com/
https://www.xfdq123.com/url.aspx?url=https://spo337.com/
http://www.bshare.cn/share?url=https://spo337.com/
https://www.nanotech-now.com/redir.cgi?dest=https://spo337.com/
https://sukhoverkhov.ru/wp-content/plugins/wp-js-external-link-info/redirect.php?blog=&url=https://spo337.com/
https://toolbarqueries.google.gm/url?q=https://spo337.com/
https://www.cosmeticchoice.com.au/index.php?route=banners/banners/clickthru&banner_id=159&expiration_setting=2&impressions=0&link=https://spo337.com/
https://toolbarqueries.google.ae/url?sa=i&url=https://spo337.com/
https://www.bausch.in/redirect/?url=https://spo337.com/
https://www.bausch.com.ph/redirect/?url=https://spo337.com/
http://www.wittstock.chemie.uni-oldenburg.de/agef/link_extern.html?link=https://spo337.com/
https://uk.kindofbook.com/redirect.php/?red=https://spo337.com/
https://kakaku-navi.net/items/detail.php?url=https://spo337.com/
https://www.viidii.info/?https://spo337.com/
https://www.cricbattle.com/register.aspx?returnurl=https://spo337.com/
https://www.havanautos.com/dtcspot/public/banner_redirect.aspx?idca=343&ids=86839669&cp=167&idcli=0&ci=1&p=https://spo337.com/
https://linkerload.com/url/go.php?url=https://spo337.com/
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=https://spo337.com/
https://aquajeux.com/produits/controle-et-gestion-d-eau/activation-electronique/pado-329?r=https://spo337.com/
http://www.howtotrainyourdragon.co.nz/notice.php?url=https://spo337.com/
https://www.bausch.com.tw/redirect/?url=https://spo337.com/
http://www.shippingchina.com/pagead.php?id=RW4uU2hpcC5tYWluLjE=&tourl=https://spo337.com/
https://toolbarqueries.google.com.tw/url?q=https://spo337.com/
http://janeyleegrace.worldsecuresystems.com/redirect.aspx?destination=https://spo337.com/
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=https://spo337.com/
https://www.sigel.de/en_gb/Weiterempfehlen?url=https://spo337.com/
https://www.apegs.ca/Portal/Pages/sign-up-member?returnUrl=https://spo337.com/
https://namathis.com/verpagina_header.php?pagina=https://spo337.com/
https://api.mymosey.com/forward?url=https://spo337.com/&vendor=KLOOK
https://g0.more.game.tw/ogames.php?l=210&t=280&w=910&h=750&s=https://spo337.com/
https://www.lionscup.dk/?side_unique=4fb6493f-b9cf-11e0-8802-a9051d81306c&s_id=30&s_d_id=64&go=https://spo337.com/
https://toolbarqueries.google.dk/url?q=https://spo337.com/
http://aps.sn/spip.php?page=clic&id_publicite=366&id_banniere=6&from=/actualites/sports/lutte/article/modou-lo-lac-de-guiers-2-l-autre-enjeu&redirect=https://spo337.com/
https://pse5-esd5.aadnc-aandc.gc.ca/pubcbw/publication/display_file.aspx?link=https://spo337.com/
http://kreepost.com/go/?https://spo337.com/
https://www.1upfun.com/redirect.aspx?url=https://spo337.com/
http://mailer.dt.co.kr/bin/checker?mode=5&module=11&mailidx=4275&dmidx=0&emidx=0&service=0&etime=20080227100001&seqidx=18963&objidx=16&url=https://spo337.com/
https://www.matchesfashion.com/us/affiliate?url=https://spo337.com/
https://im.tonghopdeal.net/pic.php?q=https://spo337.com/
https://toolbarqueries.google.com.bn/url?q=https://spo337.com/
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=https://spo337.com/
https://leadertoday.org/topframe2014.php?goto=https://spo337.com/
https://spb-medcom.ru/redirect.php?https://spo337.com/
https://www.info-realty.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.sermemole.com/public/serbook/redirect.php?url=https://spo337.com/
https://www.hradycz.cz/redir.php?b=445&t=https://spo337.com/
https://heaven.porn/te3/out.php?u=https://spo337.com/
https://toolbarqueries.google.com.ar/url?sa=i&url=https://spo337.com/
http://prlog.ru/backlink/https://spo337.com/
https://www.ntnu.edu.tw/frame.php?url=https://spo337.com/
http://park3.wakwak.com/~yadoryuo/cgi-bin/click3/click3.cgi?cnt=chalet-main&url=https://spo337.com/
https://adminstation.ru/verification/?url=https://spo337.com/
https://toolbarqueries.google.bg/url?q=https://spo337.com/
https://weblib.lib.umt.edu/redirect/proxyselect.php?url=//https://spo337.com/
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=https://spo337.com/
http://www.jtrchina.com/m2c/2/s_date0.jsp?tree_id=0&sdate=2020-03-09&url=https://spo337.com/
http://weekly.chosun.com/protect_weekly/redirect.asp?url=https://spo337.com/
http://www.paladiny.ru/go.php?url=https://spo337.com/
https://toolbarqueries.google.com.ng/url?sa=i&url=https://spo337.com/
https://maps.google.cv/url?q=https://spo337.com/
https://www.datum.tv/ra.asp?url=https://spo337.com/
https://vssillc.asureforce.net/redirect.aspx?redirecturl=https://spo337.com/
http://2ch-ranking.net/redirect.php?url=https://spo337.com/
https://www.google.mk/url?q=https://spo337.com/
https://mycounter.com.ua/go.php?https://spo337.com/
http://db.studyincanada.ca/forwarder.php?f=https://spo337.com/
https://live.warthunder.com/away/?to=https://spo337.com/
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=https://spo337.com/
http://www.yoger.com.cn/union/transfer.php?uID=10125&url=https://spo337.com/
http://www.016788.com/gourl.asp?url=https://spo337.com/
https://maps.google.bt/url?q=https://spo337.com/
https://guru.sanook.com/?URL=https://spo337.com/
https://www.badaweb.com/detall.php?uid=20000530190000-002&control=hoQChn9YtFJwg&idioma=0&keyword=P%E0ginaPrincipaldeBW&cat=&ciutat=5&url=https://spo337.com/
https://tool.365jz.com/alexa/https://spo337.com/
https://www.juina.mt.gov.br/atalhoconta.php?id=7&link=https://spo337.com/
https://www.bb.com.tr/util/change-language.php?lng=EN&url=https://spo337.com/
http://www.01caijing.com/go.htm?url=https://spo337.com/
아시안커넥트 먹튀검증 https://spo337.com/
머니라인247 먹튀검증 https://spo337.com/
황룡카지노 먹튀검증 https://spo337.com/
안전 온라인카지노 추천 https://spo337.com/
안전 카지노사이트 추천 https://spo337.com/
실시간 라이브배팅 https://spo337.com/
해외배팅사이트 추천 https://spo337.com/
해외스포츠배팅사이트 추천 https://spo337.com/
해외배팅사이트에이전시 추천 https://spo337.com/
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://spo337.com/
www.joeshouse.org/booking?link=https://spo337.com/&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=https://spo337.com/
swra.backagent.net/ext/rdr/?https://spo337.com/
namiotle.pl/?wptouch_switch=mobile&redirect=https://spo337.com/
golfpark.jp/banner/counter.aspx?url=https://spo337.com/
www.mexicolore.co.uk/click.php?url=https://spo337.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=https://spo337.com/
www.invisalign-doctor.in/api/redirect?url=https://spo337.com/
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=https://spo337.com/
www.immunallergo.ru/bitrix/redirect.php?goto=https://www.https://spo337.com/
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=https://spo337.com/
trackingapp4.embluejet.com/Mod_Campaigns/tracking.asp?idem=31069343&em=larauz@untref.edu.ar&ca=73143&ci=0&me=72706&of=581028&adirecta=0&url=https://spo337.com/
smils.ru/bitrix/redirect.php?goto=https://spo337.com/
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=https://spo337.com/
cipresso.ru/bitrix/redirect.php?goto=https://spo337.com/
www.vacacionartravel.com/DTCSpot/public/banner_redirect.aspx?idca=286&ids=75665176&cp=167&idcli=0&ci=2&p=https://spo337.com/
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=https://spo337.com/
www.malles-bertault.com/?change_langue=en&url=http%253a%252f%252fhttps://spo337.com/
www.beeicons.com/redirect.php?site=https://spo337.com/
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=https://spo337.com/
www.cccowe.org/lang.php?lang=en&url=https://spo337.com/
www.mytokachi.jp/index.php?type=click&mode=sbm&code=2981&url=https://spo337.com/
www.wqketang.com/logout?goto=https://spo337.com/
access.bridges.com/externalRedirector.do?url=https://spo337.com/
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://spo337.com/
bot.buymeapie.com/recipe?url=https://spo337.com/
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://spo337.com/
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=https://spo337.com/
quartiernetz-friesenberg.ch/links-go.php?to=https://spo337.com/
www.maultalk.com/url.php?to=https://spo337.com/
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=https://spo337.com/
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=https://spo337.com/
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=https://spo337.com/
http://getdatasheet.com/url.php?url=https://spo337.com/
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://spo337.com/
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=https://spo337.com/
www.aldolarcher.com/tools/esstat/esdown.asp?File=https://spo337.com/
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&nochat=1&domain=https://spo337.com/
http://blackwhitepleasure.com/cgi-bin/atx/out.cgi?trade=https://spo337.com/
azlan.techdata.com/InTouch/GUIBnrT3/BnrTrackerPublic.aspx?CountryCode=18&BannerLangCulture=nl-nl&URL=https://spo337.com/&Target=2&BannerId=41919&Zoneid=281&Parameters=&cos=Azlan
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44zoneid=1cb=7743e8d201oadest=https://spo337.com/
https://www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=https://spo337.com/
www.lzmfjj.com/Go.asp?URL=https://spo337.com/
http://alexmovs.com/cgi-bin/atx/out.cgi?id=148&tag=topatx&trade=https://spo337.com/
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2bannerid=40zoneid=16cb=fc1d72225coadest=https://spo337.com/
psylive.ru/success.aspx?id=0&goto=https://spo337.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=https://spo337.com/
www.mybunnies.net/te3/out.php?u=https://spo337.com/
lubaczowskie.pl/rdir/?l=https://spo337.com/&lid=1315
www.kowaisite.com/bin/out.cgi?id=kyouhuna&url=https://spo337.com/
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=https://spo337.com/
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=https://spo337.com/
https://asstomouth.guru/out.php?url=https://spo337.com/
advtest.exibart.com/adv/adv.php?id_banner=7201&link=https://spo337.com/
https://thairesidents.com/l.php?b=85&p=2,5&l=https://spo337.com/
www.latestnigeriannews.com/link_channel.php?channel=https://spo337.com/
www.haogaoyao.com/proad/default.aspx?url=https://spo337.com/
globalmedia51.ru/bitrix/redirect.php?goto=https://spo337.com/
citysafari.nl/Home/setCulture?language=en&returnUrl=https://spo337.com/
es.catholic.net/ligas/ligasframe.phtml?liga=https://spo337.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://spo337.com/
www.webshoptrustmark.fr/Change/en?returnUrl=https://spo337.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=https://spo337.com/
e.ourger.com/?c=scene&a=link&id=47154371&url=https://spo337.com/
zh-hk.guitarians.com/home/redirect/ubid/1015?r=https://spo337.com/
www.mastercleaningsupply.com/trigger.php?r_link=https://spo337.com/
lrnews.ru/xgo.php?url=https://spo337.com/
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://https://spo337.com/
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=https://spo337.com/
spb-medcom.ru/redirect.php?https://spo337.com/
forest.ru/links.php?go=https://spo337.com/
reefcentral.ru/bitrix/rk.php?goto=https://spo337.com/
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=https://spo337.com/
https://trackdaytoday.com/redirect-out?url=https://spo337.com/
https://bigjobslittlejobs.com/jobclick/?RedirectURL=https://spo337.com/&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2bannerid=4zoneid=4cb=68dbdae1d1oadest=https://spo337.com/
www.obertaeva.com/include/get.php?go=https://spo337.com/
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=https://spo337.com/
https://zueeen123.blogspot.com/2024/01/for-its-2023-games-wagering-industry.html
https://site-6828921-2394-9309.mystrikingly.com/blog/to-be-a-really-successful-season-in-sports-wagering-sanctioning-don-t-expect
https://leejk777.blogspot.com/2024/01/ohios-most-memorable-year-was-news.html
https://casino999.mystrikingly.com/blog/terrible-beats-of-2023-in-the-most-obviously-awful-games-wagering
https://parkjk777.blogspot.com/2024/01/states-revision-3-certainly.html
https://site-4763676-9520-5980.mystrikingly.com/blog/top-500-million-handle-for-fifth-time-in-new-york-portable-sportsbooks
https://emiko1231.blogspot.com/2024/01/wizards-leaving-dc-could-fundamentally.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-in-golden-state-bettors-not-seeing-green
https://yoo-777.blogspot.com/2024/01/will-wagering-be-lawful-in-california.html
https://daddys-lucky000.mystrikingly.com/blog/arizona-puts-bow-on-unrivaled-october-of-sports-wagering-in-u-s
https://jay-777.blogspot.com/2024/01/which-sports-wagering-administrators.html
https://lucky-razi-777.mystrikingly.com/blog/statistical-surveying-shows-betting-minimal-impacted-notwithstanding-expansion
https://jody222000.blogspot.com/2024/01/ex-jags-worker-having-to-carry-out.html
https://site-6861405-2997-4499.mystrikingly.com/blog/massachusetts-draftkings-overstepped-state-regulation-in-tolerating
https://zueeen123.blogspot.com/2024/01/sports-wagering-rules-in-north-carolina.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-zeroing-in-additional-on-the-most-proficient-method-to-address-badgering
https://leejk777.blogspot.com/2024/01/renunciation-of-entain-chief-might.html
https://casino999.mystrikingly.com/blog/has-the-opportunity-come-to-boost-card-sharks-to-utilize-mindful-betting
https://parkjk777.blogspot.com/2024/01/kentucky-breaks-from-entryways-with-340.html
https://site-4763676-9520-5980.mystrikingly.com/blog/vermont-lottery-chooses-draftkings-aficionados-and-fanduel-for-jan-11-send
https://emiko1231.blogspot.com/2024/01/sportsbook-administrators-answer-ohio.html
https://site-6861241-1631-6047.mystrikingly.com/blog/could-hard-rock-wager-florida-outperform-all-of-new-york-in-month-to-month
https://yoo-777.blogspot.com/2024/01/ideal-time-overs-monday-night.html
https://daddys-lucky000.mystrikingly.com/blog/nfl-week-14-sports-wagering-men-not-engaging-in-sexual-relations-real
https://jay-777.blogspot.com/2024/01/cuban-adelson-exchange-could-hold.html
https://lucky-razi-777.mystrikingly.com/blog/report-unlv-new-mexico-examined-for-dubious-betting-action
https://jody222000.blogspot.com/2024/01/fanduel-and-draftkings-took-on-virginia.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-rests-on-pga-visit-for-2024-north-carolina-market-access
https://zueeen123.blogspot.com/2024/01/7-of-greatest-possible-advantages-of.html
https://site-6828921-2394-9309.mystrikingly.com/blog/pot-in-las-vegas-club-it-s-up-for-conversation
https://leejk777.blogspot.com/2024/01/sports-wagering-bankroll-executives.html
https://casino999.mystrikingly.com/blog/the-primary-post-this-is-sportshandle
https://parkjk777.blogspot.com/2024/01/where-significant-games-associations.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-101-nfl-lines-chances-point-spreads-and-that-s-only-the
https://emiko1231.blogspot.com/2024/01/nj-representative-leonard-spear-on.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sportshandle-s-brett-smiley-talks-sports-wagering-with-chris-moore-of-cbs
https://yoo-777.blogspot.com/2024/01/a-breakdown-of-draftkings-2023-tycoon.html
https://daddys-lucky000.mystrikingly.com/blog/sportshandle-talks-sports-wagering-authorization-with-cbs-sports-radio-s-bill
https://jay-777.blogspot.com/2024/01/new-jerseys-briefs-in-high-court-sports.html
https://lucky-razi-777.mystrikingly.com/blog/ron-paul-pens-section-tearing-charlie-imprint-s-web-based-gaming-boycott
https://jody222000.blogspot.com/2024/01/matthew-and-mikey-of-espn-in-lexington.html
https://site-6861405-2997-4499.mystrikingly.com/blog/supercontest-legend-james-salinas-standards-for-sports-wagering-achievement
https://zueeen123.blogspot.com/2024/01/supercontest-legend-james-salinas.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-jersey-senator-pallone-submits-amicus-brief-in-sports-wagering-case-in
https://leejk777.blogspot.com/2024/01/20-states-join-new-jersey-to-help.html
https://casino999.mystrikingly.com/blog/michigan-discussions-bill-that-would-legitimize-internet-gaming
https://parkjk777.blogspot.com/2024/01/sports-regulation-teacher-on-wagering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-would-be-simple-expansion-for-atlantic-city-gambling-clubs
https://emiko1231.blogspot.com/2024/01/logical-games-gaining-nyx-gaming-in.html
https://site-6861241-1631-6047.mystrikingly.com/blog/richard-sherman-says-nfl-injury-reports-are-explicitly-for-players
https://yoo-777.blogspot.com/2024/01/the-total-imbeciles-manual-for-sports.html
https://daddys-lucky000.mystrikingly.com/blog/there-s-zero-amazing-sections-staying-in-westgate-las-vegas-supercontest
https://jay-777.blogspot.com/2024/01/new-jersey-delegate-straightforward.html
https://lucky-razi-777.mystrikingly.com/blog/new-survey-larger-part-of-americans-backing-legitimizing-sports-wagering-for
https://jody222000.blogspot.com/2024/01/this-wagering-resembles-in-london-for.html
https://site-6861405-2997-4499.mystrikingly.com/blog/longshot-covers-produce-down-week-in-westgate-las-vegas-supercontest
https://zueeen123.blogspot.com/2024/01/nba-legend-wizardry-johnson-predicts.html
https://site-6828921-2394-9309.mystrikingly.com/blog/the-ascent-of-esports-and-wagering-opportunity-and-entanglements
https://leejk777.blogspot.com/2024/01/crazy-bosses-redskins-last-play-flipped.html
https://casino999.mystrikingly.com/blog/the-south-oaks-betting-screen-take-the-issue-betting-test
https://parkjk777.blogspot.com/2024/01/sports-regulation-teacher-on-wagering.html
https://site-4763676-9520-5980.mystrikingly.com/blog/the-chernin-gathering-pushes-all-in-on-sports-wagering-and-dream-with
https://emiko1231.blogspot.com/2024/01/nfl-week-5-wagering-recap-street-groups.html
https://site-6861241-1631-6047.mystrikingly.com/blog/how-three-bettors-benefitted-from-one-wagering-ticket-thanks-to-giancarlo
https://yoo-777.blogspot.com/2024/01/new-jersey-representatives-urge-house.html
https://daddys-lucky000.mystrikingly.com/blog/gaming-regulation-master-predicts-new-jersey-triumph-in-high-court-sports
https://jay-777.blogspot.com/2024/01/legitimate-master-assesses-high-court.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-passes-bill-that-would-sanction-sports-wagering-yet-with-a
https://jody222000.blogspot.com/2024/01/previous-wr-mike-pritchard-talks-dark.html
https://site-6861405-2997-4499.mystrikingly.com/blog/long-term-redskins-te-chris-cooley-gives-player-s-perspective-on-sports-wagering
https://zueeen123.blogspot.com/2024/01/maryland-legislator-and-paper-needs-to.html
https://site-6828921-2394-9309.mystrikingly.com/blog/andrew-brandt-on-the-nfl-and-sports-wagering-dream-helps-overcome-any-barrier
https://leejk777.blogspot.com/2024/01/today-is-only-seventeenth-games.html
https://casino999.mystrikingly.com/blog/previous-wr-mike-pritchard-talks-dark-horses-and-sports-wagering-future-for
https://parkjk777.blogspot.com/2024/01/on-sports-wagering-long-term-redskins.html
https://site-4763676-9520-5980.mystrikingly.com/blog/supporting-games-wagering-boycott-trump-named-lawyer-documents-high-court-brief
https://emiko1231.blogspot.com/2024/01/celtics-attorney-explains-adam-silvers.html
https://site-6861241-1631-6047.mystrikingly.com/blog/7-nfl-wagering-notes-at-the-season-s-close-halfway-point
https://yoo-777.blogspot.com/2024/01/why-nfl-really-needs-to-lose-high-court.html
https://daddys-lucky000.mystrikingly.com/blog/dodgers-versus-astros-worldwide-championship-wagering-lines-and-props
https://jay-777.blogspot.com/2024/01/new-jersey-flames-back-at-associations.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-authorizes-sports-wagering-forthcoming-change-in-government
https://jody222000.blogspot.com/2024/01/sports-wagering-usa-important-points.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sports-wagering-gathering-action-items-money-might-be-above-all-else-however
https://zueeen123.blogspot.com/2024/01/unlv-meeting-audit-ncaa-actually.html
https://site-6828921-2394-9309.mystrikingly.com/blog/antitrust-tripwires-lawful-master-makes-sense-of-sports-wagering-information
https://leejk777.blogspot.com/2024/01/a.html
https://casino999.mystrikingly.com/blog/james-holzhauer-resists-pattern-gets-peril-rewards-before-country-learns-of
https://parkjk777.blogspot.com/2024/01/imperfections-and-everything-il-senate.html
https://site-4763676-9520-5980.mystrikingly.com/blog/illinois-sports-wagering-heads-to-senate-for-conclusive-endorsement
https://emiko1231.blogspot.com/2024/01/illinois-meeting-expanded-and-sports.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nba-finals-the-same-old-thing-for-vegas-books-regardless-of-critical
https://yoo-777.blogspot.com/2024/01/where-to-play-online-sportsbooks-and.html
https://daddys-lucky000.mystrikingly.com/blog/the-street-ahead-for-the-rotogrinders-organization
https://jay-777.blogspot.com/2024/01/us-open-wagering-review-streams-koepka.html
https://lucky-razi-777.mystrikingly.com/blog/montana-administrators-neglect-to-supersede-lead-representative-on-sports
https://jody222000.blogspot.com/2024/01/legitimate-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/oregon-representatives-attempting-to-kill-sports-wagering
https://zueeen123.blogspot.com/2024/01/westgate-supercontest-players-score.html
https://site-6828921-2394-9309.mystrikingly.com/blog/celtics-attorney-explains-adam-silver-s-backing-for-authorized-sports-wagering
https://leejk777.blogspot.com/2024/01/7-nfl-wagering-notes-at-seasons-close.html
https://casino999.mystrikingly.com/blog/worldwide-championship-wagering-lines-and-props-of-dodgers-versus-astros
https://parkjk777.blogspot.com/2024/01/sports-wagering-usa-action-items-part.html
https://site-4763676-9520-5980.mystrikingly.com/blog/sports-wagering-gathering-focal-points-money-might-be-top-dog-yet
https://emiko1231.blogspot.com/2024/01/a-background-marked-by-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/just-around-the-corner-the-debut-sports-wagering-usa-meeting
https://yoo-777.blogspot.com/2024/01/tps-report-nfl-week-10-picks.html
https://daddys-lucky000.mystrikingly.com/blog/house-legal-executive-director-and-sports-wagering-rival-weave-goodlatte-to
https://jay-777.blogspot.com/2024/01/might-draftkings-at-some-point-truly.html
https://lucky-razi-777.mystrikingly.com/blog/sportshandle-talks-sports-wagering-with-jody-mcdonald-on-cbs-sports-radio
https://jody222000.blogspot.com/2024/01/new-jersey-congressperson-beam-lesniak.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nj-senator-sports-wagering-defender-candid-lobiondo-to-resign
https://zueeen123.blogspot.com/2024/01/michigan-could-prepared-itself-for.html
https://site-6828921-2394-9309.mystrikingly.com/blog/longshots-packers-power-nice-week-in-westgate-las-vegas-supercontest
https://leejk777.blogspot.com/2024/01/sitting-tight-for-sports-wagering-at-us.html
https://casino999.mystrikingly.com/blog/here-s-sound-for-oral-contention-in-new-jersey-s-high-court-sports-wagering-case
https://parkjk777.blogspot.com/2024/01/what-global-soccer-can-instruct-us.html
https://site-4763676-9520-5980.mystrikingly.com/blog/massachusetts-peering-toward-sports-wagering-in-wake-of-high-legal-dispute
https://emiko1231.blogspot.com/2024/01/senator-pallone-acquaints-bill-with.html
https://site-6861241-1631-6047.mystrikingly.com/blog/las-vegas-supercontest-players-score-enormous-in-week-13-as-canines-return
https://jay-777.blogspot.com/2024/01/mississippi-locked-and-stacked-for.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-administrators-hope-to-authorize-sports-wagering-put-arrangement-on
https://jody222000.blogspot.com/2024/01/somewhat-less-discussion-somewhat-more.html
https://site-6861405-2997-4499.mystrikingly.com/blog/the-ascent-and-energy-of-in-play-wagering-made-sense-of-by-master
https://zueeen123.blogspot.com/2024/01/indiana-sports-wagering-bill-contains.html
https://site-6828921-2394-9309.mystrikingly.com/blog/congress-a-gem-ball-and-the-debate-over-sports-wagering
https://leejk777.blogspot.com/2024/01/nfl-trump-card-end-of-week-wagering.html
https://casino999.mystrikingly.com/blog/vegas-legend-dave-cokin-joins-the-support-why-i-bet-with-jimmy-shapiro
https://parkjk777.blogspot.com/2024/01/how-might-jeff-meetings-enemy-of-weed.html
https://site-4763676-9520-5980.mystrikingly.com/blog/georgia-legislators-will-push-for-gambling-clubs-in-18-regardless-of-resistance
https://emiko1231.blogspot.com/2024/01/nfl-2017-normal-season-wagering-recap.html
https://site-6861241-1631-6047.mystrikingly.com/blog/indiana-legislator-wanting-to-support-bill-sanctioning-games-wagering-in-state
https://yoo-777.blogspot.com/2024/01/west-virginia-officials-reestablish.html
https://daddys-lucky000.mystrikingly.com/blog/nfl-divisional-round-wagering-recap-lookahead-to-titles
https://jay-777.blogspot.com/2024/01/effect-of-1-duty-on-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/new-york-senate-to-hold-hearing-on-capability-of-sports-wagering-in-the-state
https://jody222000.blogspot.com/2024/01/should-indianas-proposed-1-games.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nba-pushes-for-legitimate-games-wagering-in-new-york-yet-needs-slice-of-the-pie
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-legalized.html
https://site-6828921-2394-9309.mystrikingly.com/blog/illinois-enters-legitimate-games-wagering-bill-fight-as-congressperson
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-strategy.html
https://casino999.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-journey.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-sports-wagering-bill-would-allow-betting-at-five-club-and-on
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-mastery.html
https://site-6828921-2394-9309.mystrikingly.com/blog/fanduel-closures-2023-with-top-games-wagering-portion-of-the-overall-industry
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-skill.html
https://casino999.mystrikingly.com/blog/no-less-than-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-guide.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-wagering-bills-acquire-from-ohio-maryland-regulation
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-articles-wins.html
https://site-6861241-1631-6047.mystrikingly.com/blog/a-hawks-fan-stands-up-to-the-uncommon-blended-profound-support-bet
https://bettingstratigicarticle.blogspot.com/2024/01/betting-stratigic-articles-decision.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-ultimate.html
https://lucky-razi-777.mystrikingly.com/blog/florida-gaming-magistrate-sports-bettors-show-enthusiasm-in-first-month
https://gameswageringarticles.blogspot.com/2024/01/blog-post.html
https://site-6861405-2997-4499.mystrikingly.com/blog/versatile-games-betting-authoritatively-dispatches-in-vermont
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-guide.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-an-idea-for-gubernatorial-up-and-comers
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-successful.html
https://casino999.mystrikingly.com/blog/colorado-clears-50-million-in-september-sports-betting-income
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-long-term-success.html
https://site-4763676-9520-5980.mystrikingly.com/blog/tennessee-observes-two-years-of-lawful-portable-games-betting
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-articles.html
https://site-6861241-1631-6047.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-versatile-betting-send-off
https://bettingstratigicarticle.blogspot.com/2024/01/betting-tratigic-article-basic-bet.html
https://daddys-lucky000.mystrikingly.com/blog/new-york-wagering-handle-arrives-at-most-elevated-point-since-college-basketball
https://sportswageringinfo.blogspot.com/2024/01/live-sports-wagaring-info.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-controller-nixes-draftkings-solicitation-for-widespread-day-for
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-defying-crowd.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maryland-bettors-draw-one-stage-nearer-to-lawful-versatile-betting
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-underdog.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-an-idea-for-gubernatorial-competitors
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-multiple-bets.html
https://casino999.mystrikingly.com/blog/ontario-dispatches-face-to-face-sports-wagering-at-retail-club-in-area
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-point-spread.html
https://site-4763676-9520-5980.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-portable-betting-send-off
https://bestsportsbettingarticles.blogspot.com/2024/01/best%20sports%20betting%20articles.html
https://site-6861241-1631-6047.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bettingstratigicarticle.blogspot.com/2024/01/betting-strategic-articles-disciplined-mindset.html
https://daddys-lucky000.mystrikingly.com/blog/betr-acquires-sports-betting-business-sector-access-in-three-additional-states
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-profits.html
https://lucky-razi-777.mystrikingly.com/blog/nascar-prepared-to-exploit-north-carolina-sports-wagering
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-specialized.html
https://site-6861405-2997-4499.mystrikingly.com/blog/somewhere-around-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-story-proposition-bets.html
https://site-6828921-2394-9309.mystrikingly.com/blog/north-carolina-online-games-betting-to-send-off-on-walk-11
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-strategy_01802557671.html
https://casino999.mystrikingly.com/blog/reports-iowa-specialist-blamed-for-warrantless-sports-wagering-search
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-analysis.html
https://site-4763676-9520-5980.mystrikingly.com/blog/responding-to-key-inquiries-regarding-maryland-s-portable-betting-send-off
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-markets.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pennsylvania-finishes-memorable-year-with-record-month-to-month-sports
https://bettingstratigicarticle.blogspot.com/2024/01/betting-strategic-articles-influences.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-mobile-tips.html
https://lucky-razi-777.mystrikingly.com/blog/rocha-california-drive-will-encourage-harm-the-brand-of-versatile-games
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-articles-supercharge.html
https://site-6861405-2997-4499.mystrikingly.com/blog/is-america-terrible-with-awful-games-bettors
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-overview.html
https://site-6828921-2394-9309.mystrikingly.com/blog/ohio-legislator-needs-to-school-himself-partners-on-sports-wagering
https://sportsbettingindustry.blogspot.com/2024/01/sports-betting-industry-beginner.html
https://casino999.mystrikingly.com/blog/lawful-games-wagering-in-the-u-s-the-english-are-coming-the-english-are
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-modern-data.html
https://site-4763676-9520-5980.mystrikingly.com/blog/hollywood-club-set-to-offer-west-virginia-sports-wagering-before-sept-1
https://bestsportsbettingarticles.blogspot.com/2024/01/best-sports-betting-different-types.html
https://site-6861241-1631-6047.mystrikingly.com/blog/delaware-gambling-clubs-state-to-partake-in-450-000-after-second-month-of
https://bettingstratigicarticle.blogspot.com/2024/01/sustanable-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/connecticut-lead-representative-gives-officials-cutoff-time-to-follow-up-on
https://sportswageringinfo.blogspot.com/2024/01/sports-real-time-wagering-info-.html
https://lucky-razi-777.mystrikingly.com/blog/what-s-in-store-at-the-book-sportsbook-coming-soon-to-the-horseshoe-tunica
https://gameswageringarticles.blogspot.com/2024/01/games-wagering-article-players.html
https://site-6861405-2997-4499.mystrikingly.com/blog/pennsylvania-s-parx-gambling-club-and-gan-report-sports-wagering-organization
https://onlinesportsbettingstory.blogspot.com/2024/01/mobile-online-sports-beting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/hawaii-s-statewide-versatile-bill-is-feeling-the-loss-of-a-few-critical-parts
https://sportsbettingindustry.blogspot.com/2024/01/common-mistake-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/what-s-the-games-wagering-worth-of-a-mentor
https://articleinsportsbetting.blogspot.com/2024/01/legal-landscape-article-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/report-barstool-sports-to-go-into-advertising-concurrence-with-draftkings
https://bestsportsbettingarticles.blogspot.com/2024/01/impact-psychology-best-sport-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/fanduel-starts-offering-monetary-proficiency-administrations-to-bettors
https://bettingstratigicarticle.blogspot.com/2024/01/opportunities-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/little-north-carolina-universities-excited-for-wagering-duty-bonus
https://sportswageringinfo.blogspot.com/2024/01/sports-wagering-info-odds.html
https://lucky-razi-777.mystrikingly.com/blog/pennsylvania-finishes-notable-year-with-record-month-to-month-sports-betting
https://gameswageringarticles.blogspot.com/2024/01/dynamic-games-wagering-articles.html
https://site-6861405-2997-4499.mystrikingly.com/blog/virginia-bill-would-legitimize-wagering-in-state-school-groups
https://onlinesportsbettingstory.blogspot.com/2024/01/asian-handicap-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/mlb-creates-absurd-new-contention-for-cut-of-sports-wagering-bets
https://sportsbettingindustry.blogspot.com/2024/01/fantasy-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/connecticut-considering-new-advertisement-club-and-sports-wagering-bill
https://articleinsportsbetting.blogspot.com/2024/01/article-sports-betting-profit.html
https://site-4763676-9520-5980.mystrikingly.com/blog/west-virginia-official-talks-about-state-s-games-wagering-bill-and-future-of
https://bestsportsbettingarticles.blogspot.com/2024/01/consideration-sports-betting-best-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/why-sports-expectation-markets-are-like-onions-and-film-industry-film-receipts
https://bettingstratigicarticle.blogspot.com/2024/01/technology-betting-strategic-article.html
https://daddys-lucky000.mystrikingly.com/blog/iowa-bill-to-authorize-sports-wagering-advances-subtleties-need-pressing
https://sportswageringinfo.blogspot.com/2024/01/future-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/nevada-sportsbooks-end-on-a-positive-note-in-december-for-record-249-million
https://gameswageringarticles.blogspot.com/2024/01/blog-post_31.html
https://site-6861405-2997-4499.mystrikingly.com/blog/kansas-bounces-on-board-lawful-games-wagering-regulation-trend
https://onlinesportsbettingstory.blogspot.com/2024/01/online-sports-betting-pro-level.html
https://site-6828921-2394-9309.mystrikingly.com/blog/sports-wagering-bankroll-the-executives-benefits-victors-and-discipline-1623a8ef-e6da-4dd6-bedb-5596e78ec1af
https://sportsbettingindustry.blogspot.com/2024/01/game-changing-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/sports-wagering-nfl-lines-chances-point-spreads-and-then-some
https://articleinsportsbetting.blogspot.com/2024/01/optimal-wins-sports-betting-.html
https://site-4763676-9520-5980.mystrikingly.com/blog/new-jersey-representative-pallone-submits-amicus-brief-in-sports-wagering
https://bestsportsbettingarticles.blogspot.com/2024/02/best-odds-earnings-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/american-gaming-affiliation-records-brief-in-sports-wagering-case-in-high-court
https://bettingstratigicarticle.blogspot.com/2024/02/essential-tips-sports-betting.html
https://daddys-lucky000.mystrikingly.com/blog/n-j-representative-leonard-spear-on-sports-wagering-jersey-merits
https://sportswageringinfo.blogspot.com/2024/02/sports-wagering-triumph.html
https://lucky-razi-777.mystrikingly.com/blog/7-of-the-greatest-possible-advantages-of-lawful-managed-sports-wagering
https://gameswageringarticles.blogspot.com/2024/02/games-wagering-victory-tips.html
https://site-6861405-2997-4499.mystrikingly.com/blog/bettor-at-focus-of-alabama-baseball-embarrassment-has-to-deal-with-upwards-of
https://onlinesportsbettingstory.blogspot.com/2024/02/mastering-online-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-sports-wagering-bill-starts-development-through-senate
https://sportsbettingindustry.blogspot.com/2024/02/successful-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/looking-for-bettors-and-administrators-for-wide-arriving-at-industry-reviews
https://articleinsportsbetting.blogspot.com/2024/02/savvy-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/california-sports-wagering-defender-brings-an-end-to-drive-exertion
https://bestsportsbettingarticles.blogspot.com/2024/02/winning-streak-sports-betting-article.html
https://site-6861241-1631-6047.mystrikingly.com/blog/something-like-two-arizona-betting-licenses-to-go-available-to-all-in-february
https://bettingstratigicarticle.blogspot.com/2024/02/mastering-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/virginia-bill-would-authorize-wagering-in-state-school-groups-f8de9e02-c6a6-495d-9ec9-14a31daf9d51
https://onlinesportsbettingstory.blogspot.com/2024/02/changing-sports-betting-story.html
https://site-6828921-2394-9309.mystrikingly.com/blog/georgia-lawmaker-once-again-introduces-sports-wagering-bill
https://sportsbettingindustry.blogspot.com/2024/02/mastering-sports-betting-industry.html
https://casino999.mystrikingly.com/blog/rocha-california-drive-will-assist-harm-the-brand-of-portable-games-wagering
https://articleinsportsbetting.blogspot.com/2024/02/mastering-revolutionary-sports-betting.html
https://site-4763676-9520-5980.mystrikingly.com/blog/is-america-crummy-with-awful-games-bettors
https://bestsportsbettingarticles.blogspot.com/2024/02/best-tip-sports-betting.html
https://site-6861241-1631-6047.mystrikingly.com/blog/vermont-s-three-portable-sportsbooks-all-set-with-send-off-advancements
https://bettingstratigicarticle.blogspot.com/2024/02/expert-betting-strategic.html
https://daddys-lucky000.mystrikingly.com/blog/media-note-pad-where-might-the-telecom-companies-be-without-sportsbook
https://sportswageringinfo.blogspot.com/2024/02/successful-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/yearlings-texans-same-game-parlay-expects-pass-cheerful-undertaking
https://gameswageringarticles.blogspot.com/2024/02/secret-success-games-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/maturing-superteams-among-wagering-top-choices-to-bring-home-nba-championship
https://onlinesportsbettingstory.blogspot.com/2024/02/proven-winnings-online-sports-betting.html
https://site-6828921-2394-9309.mystrikingly.com/blog/climate-abbreviated-pga-visit-occasion-sparkles-sports-wagering-discussion
https://sportsbettingindustry.blogspot.com/2024/02/mastering%20strategies-sports-betting.html
https://casino999.mystrikingly.com/blog/an-hour-endeavors-to-handle-sports-wagering-thrashes-about-all-things-being
https://articleinsportsbetting.blogspot.com/2024/02/art-sports-betting-article.html
https://site-4763676-9520-5980.mystrikingly.com/blog/ncaa-solicitations-restriction-on-school-player-prop-wagers-in-ohio
https://bestsportsbettingarticles.blogspot.com/2024/02/unbeatable-sports%20betting-tips.html
https://site-6861241-1631-6047.mystrikingly.com/blog/liv-golf-wagering-markets-presented-in-additional-states-for-2024-season
https://bettingstratigicarticle.blogspot.com/2024/02/profit-expert-betting-stratigic.html
https://daddys-lucky000.mystrikingly.com/blog/mississippi-online-games-wagering-bill-clears-house-missouri-bill-escapes-panel
https://sportswageringinfo.blogspot.com/2024/02/Winning-Sports-Wagering.html
https://lucky-razi-777.mystrikingly.com/blog/swift-at-the-super-bowl-can-t-wager-on-it
https://gameswageringarticles.blogspot.com/2024/02/strategies-excel-ports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-betr-getting-ready-to-leave-massachusetts-market
https://zueeen123.blogspot.com/2023/11/cavalier-of-california-sports-wagering.html
https://site-6828921-2394-9309.mystrikingly.com/blog/from-activity-organization-nfl-smartest-options-for-week-8
https://leejk777.blogspot.com/2023/11/for-wagering-on-golf-pga-suspends-two.html
https://casino999.mystrikingly.com/blog/21-rule-in-massachusetts-a-potential-problem-for-espn-devotees
https://parkjk777.blogspot.com/2023/11/betway-pulls-out-application-for-online.html
https://site-4763676-9520-5980.mystrikingly.com/blog/first-nhl-player-to-get-betting-suspension-shane-pinto-supposedly-suspended
https://emiko1231.blogspot.com/2023/11/liv-golf-needs-major-wagering-interest.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sports-wagering-again-legitimate-yet-not-live-in-florida-as-legal-disputes
https://yoo-777.blogspot.com/2023/11/heres-reason-media-review-gave-edge-to.html
https://daddys-lucky000.mystrikingly.com/blog/does-house-cash-or-expertise-make-sports-bettors-want-more-and-more
https://jay-777.blogspot.com/2023/11/versatile-wagering-authorization.html
https://lucky-razi-777.mystrikingly.com/blog/early-inquiries-emerge-around-tennessee-s-wagering-expense-design
https://jody222000.blogspot.com/2023/11/this-year-with-astros-wagers-sleeping.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nba-prospects-wagers-we-love-for-2023-2024
https://zueeen123.blogspot.com/2023/12/florida-high-court-stretches-out.html
https://site-6828921-2394-9309.mystrikingly.com/blog/at-arizona-pga-visit-objective-draftkings-opens-retail-sportsbook
https://leejk777.blogspot.com/2023/12/driving-investor-thinks-espn-bet-will.html
https://casino999.mystrikingly.com/blog/g2e-consents-to-installment-settlement-for-israel-based-lsports-in-the-midst
https://parkjk777.blogspot.com/2023/12/american-gaming-affiliation-proceeds.html
https://site-4763676-9520-5980.mystrikingly.com/blog/desantis-solicitations-expansion-on-the-off-chance-that-that-could-direct
https://emiko1231.blogspot.com/2023/12/following-up-high-court-administering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/espn-bet-divulges-official-logo-in-front-of-november-send-off
https://yoo-777.blogspot.com/2023/12/record-promotion-offers-aided-lift.html
https://daddys-lucky000.mystrikingly.com/blog/draftkings-crawls-toward-maryland-retail-sportsbook-opening
https://jay-777.blogspot.com/2023/12/massachusetts-gaming-commission-hopes.html
https://lucky-razi-777.mystrikingly.com/blog/north-carolina-lottery-pushes-ahead-with-fundamental-games-wagering-rules
https://jody222000.blogspot.com/2023/12/media-note-pad-ascent-of-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/new-jersey-sports-betting-handle-floods-to-1-3-billion-in-september
https://zueeen123.blogspot.com/2023/12/sportradar-virtuoso-games-hit-with.html
https://site-6828921-2394-9309.mystrikingly.com/blog/25-billion-in-post-paspa-sports-betting-handle-in-illinois-outperforms
https://leejk777.blogspot.com/2023/12/there-are-2-key-business-sectors-it.html
https://casino999.mystrikingly.com/blog/more-youthful-bettors-have-more-assorted-betting-propensities-than-more
https://parkjk777.blogspot.com/2023/12/from-sending-off-hard-rock-bet-high.html
https://site-4763676-9520-5980.mystrikingly.com/blog/kansas-sets-new-high-for-sports-betting-handle-at-219-3-million
https://emiko1231.blogspot.com/2023/12/portable-handle-record-with-1692.html
https://site-6861241-1631-6047.mystrikingly.com/blog/houston-is-preferred-over-its-division-adversary-in-an-all-texas-al-series
https://yoo-777.blogspot.com/2023/12/ncaas-disposition-toward-sports.html
https://daddys-lucky000.mystrikingly.com/blog/which-live-characters-will-advance-betting-at-the-point-when-espn-bet-shows-up
https://jay-777.blogspot.com/2023/12/sports-wagering-controllers-acclimate.html
https://lucky-razi-777.mystrikingly.com/blog/nhl-abounding-with-incredible-connors-to-wager-on-as-season-starts
https://jody222000.blogspot.com/2023/12/new-longshot-dream-asset-will-support.html
https://site-6861405-2997-4499.mystrikingly.com/blog/greatest-administrators-astounded-to-find-out-about-potential-california
https://zueeen123.blogspot.com/2023/12/mindful-betting-development-new.html
https://site-6828921-2394-9309.mystrikingly.com/blog/astonished-to-catch-wind-of-potential-california-sports-wagering-drive
https://leejk777.blogspot.com/2023/12/espn-bet-is-lock-to-send-off-by-this.html
https://casino999.mystrikingly.com/blog/what-s-on-draft-nfl-week-6-nhl-season-opener-and-2023-worldwide-gaming
https://parkjk777.blogspot.com/2023/12/an-early-groundwork-school-football.html
https://site-4763676-9520-5980.mystrikingly.com/blog/activity-organization-s-nfl-smartest-options-for-week-5
https://emiko1231.blogspot.com/2023/12/overcomes-and-dodgers-are-greatest-mlb.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nevada-rep-commends-nfl-for-patched-up-sports-wagering-strategy-however
https://yoo-777.blogspot.com/2023/12/around-sports-formally-opens-second.html
https://daddys-lucky000.mystrikingly.com/blog/georgia-remains-school-football-wagering-number-one-after-unsteady-september
https://jay-777.blogspot.com/2023/12/ncaa-hopes-to-return-to-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/maine-controller-going-for-the-gold-computerized-send-off
https://jody222000.blogspot.com/2023/12/finally-around-is-prepared-to-play-in.html
https://site-6861405-2997-4499.mystrikingly.com/blog/potential-chances-to-hop-on-line-development-in-bills-jags-falcons-rams
https://zueeen123.blogspot.com/2023/12/whats-in-store-at-meadowlands-fanduel.html
https://site-6828921-2394-9309.mystrikingly.com/blog/timetable-of-state-and-sportsbook-improvements-u-s-sports-wagering-in-2018
https://leejk777.blogspot.com/2023/12/the-week-in-sports-wagering-and-sports.html
https://casino999.mystrikingly.com/blog/to-restrict-portable-wagering-to-on-premises-pga-visit-concurs-it-d-be-absurd
https://parkjk777.blogspot.com/2023/12/fanduel-scores-arrangement-to-oversee.html
https://site-4763676-9520-5980.mystrikingly.com/blog/mailbag-mythbusting-the-kitchen-sink-rules
https://emiko1231.blogspot.com/2023/12/hammerin-hank-goldberg-offers-accounts.html
https://site-6861241-1631-6047.mystrikingly.com/blog/pittsburgh-privateers-take-sports-wagering-respectability-expense-to
https://yoo-777.blogspot.com/2023/12/monitoring-west-virginias-games.html
https://daddys-lucky000.mystrikingly.com/blog/the-unlawful-betting-organizations-act-and-sports-wagering-mailbag-mythbusting
https://jay-777.blogspot.com/2023/12/new-jersey-sports-wagering-dispatches.html
https://lucky-razi-777.mystrikingly.com/blog/pounding-hank-goldberg-joins-the-support-why-i-bet-with-jimmy-shapiro
https://jody222000.blogspot.com/2023/12/in-any-case-so-what-in-blazes-is.html
https://site-6861405-2997-4499.mystrikingly.com/blog/mailbag-mythbusters-uigea-and-sports-wagering-made-sense-of
https://zueeen123.blogspot.com/2023/12/furthermore-pickem-dream-is-restricted.html
https://site-6828921-2394-9309.mystrikingly.com/blog/to-rule-virginia-sports-betting-scene-fanduel-proceeds
https://leejk777.blogspot.com/2023/12/income-on-ascent-again-in-ohio-wagering.html
https://casino999.mystrikingly.com/blog/dodgers-and-every-other-person-as-nl-special-case-week-starts-wagering
https://parkjk777.blogspot.com/2023/12/activity-organizations-games-bet-nfl.html
https://site-4763676-9520-5980.mystrikingly.com/blog/nfl-rolls-out-enormous-improvements-to-betting-arrangement
https://emiko1231.blogspot.com/2023/12/its-ideal-time-again-as-sanders.html
https://site-6861241-1631-6047.mystrikingly.com/blog/retail-book-in-illinois-around-sports-dispatches-versatile-application
https://yoo-777.blogspot.com/2023/12/virginia-sportsbooks-beat-bettors-up.html
https://daddys-lucky000.mystrikingly.com/blog/court-choice-clears-way-for-hard-rock-bet-to-send-off-in-florida-however
https://jay-777.blogspot.com/2023/12/caesars-approaches-send-off-of-firebets.html
https://lucky-razi-777.mystrikingly.com/blog/in-maryland-draftkings-draws-nearer-to-opening-retail-sportsbook
https://jody222000.blogspot.com/2023/12/live-blog-whats-going-on-kentucky.html
https://site-6861405-2997-4499.mystrikingly.com/blog/europe-a-thin-ryder-cup-wagering-number-one
https://zueeen123.blogspot.com/2023/12/hard-rock-bet-might-be-fastest-and.html
https://site-6828921-2394-9309.mystrikingly.com/blog/north-carolina-commission-sure-of-portable-betting-send-off-by-june-cutoff-time
https://leejk777.blogspot.com/2023/12/canelo-means-to-join-boxings-uncommon.html
https://casino999.mystrikingly.com/blog/florida-parimutuels-train-in-on-seminole-minimal-in-state-court
https://parkjk777.blogspot.com/2023/12/the-arizona-division-of-gaming-detailed.html
https://site-4763676-9520-5980.mystrikingly.com/blog/north-carolina-versatile-betting-authorizing-change-draws-in-industry
https://emiko1231.blogspot.com/2023/12/ontario-partners-need-clearness-on-most.html
https://site-6861241-1631-6047.mystrikingly.com/blog/nfl-virtuoso-games-reveal-stream-and-bet-stage-through-betvision
https://yoo-777.blogspot.com/2023/12/in-most-recent-documenting-doi-advises.html
https://daddys-lucky000.mystrikingly.com/blog/two-of-seven-stages-in-kentucky-will-permit-wagering-at-age-18-beginning
https://jay-777.blogspot.com/2023/12/schuetz-substantial-mistakes-in-sports.html
https://lucky-razi-777.mystrikingly.com/blog/school-brotherhood-accomplices-with-psychological-wellness-supplier-to-check
https://jody222000.blogspot.com/2023/12/activity-organizations-nfl-smartest.html
https://site-6861405-2997-4499.mystrikingly.com/blog/nfl-week-3-alexander-mattison-murmurs-and-whenever-score-wagers
https://zueeen123.blogspot.com/2023/12/i-need-you-to-quant-me-super-advanced.html
https://site-6828921-2394-9309.mystrikingly.com/blog/new-sumsub-stage-permits-sportsbooks-to-handle-mindful-betting
https://leejk777.blogspot.com/2023/12/for-versatile-betting-send-off-north.html
https://casino999.mystrikingly.com/blog/for-web-based-betting-in-kentucky-sportsbooks-carrying-out-pre-send-off-offers
https://parkjk777.blogspot.com/2023/12/with-same-game-parlay-potential-week-3.html
https://site-4763676-9520-5980.mystrikingly.com/blog/report-d-c-lottery-cutoff-points-retail-bets-after-cafe-bettor-wins-huge
https://emiko1231.blogspot.com/2023/12/also-bettors-improved-pennsylvania.html
https://site-6861241-1631-6047.mystrikingly.com/blog/furthermore-presumably-substantially-more-portable-games-wagering-in
https://yoo-777.blogspot.com/2023/12/about-his-betting-compulsion-phil.html
https://daddys-lucky000.mystrikingly.com/blog/for-sports-wagering-and-igaming-asset-roundhill-to-change-fundamental-file
https://jay-777.blogspot.com/2023/12/north-carolina-financial-plan.html
https://lucky-razi-777.mystrikingly.com/blog/massachusetts-sports-betting-scene-draftkings-keeps-on-overwhelming
https://jody222000.blogspot.com/2023/12/gross-income-for-sports-betting.html
https://site-6861405-2997-4499.mystrikingly.com/blog/fanduel-sets-single-week-portable-income-record-in-new-york
https://zueeen123.blogspot.com/2023/12/will-prohibet-give-one-quit-shopping-to.html
https://site-6828921-2394-9309.mystrikingly.com/blog/how-do-stodgy-noses-need-to-manage-capable-betting
https://leejk777.blogspot.com/2023/12/five-significant-administrators-submit.html
https://casino999.mystrikingly.com/blog/sports-wagering-authorizing-development-in-maryland-legislator-looks-for-retail
https://parkjk777.blogspot.com/2023/12/prime-sportsbook-set-to-start-up-in.html
https://site-4763676-9520-5980.mystrikingly.com/blog/geocomply-reports-242-million-nfl-week-1-wagering-exchanges-up-56-over-a
https://emiko1231.blogspot.com/2023/12/with-around-90-of-2023-mlb-season.html
https://site-6861241-1631-6047.mystrikingly.com/blog/looks-for-retail-sports-wagering-permitting-development-in-maryland-official
https://yoo-777.blogspot.com/2023/12/sports-betting-invites-any-and-all.html
https://daddys-lucky000.mystrikingly.com/blog/superteams-supercharge-wnba-wagering-interest-as-end-of-the-season-games-start
https://jay-777.blogspot.com/2023/12/colorado-becomes-6th-state-to.html
https://lucky-razi-777.mystrikingly.com/blog/in-any-case-most-don-t-utilize-apparatuses-bettors-have-a-lot-of-experience
https://jody222000.blogspot.com/2023/12/chances-development-mirrors-colorados.html
https://site-6861405-2997-4499.mystrikingly.com/blog/rodgers-possibly-season-finishing-injury-rocks-planes-prospects-chances
https://zueeen123.blogspot.com/2023/12/maryland-bettors-at-last-thump-hold.html
https://site-6828921-2394-9309.mystrikingly.com/blog/media-scratch-pad-fanduel-television-s-new-cousin-sal-shows-join-swarmed
https://leejk777.blogspot.com/2023/12/draftkings-posts-then-at-that-point.html
https://casino999.mystrikingly.com/blog/north-carolina-lottery-looks-for-sports-betting-authorizing-chief
https://parkjk777.blogspot.com/2023/12/in-sports-betting-income-for-2023-new.html
https://site-4763676-9520-5980.mystrikingly.com/blog/activity-organization-s-nfl-smartest-choices-in-sports-wagering
https://emiko1231.blogspot.com/2023/12/allowed-to-play-supercontest-now.html
https://site-6861241-1631-6047.mystrikingly.com/blog/devotees-sportsbook-promotion-code-arrangement-awards-official-group-jersey
https://yoo-777.blogspot.com/2023/12/kansas-sees-ups-and-downs-in-initial.html
https://daddys-lucky000.mystrikingly.com/blog/missouri-polling-form-proposition-documented-would-send-choice-to-authorize
https://jay-777.blogspot.com/2023/12/friday-live-blog-following-games.html
https://lucky-razi-777.mystrikingly.com/blog/nfl-week-1-notes-from-a-non-wagering-master-and-a-ton-of-alexander-mattison
https://jody222000.blogspot.com/2023/12/iowa-controller-proposes-refreshed.html
https://site-6861405-2997-4499.mystrikingly.com/blog/thursday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable
https://zueeen123.blogspot.com/2023/12/kansas-sees-ups-and-downs-in-initial.html
https://site-6828921-2394-9309.mystrikingly.com/blog/friday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable-week
https://leejk777.blogspot.com/2023/12/refreshed-wagering-rules-after-school.html
https://casino999.mystrikingly.com/blog/thursday-live-blog-following-games-wagering-news-as-nfl-s-most-memorable
https://parkjk777.blogspot.com/2023/12/what-around-stress-little-overlay.html
https://site-4763676-9520-5980.mystrikingly.com/blog/draftkings-offering-prop-on-absolute-nfl-focuses-for-2023-season
https://emiko1231.blogspot.com/2023/12/heres-where-youll-have-option-to-place.html
https://site-6861241-1631-6047.mystrikingly.com/blog/some-iowa-iowa-state-competitors-acknowledge-betting-supplication-arrangements
https://yoo-777.blogspot.com/2023/12/schuetz-football-is-back-fail-to.html
https://daddys-lucky000.mystrikingly.com/blog/betr-dispatches-its-genuine-cash-portable-sportsbook-in-virginia
https://jay-777.blogspot.com/2023/12/wednesday-live-blog-following-games.html
https://lucky-razi-777.mystrikingly.com/blog/approved-for-departure-sports-handle-staff-picks-for-2023-nfl-season
https://jody222000.blogspot.com/2023/12/for-week-1-worth-street-canine-titans.html
https://site-6861405-2997-4499.mystrikingly.com/blog/acuna-or-betts-for-mvp-you-choose-on-the-grounds-that-we-can-t
https://zueeen123.blogspot.com/2023/12/espn-moves-sports-wagering-project.html
https://site-6828921-2394-9309.mystrikingly.com/blog/prospects-payouts-put-drag-on-july-sportsbook-income-in-nevada
https://leejk777.blogspot.com/2023/12/north-carolina-lottery-making-progress.html
https://casino999.mystrikingly.com/blog/ontario-carries-out-more-severe-betting-publicizing-rules
https://parkjk777.blogspot.com/2023/12/schuetz-business-passed-up-great.html
https://site-4763676-9520-5980.mystrikingly.com/blog/arizona-awards-sports-wagering-permit-to-bet365-as-seventeenth-administrator
https://emiko1231.blogspot.com/2023/12/nascar-re-ups-with-sportradar-signs.html
https://site-6861241-1631-6047.mystrikingly.com/blog/sporttrade-dispatches-in-colorado-as-upstart-novig-seeks-after-permit
https://yoo-777.blogspot.com/2023/12/huge-ten-football-to-give-game-day.html
https://daddys-lucky000.mystrikingly.com/blog/there-s-no-need-to-focus-on-everyone-beginning-a-similar-page-it-s-about
https://jay-777.blogspot.com/2023/12/wagering-markets-give-djokovic-alcaraz.html
https://lucky-razi-777.mystrikingly.com/blog/the-bettors-annus-horribilis-a-year-of-betting-across-the-u-s
https://jody222000.blogspot.com/2023/12/maryland-lottery-fines-draftkings-94400.html
https://site-6861405-2997-4499.mystrikingly.com/blog/sporttrade-gets-second-administrator-permit-this-one-in-colorado
https://zueeen123.blogspot.com/2023/12/south-dakota-lead-representative-signs.html
https://site-6828921-2394-9309.mystrikingly.com/blog/connecticut-lead-representative-arrives-at-sports-wagering-manage-clans
https://leejk777.blogspot.com/2023/12/north-dakota-official-looks-for-two.html
https://casino999.mystrikingly.com/blog/maryland-sports-wagering-online-md-sportsbooks-and-join-offers
https://parkjk777.blogspot.com/2023/12/wyoming-officials-turn-around-course-as.html
https://site-4763676-9520-5980.mystrikingly.com/blog/wyoming-house-votes-against-lawful-games-wagering
https://emiko1231.blogspot.com/2023/12/texas-electors-need-sports-wagering.html
https://site-6861241-1631-6047.mystrikingly.com/blog/connecticut-sports-wagering-injured-last-week-yet-wagering-in-time-for-nfl
https://yoo-777.blogspot.com/2023/12/georgia-senate-supports-sports-wagering.html
https://daddys-lucky000.mystrikingly.com/blog/take-a-few-to-get-back-some-composure-the-week-in-sports-wagering
https://jay-777.blogspot.com/2023/12/arizona-house-sends-sports-wagering.html
https://lucky-razi-777.mystrikingly.com/blog/south-dakota-administrators-send-sports-wagering-bill-to-lead-representative
https://jody222000.blogspot.com/2023/12/things-turn-severe-in-battle-for.html
https://site-6861405-2997-4499.mystrikingly.com/blog/these-states-have-sports-wagering-on-house-senate-floors-yet-will-any-move
https://zueeen123.blogspot.com/2023/12/fanduel-currently-taking-pickleball.html
https://site-6828921-2394-9309.mystrikingly.com/blog/yippee-sportsbook-opens-up-at-the-venetian-hotel-las-vegas
https://leejk777.blogspot.com/2023/12/schuetz-meeting-hello-wagering-slamming.html
https://casino999.mystrikingly.com/blog/usa-s-true-j-v-group-expected-to-win-fiba-world-cup
https://parkjk777.blogspot.com/2023/12/college-of-iowa-competitors-learn.html
https://site-4763676-9520-5980.mystrikingly.com/blog/u-s-business-sports-betting-handle-outperforms-250-billion-post-paspa
https://emiko1231.blogspot.com/2023/12/superbook-bar-and-cafe-at-camden-yards.html
https://site-6861241-1631-6047.mystrikingly.com/blog/enthusiasts-sportsbook-cleared-for-send-off-soon-in-pennsylvania
https://yoo-777.blogspot.com/2023/12/industry-insiders-dave-portnoy-perhaps.html
https://daddys-lucky000.mystrikingly.com/blog/contention-carries-same-game-parlays-to-esports-wagering
https://jay-777.blogspot.com/2023/12/media-note-pad-distrust-has-large.html
https://lucky-razi-777.mystrikingly.com/blog/fan-sportsbook-dispatches-official-application-in-four-states
https://jody222000.blogspot.com/2023/12/whether-overs-or-unders-there-are-ncaa.html
https://site-6861405-2997-4499.mystrikingly.com/blog/wynnbet-leave-means-licenses-will-be-accessible-in-arizona-and-virginia
https://zueeen123.blogspot.com/2023/12/caesars-probably-will-not-have-retail.html
https://site-6828921-2394-9309.mystrikingly.com/blog/devotees-starts-taking-retail-wagers-in-ohio-rapidly-after-send-off-of
https://leejk777.blogspot.com/2023/12/arizonas-june-sports-betting-handle-up.html
https://casino999.mystrikingly.com/blog/parlays-power-new-jersey-sportsbooks-to-third-consecutive-10-month-to-month
https://parkjk777.blogspot.com/2023/12/media-journal-wariness-has-large.html
https://site-4763676-9520-5980.mystrikingly.com/blog/most-recent-court-recording-focuses-to-no-florida-sports-wagering-before-nfl
https://emiko1231.blogspot.com/2023/12/most-recent-court-documenting-focuses.html
https://site-6861241-1631-6047.mystrikingly.com/blog/massachusetts-sportsbooks-guarantee-30-1-million-in-income-for-july
https://yoo-777.blogspot.com/2023/12/expected-to-send-off-in-three-states-by.html
https://daddys-lucky000.mystrikingly.com/blog/sports-wagering-s-privileged-hugs-state-of-the-art-innovation
https://jay-777.blogspot.com/2023/12/schools-stress-sports-betting.html
https://lucky-razi-777.mystrikingly.com/blog/illinois-sportsbooks-proceed-solid-2023-with-54-7-million-income-for-junethe
https://jody222000.blogspot.com/2023/12/wynn-resorts-intends-to-close-wynnbet.html
https://site-6861405-2997-4499.mystrikingly.com/blog/draftkings-almost-overwhelms-fanduel-for-best-position-in-new-york-income
https://zueeen123.blogspot.com/2023/12/us-sports-betting-handle-outperforms.html
https://site-6828921-2394-9309.mystrikingly.com/blog/has-the-opportunity-come-to-boost-players-to-utilize-capable-betting-devices
https://leejk777.blogspot.com/2023/12/the-most-horrendously-awful-games.html
https://casino999.mystrikingly.com/blog/north-carolina-sports-wagering-regulation-course-of-events-and-most
https://parkjk777.blogspot.com/2023/12/seven-significant-administrators-have.html
https://site-4763676-9520-5980.mystrikingly.com/blog/vacillate-expects-fierce-opposition-from-espn-bet-is-on-target-for-u-s-posting
https://emiko1231.blogspot.com/2023/12/sports-wagering-or-dream-sports.html
https://site-6861241-1631-6047.mystrikingly.com/blog/will-espn-bet-shake-up-the-highest-point-of-the-sportsbook-rankings
https://yoo-777.blogspot.com/2023/12/all-in-one-resource-for-betting-media.html
https://daddys-lucky000.mystrikingly.com/blog/barstool-sportsbook-to-be-rebranded-as-espn-bet
https://jay-777.blogspot.com/2023/12/the-delight-of-line-shopping-on-nfl-win.html
https://lucky-razi-777.mystrikingly.com/blog/michigan-ohio-state-enormous-ten-wagering-top-choices-entering-2023
https://jody222000.blogspot.com/2023/12/robins-satisfied-with-sports-wagering.html
https://site-6861405-2997-4499.mystrikingly.com/blog/as-nfl-season-approaches-enthusiasts-draws-nearer-to-new-york-sports
https://zueeen123.blogspot.com/2023/12/raise-ruckus-around-town-for-its-2023.html
https://site-6828921-2394-9309.mystrikingly.com/blog/versatile-games-wagering-goes-live-in-delaware-by-means-of-betrivers
https://leejk777.blogspot.com/2023/12/wounds-select-outs-make-florida-state.html
https://casino999.mystrikingly.com/blog/north-carolina-portable-wagering-send-off-conceivable-before-college-basketball
https://parkjk777.blogspot.com/2023/12/nevada-sports-betting-handle-basically.html
https://site-4763676-9520-5980.mystrikingly.com/blog/try-not-to-expect-2024-to-be-a-really-successful-season-in-sports-wagering
https://emiko1231.blogspot.com/2023/12/wounds-select-outs-make-florida-state.html
https://site-6861241-1631-6047.mystrikingly.com/blog/as-sports-wagering-introductions-go-ohio-s-most-memorable-year-was-a
https://yoo-777.blogspot.com/2023/12/the-most-horrendously-awful-games.html
https://daddys-lucky000.mystrikingly.com/blog/ask-a-bookmaker-glancing-back-at-2023-and-toward-2024
https://jay-777.blogspot.com/2023/12/florida-parimutuels-states-change-3.html
https://lucky-razi-777.mystrikingly.com/blog/powers-of-fate-marked-and-lined-up-in-l-a-have-pushed-dodgers-chances-to
https://jody222000.blogspot.com/2023/12/sports-wagering-in-2023-hi-to-espn-bet.html
https://site-6861405-2997-4499.mystrikingly.com/blog/send-off-of-espn-bet-aficionados-tops-rundown-of-2023-games-wagering
https://adricewines.wine/blogs/news/first-wine-contest-and-already-winning?comment=133853741333#comments
https://wildcraftbrewery.co.uk/blogs/recipes/wild-eye-pa-fruit-cake-recipe?comment=127693226166#comments
https://adricewines.wine/blogs/news/first-wine-contest-and-already-winning?comment=133853806869#comments
https://marysmallwood.com/blogs/news/summers-in-jackson-hole-aint-it-grand?comment=129574174817#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130551087341#comments
https://greenfoods.com/blogs/news/7-benefits-of-barley-juice-powder?comment=129952022691#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/5-ways-to-brew-coffee-at-home?comment=131708649560#comments
https://www.truselforganics.com/blogs/news/skin-purging-or-breaking-out?comment=130744615086#comments
https://www.truselforganics.com/blogs/news/skin-purging-or-breaking-out?comment=130744647854#comments
https://lajashley.com/blogs/the-chemist-speaks/panthenol?comment=127174574261#comments
https://burnbear.com/blogs/journal/a-new-direction?comment=133983174937#comments
https://www.scubatoo.net/blogs/news/114188100-cleaning-your-new-mask?comment=124843262102#comments
https://danabronfman.com/blogs/blog/met-gala-2017-dedicated-to-comme-des-garcons-s-rei-kawakubo?comment=124050669604#comments
https://swannies.co/blogs/digest/top-5-greatest-golf-movies?comment=131045097745#comments
https://xavierfoley.com/blogs/xavier-foleys-blog/how-to-get-more-ticket-sales?comment=128977698915#comments
https://www.riverwoodtradingco.com/blogs/news/2021-shows?comment=130887352488#comments
https://toadhollownj.com/blogs/news/making-christmas-cards?comment=135359234342#comments
https://houstonskateboards.com/blogs/news/missouri-city-skatepark-grand-opening-with-houston-skateboards?comment=127782223938#comments
https://freckbeauty.com/blogs/ooze/color-correcting-w-%F0%9D%98%8A%F0%9D%98%8F%F0%9D%98%8C%F0%9D%98%8C%F0%9D%98%92%F0%9D%98%9A%F0%9D%98%93%F0%9D%98%90%F0%9D%98%94%F0%9D%98%8C?comment=129471774906#comments
https://amyssecretkitchen.com/blogs/news/fathers-day-creation?comment=131106209890#comments
https://informaltea.co.nz/blogs/news/this-week-in-tea-1?comment=129355612318#Comments
https://sigiskin.com/blogs/news/why-do-you-need-dream-capsule-in-your-night-routine?comment=133623841077#comments
https://raw-me.com/ar/blogs/news/7-tips-to-start-living-a-plant-based-lifestyle?comment=131615457369#comments
https://physiclo.com/blogs/news/get-fit-in-2017-giveaway?comment=134112477261#comments
https://milohshop.com/blogs/news/the-story-of-miloh?comment=130344255726#comments
https://deadonpaper.com/blogs/news/11312645-calaveras-shipping-yay?comment=130114453615#Comments
https://teamfarang.com/blogs/news/luca-playlist?comment=133723062588#comments
https://shopfantasyguides.com/blogs/articles/buffalo-bills?comment=125376528468#comments
https://wheretonext.ph/blogs/stories/iceland?comment=130476540146#comments
https://knitonecrochettoo.com/blogs/news/peony-blues-tee?comment=126648811707#comments
https://stinkydog.com/blogs/news/stinky-and-i-have-been-working-like-crazy?comment=128821756063#comments
https://www.reagansanai.com/blogs/news/new-product-alert?comment=130779152597#comments
https://www.tomsonelectronics.com/blogs/news/how-to-set-up-raspberry-pi-pico-rp2040-microcontroller?comment=130344288494#comments
https://covenandco.com/blogs/news/flower-doughnuts?comment=122026131626#comments
https://www.ossboardsupply.com/blogs/news/palace-normcore?comment=131106242658#Comments
https://smoodies.in/blogs/news/smoodies-in-mumbai?comment=129791492179#comments
https://radiateportablecampfire.com/blogs/beyond-the-campfire/camping-essentials-the-best-products-for-your-next-camping-trip?comment=131078193399#comments
https://www.jenni-smith.co.uk/blogs/news/happy-international-womens-day?comment=130501148743#comments
https://www.bellawren.ca/blogs/bellas-blogs/how-sustainable-is-linen?comment=130084962516#comments
https://pseudolabs.com/blogs/in-pseudo/seen-in-pseudo-vol-83?comment=130476572914#comments
https://www.arenlace.com/blogs/blog/tips-to-help-newlyweds-manage-the-holidays?comment=130770960463#comments
https://ferriswheelpress.com/blogs/fan-art/october-22-fan-art-rollup?comment=130659713118#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134115098906#comments
https://www.gussloan.com/blogs/gus-sloan-the-blog/not-your-average-summer-style-guide?comment=130696183923#comments
https://ahwgeorgiaezra.com/blogs/build/our-home-renovation-2-0-part-3?comment=132803297331#comments
https://groundedfactory.com/blogs/news/how-to-find-and-prepare-for-a-yoga-teacher-training?comment=134372852056#comments
https://www.nikkiwilliams.com/blogs/news/interview-with-hawkins-co?comment=134219268395#comments
https://www.spicyelement.com/blogs/recipe/grilled-salmon-head?comment=125775642820#comments
https://www.katiesplates.com/blogs/sherry-greeks-blog-katies-plates-official-registered-dietitian/gluten-free?comment=131377004801#Comments
https://formula2skincare.com/blogs/news/beauty-hacks-with-formula-2-skin-care-cream?comment=130696249459#comments
https://kryptoneconsultingltd.weebly.com/articles/procedure-of-buying-land-in-kenya-a-presentation
https://www.dohertyphotography.com/blogs/a-year-underwater/la-paz-and-cabo?comment=133915082886#comments
https://www.ghanzibrand.com/blogs/news-1/offshoot-project-with-remi-fargier?comment=135684325711#comments
https://glitzandspursboutique.com/blogs/news/tiered-lace-babydoll-top?comment=129451556917#comments
https://www.skinnymetea.com.au/blogs/smtblog/the-best-supplements-to-take-for-your-health?comment=134112510029#comments
https://elkshape.com/blogs/articles/archery-shoulder-exercises?comment=133508235579#comments
https://www.sportstakeoff.com/blogs/look-at-this/dirty-but-yet-smartest-cheap-shot-ive-ever-seen?comment=135767785811#comments
https://www.babybento.com.au/blogs/recipes/sushi?comment=128658866258#comments
https://absolutemerch.com/blogs/news/good-cause?comment=135030309175#comments
https://www.homadic.com/blogs/news/delegates-of-german-federal-ministry-of-environment-visit?comment=135887520076#comments
https://hourloop.com/blogs/news/i-want-much-more-than-this-provincial-life-beauty-and-the-beast-1740-to-2017?comment=128067305530#comments
https://www.tonewoodmaple.com/blogs/news/5885643-tonewood-maple-sugarmaker-david-hartshorn?comment=131207888963#comments
https://tinyrabbithole.com/blogs/news-1/make-naiise-handmade-plushie-at-naiise?comment=130695037147#comments
https://aandscovers.com/blogs/news/is-it-just-me-or-are-magnets-really-attractive?comment=134690865460#comments
https://smartygirlbrand.com/blogs/news/why-does-smartygirl-use-so-much-pink-and-purple?comment=124907159598#comments
https://www.utzmarket.com/blogs/musings/utz-market-n-c-leather?comment=126693114028#comments
https://hardnightgoodmorning.com/blogs/hard-night-good-morning-blog/8863659-the-secret-behind-barley?comment=130006155334#comments
https://jinmee.co.uk/blogs/news/aha-vs-bha-what-s-the-difference?comment=132803493939#comments
https://hovdenwear.com/blogs/news/hovden-art?comment=126938448056#comments
https://shop.nucoconut.com/blogs/recipes/moroccan-cigars-aip-paleo-keto?comment=131106275426#comments
https://retreatyourself.com/blogs/news/mystery-box-brands-october-2022-edition?comment=131615588441#comments
http://www.justindoran.ie/blog/new-tutorials-added
https://cincyshirts.com/blogs/news/free-fc-cincinnati-opening-match-commemorative-tee?comment=127723667610#comments
https://gardenersorchardandbakery.com/blogs/news/farm-fresh-tomatoes-and-missouri-peaches-are-here?comment=122830258221#comments
https://bohochicfiberco.com/blogs/news/87446145-handmade-wardrobe?comment=127782322242#comments
https://www.nortonhousequilting.com/blogs/news/februarys-quilting-your-legacy-monthly-challenge-is-u-f-o-projects?comment=131337683160#comments
https://mehperu.com/en/blogs/blog-meh/no-todos-los-lunes-son-buenos-1?comment=133047550247#comments
https://lagom142.com/blogs/news/plastic-free?comment=134156910893#comments
https://playmobi.com/blogs/blog/kids-and-pets?comment=129258586155#comments
https://www.head-lites.com/blogs/the-sbark/45834241-guiding-lites?comment=125475848381#comments
https://www.emergencylights.net/blogs/blog/title-24-light-switches?comment=131387064556#comments
https://naattumarundhukadai.com/blogs/news/tummy-trim-gel-visible-sliming-on-7th-day-onwords?comment=131254976770#comments
https://yatesprecision.com/blogs/from-the-breakroom/machinist-survey-with-ben-jenkinson-of-jimko-machine?comment=127723700378#comments
https://sofragrance.com/blogs/news/so-sorry-not-sorry-girls-night-in?comment=130695069915#comments
https://www.kassausa.com/blogs/inspiration/how-to-make-your-own-chalkboard-wall-calendar?comment=131377070337#comments
https://shitthatiknit.com/blogs/news/beneath-the-beanie-ashleigh-stone?comment=130344321262#comments
https://saltypaloma.com/blogs/salty-talk/salty-paloma-holiday-party-2018?comment=133742428451#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128658931794#comments
https://misajewelry.com/blogs/blog/18894159-oscar-faves?comment=122026164394#comments
https://www.vbcityfc.com/blogs/news/match-preview-vbvfrd?comment=122026197162#comments
https://luangisa.com/blogs/news/for-wholesalers-interested-in-african-art-african-home-decor-jewelry-and-more-contact-luangisa-art-gallery?comment=130863366399#comments
https://www.theartdream.com/blog/black-panther-pop-up
https://woodleon.com/blogs/blog/what-swag-employees-want?comment=130452259036#comments
https://vincita.cc/blogs/news/introducing-vincita-north-point?comment=130744811694#comments
https://www.plantnmore.com/blogs/news/square-foot-gardening?comment=132484530410#comments
https://lizzie-loves.com/blogs/blog/baked-oat-berry-bars?comment=129952219299#comments
https://flowersofjoy.biz/blog/Tulips
https://www.simplysavorybyrachel.com/blogs/news/breakfast-dirty-rice?comment=128658964562#comments
http://www.yadvindermalhi.org/blog/the-ecomodernism-debate-with-mark-lynas
https://balandlaw.com/blog/guest-blog-post-by-attorney-paul-birnberg-on-security-deposits-was-mungall-v-garry-correctly-decided
https://www.believeiam.com/blogs/biablog/7319402-yoga-for-runners-the-yin-for-the-yang?comment=129433829552#comments
https://www.oddpears.com/blogs/blog/the-meditative-minimal-compositions-of-bobby-clark?comment=130148237504#comments
https://trubeautyshop.com/blogs/default-blog/what-is-a-jade-roller-beauty-benefits-and-tips?comment=125775675588#comments
https://iccwbo.uk/blogs/news/it-s-time-for-a-more-intelligent-approach-to-tackling-covid-19?comment=131003515132#comments
https://www.littlegreenpouch.com/blogs/recipes/6380464-tricked-out-bananameal?comment=124050735140#comments
https://www.savvysleepers.com/blogs/the-benefits-of-satin/savvy-sleepers-testimonials-around-the-web?comment=124907192366#comments
https://stylealertsa.com/blogs/news/thuli-mola-on-common-fashion-mistakes-women-make?comment=127814828113#comments
https://www.staranisefoods.com/blogs/recipes/brown-rice-noodles-with-broccoli-in-almond-butter-sauce?comment=121481494572#comments
https://russellroadracing.co.uk/blogs/news/obd-bench-boot-tuning-explained?comment=127145410634#comments
https://www.somata.ca/blog/rebooting-with-nutrient-therapy
https://shevoke.com/blogs/news/a-day-with-abbey-stockwell?comment=132840816802#comments
https://themodushop.com/blogs/news/thanksgiving-week-sale?comment=134196396369#comments
https://oliofood.com/blogs/olio-blog/article-1?comment=130265350389#comments
https://twowheelsbiglife.com/blogs/you-tube/hunger-pains?comment=129433895088#comments
https://doddleandco.com/blogs/parenting/bobbi-browns-self-care-advice-for-mamas?comment=125100097559#comments
https://neofilmshop.com/blogs/news/film-review-the-mule-2018-usa?comment=133675155754#comments
https://www.gardensundials.com/blogs/news/black-friday-is-on?comment=131615719513#comments
https://thefloralfixx.ca/blogs/news/valentines-day-flowers-and-gift-ideas?comment=131208052803#comments
https://topmusicarts.com/blogs/news/tips-for-new-music-producers?comment=131022586087#comments
https://www.532yoga.com/blog/born-tough
https://judgementyard.org/blogs/news/sizzla-kalonji-happy-earthday-caveman-studio?comment=128731840598#comments
https://makely.shop/blogs/inspiration/everything-you-need-to-know-about-adhesive-styrene?comment=134098223417#comments
https://tagzfoods.com/blogs/news/tagz-why?comment=131721658608#comments
https://evxocosmetics.com/blogs/news/makeup-tips-for-mature-skin?comment=133742461219#comments
https://bespokebinny.com/blogs/a-place-to-call-home/our-founder-natalie-manima-shares-how-she-has-balanced-her-phd-motherhood-practicing-as-a-therapist-and-entrepreneurship?comment=131337781464#comments
https://denishajulea.com/blogs/fashion-blog/more-than-a-bride-secrets-for-a-wonderful-union?comment=130696282227#comments
https://www.pureboardshop.com/blogs/pure-boardshop/2022-masters-game-of-skate?comment=132803821619#comments
https://www.zwaveoutlet.com/blogs/news/email-question-of-the-week-z-wave-and-zigbee-the-same-thing-or-different?comment=134115295514#comments
https://www.operationofhope.org/blogs/news/dr-kevin-healy-anesthesiologist-on-his-recent-medical-mission-to-malawi?comment=127814860881#comments
https://www.superfood-world.com/blogs/news/what-can-ceylon-cinnamon-do-for-your-health?comment=129433960624#comments
https://worldofdive.com/blogs/news/fit-kids-fit-you?comment=128369295511#comments
https://sajawedding.com/blogs/news/jennas-wedding-part-i?comment=125775741124#comments
https://inkboxartistry.com/blogs/inkbox-artistry-blog/15-tips-to-beat-the-instagram-algorithm?comment=135030604087#comments
https://olddirty.boston/blogs/news/jamaica-plain?comment=137655910463#comments
https://miracase.com/blogs/news/omg-should-i-get-the-iphone-case-for-the-xr?comment=129232142449#comments
http://www.themacroexperiment.com/blog/enjoying-food-protein-pumpkin-bread
https://measinasamoa.com.au/blogs/news/fai-mai-tyla-hina-lei-floral-design?comment=129791754323#comments
https://tournaments.spikeball.com/blogs/the-rally/will-origin-impact-finally-beat-cisek_showalter-at-midwest-regionals?comment=129433993392#comments
https://www.swordandplough.com/blogs/news/interview-with-a-brand-champion-the-proud-parent?comment=121188155531#comments
https://www.melodylanedesigns.com/blogs/news/starting-to-think-about-halloween?comment=125582737455#comments
https://palmettowinesellers.com/blogs/news/party-time?comment=133733810461#comments
https://www.vintagegrooming.com/blogs/grooming-articles-and-tutorials/does-size-matter-a-guide-to-beard-length?comment=134115393818#comments
https://www.thetroutlook.com/latest-updates/could-barriers-benefit-brook-trout
https://www.onevillagecoffee.com/blogs/ask-steve/how-to-choose-coffee-for-cold-brew?comment=131254911209#comments
https://www.pamhurst.com/blogs/news/changes-local-national-and-personal?comment=126468128886#comments
https://oceanzenbikini.com/blogs/press/founder-steph-gabriel-finalist-young-entrepreneur-year-award?comment=129232371825#comments
https://mulberrytreeathome.com/blogs/news/how-to-style-your-hallway?comment=128370573463#comments
https://www.vomfassusa.com/blogs/store-news/creamy-pesto-shrimp-with-lemon-pepper-linguine?comment=129574633569#comments
https://rockstreet-art.com/blogs/whats-new/i-live-a-life-of-colours-do-you-artist-alina-prodan-talking-about-colours-of-childhood-and-their-impact-on-her-art?comment=130501279815#comments
https://adorit.ca/blogs/news/10-years-old-what-can-we-say?comment=127782780994#comments
https://cafeliegeois.ca/blogs/actualite-nouvelles-cafe-liegeois/by-the-way-what-are-ese-pods?comment=131615916121#comments
https://www.vehiclebodyfittings.co.uk/blogs/news/50948165-vehicle-body-fittings-ambulance-fleetcare-services-ltd?comment=131321888846#comments
https://www.apollonnutrition.com/blogs/news/breaking-news-apollon-nutrition-founder-and-ceo-robert-samborsky-announces-upcoming-fight-in-the-warriors-cup?comment=130265678069#comments
https://viensavecmoi.ca/blogs/news/so-long-sweet-summer?comment=134098813241#comments
https://www.luckydipltd.co.uk/blogs/lucky-dip-blog/roald-to-rowling-the-wonka-legacy-of-literary-candy?comment=127724748954#comments
https://www.theflowernook.com.au/blogs/cut-blooms/cut-blooms?comment=125888594000#comments
https://www.petfon.com/blogs/love-letters/love-letter-from-marquis?comment=132117135606#comments
https://balanceandolavida.com/blogs/snacks-colaciones-y-postres/crema-de-almendra-casera?comment=129466597555#comments
https://www.inthemeadow.com.au/blogs/news/113068547-oh-hey-mazza?comment=137098166359#comments
https://socksoho.com/blogs/news/the-sock-tradition?comment=133966725413#comments
https://rebournebodyandhome.com/blogs/news/coping-with-trauma-tips-to-getting-through-it?comment=126938972344#comments
https://merkaba.nz/blogs/hemp-blog/origin-and-evolution-of-hemp-foods?comment=137657778239#comments
https://www.undracelesteny.com/blogs/ucnynews/what-a-time-to-be-alive-the-great-things-that-happened-in-2020?comment=129996161190#comments
https://givehersix.com/blogs/blog/60362501-welcome-to-give-her-six?comment=129452539957#comments
https://mihamade.com/blogs/stories/meet-the-maker-ana-maria?comment=130515730650#comments
https://sigiskin.com/blogs/news/why-do-you-need-dream-capsule-in-your-night-routine?comment=133624037685#comments
https://offonatangentshop.com/blogs/news/97445825-lets-get-this-thing-going?comment=129900970149#comments
https://www.garmentprinterink.com/blogs/sprint/anajet-sprint-resetting-the-service-counter?comment=130660761694#comments
https://www.creativeacademic.uk/blog/creativity-in-the-making
https://isabellacoutureshop.com/blogs/news/82533443-pre-introduction?comment=127174803637#Comments
https://covenandco.com/blogs/news/spring-strawberry-vanilla-cupcakes?comment=122026623146#comments
https://liondiamonds.nyc/blogs/blog/ultra-precision-machining-sysytem?comment=126770610249#comments
https://www.jeeperspeepers.co.uk/blogs/news/introducing-miranda?comment=128711950529#comments
https://www.aristelle.com/blogs/news/108343302-how-to-buy-lingerie-for-your-someone-special?comment=131395911980#comments
https://www.undracelesteny.com/blogs/ucnynews/singing-its-a-new-dawn-its-a-new-day?comment=129998749862#comments
https://www.truselforganics.com/blogs/news/what-exactly-is-a-face-serum?comment=130747072686#comments
https://mrstone.com/blogs/articles/safety-first-types-of-safety-equipment-stone-fabricators-and-installers-use?comment=127146360906#Comments
https://mrstone.com/blogs/articles/safety-first-types-of-safety-equipment-stone-fabricators-and-installers-use?comment=127146393674#Comments
https://www.kstuned.com/blogs/knowledge/to-spray-or-not-to-spray?comment=133686526232#comments
https://www.aprilcoffeeroasters.com/blogs/coffee-with-april-podcast/kapo-chiu?comment=135866188122#comments
https://oskerstore.com/blogs/news/5-australian-fashion-trends-to-keep-you-ahead-of-the-curve-this-summer-2018?comment=135360381222#comments
https://www.thekindstoreonline.co.uk/blogs/blog/diy-liquid-hand-soap?comment=132119429366#comments
https://www.sculpeyproducts.com/blogs/news/is-free-shipping-really-free?comment=130660827230#comments
https://greenfoods.com/blogs/news/choco-coco-martini-with-vibrant-collagen-creamer?comment=129956151459#comments
https://www.undracelesteny.com/blogs/ucnynews/spring-home-refresh?comment=129998782630#comments
https://us.gozney.com/blogs/news/roccbox-tops-the-lot-named-a-which-best-buy?comment=121279185033#comments
https://glamtheory.store/blogs/glam-school/how-to-avoid-the-makeup-devil-flashback?comment=125038460987#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134115229773#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135133331721#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133326536978#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127694995638#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135832961371#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684489551#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709468760#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128508526752#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624365365#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133629870364#comments
https://www.evogennutrition.com/blogs/fst-7/train-with-the-pro-creator-hadi-choopan-fst-7-quads-one-week-out?comment=121483558956#comments
https://gosciencecrazy.com/blogs/blog-science-crazy/how-ready-are-we-for-ready-player-one?comment=131112370404#comments
https://mevrouandco.com/blogs/news/milla-peertuin?comment=128925827224#comments
https://waterlemonkids.com/blogs/news/happy-anniversary?comment=128953516087#comments
https://vani-t.com/blogs/news/farewell-ghostly-paleness-hello-bronze-beauty?comment=130599846061#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/harmony-week?comment=131819405511#comments
https://shop.wattsfarms.co.uk/blogs/recipes/vegan-fresh-pesto-sauce?comment=121483591724#comments
https://micapeet.com/blogs/blog/ultimate-monstera-lovers-gift-guide-10-products-from-indie-brands-every-cheese-plant-enthusiast-need-in-their-lives?comment=129356136606#comments
https://www.ezscreenprint.com/blogs/diy-screen-printing-at-home/diy-t-shirt-screen-printing-video?comment=133975638121#comments
https://www.baristaspace.com/blogs/baristaspace/stephen-hawking-the-scientific-superstar-leave-the-world?comment=130007400518#comments
https://pulpprintshop.com/blogs/pulp-blog/pulp-artists?comment=131819733159#comments
https://www.onevillagecoffee.com/blogs/ask-steve/5-reasons-to-start-a-one-village-coffee-subscription-today?comment=131256123625#comments
https://www.bigbrattboutique.com/blogs/news/the-momma?comment=133754454332#comments
https://sofragrance.com/blogs/news/the-so-christmas-gift-set-guide-you-ve-been-waiting-for?comment=130695659739#comments
https://www.thelooploft.com/blogs/ryans-corner/29331073-drum-direktor-tips-tricks?comment=126744297652#comments
https://swannies.co/blogs/digest/brawl-on-the-back-9?comment=131045228817#comments
https://www.getgiddy.com/blogs/giddy-blog/not-just-your-average-hand-balm?comment=131681681634#comments
https://www.iridescentglassdesign.co.uk/blogs/news/tiz-the-season?comment=129956806819#comments
https://lastu.co/blogs/lastu-blog-and-vlog/minimalist-style-from-the-forests-walnut?comment=134935249239#comments
https://plushpawsproducts.com/blogs/news/whats-the-best-remedy-for-bad-breath-in-dogs?comment=132757750003#comments
https://banudesigns.com/blogs/ethnicfashion/bridal-accessories-2021?comment=133975736425#comments
https://mrstone.com/blogs/articles/30223425-a-buyer-s-guide-to-choosing-the-perfect-sink?comment=127146983498#Comments
https://www.pawzroad.com/blogs/news/amt0089-installation-video?comment=126469406838#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134116900941#comments
https://www.swishfashion.com.au/blogs/latest-blog-1/swish-armidale-interview?comment=131108733026#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130553086189#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135134445833#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133326864658#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135833878875#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684620623#comments
https://www.maxihome.com.sg/blogs/news/9-benefits-that-come-with-buying-new-furniture-for-your-home-and-living-room?comment=127727141018#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709730904#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128508952736#comments
https://gemkart.com/blogs/news/the-role-of-luck-in-stock-market?comment=130747990190#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624398133#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133629935900#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127695782070#comments
https://tailgatengo.com/blogs/press/now-they-re-cooking-inventive-family-develops-portable-outdoor-kitchens?comment=132757815539#comments
https://shelbykregel.com/blogs/the-green-bus-us/learning-to-receive?comment=130135359597#comments
https://accesslawonlineltd.com/blogs/case-law-brought-to-life/carlill-v-carbolic-smoke-ball-co-1893?comment=119910760583#comments
https://physiclo.com/blogs/news/get-fit-in-2017-giveaway?comment=134116966477#comments
https://historyonashirt.com/blogs/knights-without-parachutes/boarding-ship-from-iwo-jima?comment=129233584241#comments
https://www.operationofhope.org/blogs/news/burn-mission-cho-ray-hospital?comment=127815450705#comments
https://brandonsteiner.com/blogs/what-else/video-when-you-put-in-the-work-people-notice-1?comment=126694686892#comments
https://jophiel.com/blogs/style-news/132385283-how-does-a-fashion-trend-start?comment=132490330346#comments
https://dinamackney.com/blogs/trends/dina-mackney-trend-rainbows-and-flowers?comment=132163338461#comments
https://melapteh.com/blogs/news/a-feature-on-elle-cote-divoire?comment=131504537755#comments
https://amourducake.com/en/blogs/english-blog/swirls-crepes-or-spirals-crepes-with-video?comment=136178237768#comments
https://terrathelabel.com/blogs/news/ethicalclothing?comment=129952874599#comments
https://enjoytheriderecords.com/blogs/what-were-listening-to/what-were-listening-to-and-more-1?comment=130347925742#comments
https://harrisandfrank.com/blogs/news/how-to-wear-a-suit-in-the-summer-1?comment=131256353026#comments
https://www.spicyelement.com/blogs/recipe/grilled-salmon-head?comment=125776494788#comments
https://www.bysashatattooing.com/blogs/news/exclusive-interview-with-amazing-noraink?comment=127783600194#comments
https://orr.is/blogs/orrbyorr/orr-by-orr-blog-blog?comment=129575387233#comments
https://www.truselforganics.com/blogs/news/alexisjadekaiser?comment=130748481710#comments
https://jordandene.com/blogs/blog/coffee-break-monstress-volume-one?comment=130553217261#comments
https://nicolezizistudio.com/blogs/news/sweet-sneak-studios-photo-series-focuses-on-microplastics-in-the-food-chain?comment=119378477187#comments
https://sebastianmccall.com/blogs/pocketplacement/camo-chic?comment=130020901059#comments
https://cupofte.com/blogs/news/how-much-caffeine-is-in-a-cup-of-tea?comment=130780004565#comments
https://www.compawssion.com/blogs/blog/8361706-polo-proofs?comment=130600173741#comments
https://sudzbox.com/blogs/news/how-to-correctly-wash-your-detailing-towels?comment=133042405627#comments
https://www.dollymoo.com/blogs/news/6606909-fresh-batch-of-lavender?comment=127885180970#comments
https://milohshop.com/blogs/news/the-story-of-miloh?comment=130347958510#comments
https://thegentlepit.com/blogs/the-gentle-pit/why-are-pit-bulls-the-most-euthanized-breed-in-shelters?comment=133629968668#comments
https://www.sablebeauty.com/blogs/news/why-looking-after-your-most-initimate-parts-is-the-ultimate-form-of-selfcare?comment=137661677631#comments
https://www.melissaallenmoodessentials.com/blogs/news/positive-vibes-to-dance-love-and-laugh-more-in-2021?comment=127280742577#comments
https://flysupplyclothing.com/blogs/news/check-out-who-is-wearing-fly-supply-clothing?comment=132163600605#comments
https://vincita.cc/blogs/news/118603012-come-and-meet-us-at-vincita-lifestore-booth-at-central-festival-eastville?comment=130748547246#comments
https://jamhuriwear.com/blogs/jw-blog/yes-we-cannes?comment=126976426159#comments
https://kryptoneconsultingltd.weebly.com/articles/solid-waste-management-operations-process
https://www.wyldsmoke.com.au/blogs/news/113701062-the-shank-brothers-rib-athon?comment=130007466054#comments
https://alchemizefightwear.com/blogs/news/3-keys-for-survival-in-a-male-dominated-gym?comment=127695814838#comments
https://alchemizefightwear.com/blogs/news/3-keys-for-survival-in-a-male-dominated-gym?comment=127695847606#comments
https://raw-me.com/blogs/news/the-benefits-of-juice-cleansing?comment=131617587289#comments
https://www.oddpears.com/blogs/blog/113582275-minimal-considerate-slow-fashion-from-melbourne-label-scott-benedictine?comment=130149155008#comments
https://freckbeauty.com/blogs/ooze/shape-your-cheekslime?comment=129482260666#comments
https://www.sportstakeoff.com/blogs/look-at-this/football-hacks-missing-visor-clips-dont-worry-we-have-a-hack-for-that?comment=135768277331#comments
https://mamabanna.com/blogs/stories/beauty-treatments-from-mama?comment=125584015407#comments
https://cedarburgthreads.com/blogs/news/storytime-part-2-i-me-my-and-mine?comment=122027016362#comments
https://www.arenlace.com/blogs/blog/birthday-bae-gifts-tshirts?comment=130774597711#comments
https://www.skinnymetea.com.au/blogs/smtblog/7-green-diy-beauty-recipes?comment=134117523533#comments
https://bootstees.com/blogs/boots-tees-t-shirt-blog/40046401-believe-in-yourself-shirt-available-on-shirt-woot?comment=130748612782#comments
https://www.caulicure.com/blogs/lend-me-your-ear/the-reality-of-brazilian-jiu-jitsu-and-cauliflower-ear?comment=133686788376#comments
https://www.watsonandlou.com/blogs/news/a-nice-place-to-be?comment=137661743167#comments
https://globaltrendz.ca/blogs/blog/fishnet-knee-high-socks?comment=127783665730#comments
https://localpetmarket.com/blogs/news/the-truth-about-prescription-pet-food?comment=128735412310#comments
https://adilsons.org/blogs/anime/top-10-strongest-black-clover-characters?comment=133734170909#comments
https://gratefulgnome.com/blogs/news/riddle-time-50?comment=133676204330#comments
https://yatesprecision.com/blogs/from-the-breakroom/machinist-interview-003-jt-belknap-of-dfm-tool-works?comment=127727665306#comments
https://kizildenizgroup.com/ar/comment/37
https://merkaba.nz/blogs/hemp-blog/hemp-tofu-a-great-choice-for-vegans?comment=137662660671#comments
https://oskerstore.com/blogs/news/osker-bringing-australian-fashion-to-the-world?comment=135361233190#comments
https://jaderollerbeauty.com/blogs/jade-lifestyle-blog/elevating-esthetics-the-jade-roller-beauty%C2%AE-mission?comment=131507650789#comments
https://www.head-lites.com/blogs/the-sbark/45834241-guiding-lites?comment=125476372669#comments
https://backyardsafarico.com/blogs/news/introducing-our-new-pollinator-garden-the-honey-bee-habitat?comment=125403398315#comments
https://slipins.com/blogs/news/we-are-partnering-with-pow-people-of-the-water-a-wonderful-non-profit?comment=130781905049#comments
https://www.extensions-plus.com/blogs/hair-blog/how-to-achieve-bouncy-curls?comment=133687050520#comments
https://tex.com.tw/blogs/manufacture/about-jis-layout-design?comment=131504865435#comments
https://susuaccessories.com/blogs/the-susu-gal-blog/welcome-to-the-guild?comment=131504898203#comments
https://maisondemings.com/blogs/news/shop-small-with-living-soul-pottery?comment=134536462619#comments
https://www.grunt.com/blogs/news/marine-of-the-week-9?comment=125101899799#comments
https://dalmanjumpco.com/blogs/news/inside-vasco-flores-tack-locker?comment=126744428724#comments
https://bodsupport.com/blogs/news/causes-of-leg-pain-and-foot-pain?comment=128509313184#comments
https://provinggroundstraining.com/blogs/crossfit-motivation/how-to-get-good-at-crossfit-by-not-doing-crossfit?comment=128509345952#comments
https://www.cctung.com/it/challenge
https://kenyarn.com/blogs/kenyarn-blog/camp-kenyarn-2021-collaborative-shop-update?comment=133734203677#comments
https://www.radiancestl.com/blogs/news/iv-nutrient-therapy-boosting-health-drop-by-drop?comment=130781937817#comments
https://www.stockandbarrelco.com/blogs/s-b-blog/making-a-western-leather-laptop-bag?comment=130007498822#comments
https://saintphilippe.com/blogs/news/the-collection-2-new-collection-release?comment=131210149955#comments
https://www.springbreakwatches.com/blogs/dreamers/76238085-how-netflix-is-changing-the-world?comment=131163554024#comments
https://www.nundlenaturalskin.com.au/blogs/news/restock-and-save?comment=128071630906#Comments
https://viensavecmoi.ca/blogs/news/viens-avec-moi-inside-the-boutique?comment=134099829049#comments
https://www.chiclifestyle.com/blogs/news/once-upon-a-time?comment=129773830229#comments
https://tagzfoods.com/blogs/news/tagz-why?comment=131723821296#comments
https://s8tattoo.com/blogs/stencil-science/stencil-science-the-s8-stencil-printer?comment=131819700423#comments
https://rebournebodyandhome.com/blogs/news/5-reasons-to-go-vegan?comment=126940479672#comments
https://www.superfood-world.com/blogs/news/what-can-ceylon-cinnamon-do-for-your-health?comment=129434648752#comments
https://www.creativeacademic.uk/blog/creativity-in-the-making
https://www.theflyingkids.com/blogs/bloggers/an-ordinary-family-of-6?comment=128823034015#comments
https://www.sculpeyproducts.com/blogs/news/is-free-shipping-really-free?comment=130662236254#comments
https://greenfoods.com/blogs/news/choco-coco-martini-with-vibrant-collagen-creamer?comment=129956905123#comments
https://www.undracelesteny.com/blogs/ucnynews/spring-home-refresh?comment=129999208614#comments
https://us.gozney.com/blogs/news/roccbox-tops-the-lot-named-a-which-best-buy?comment=121279840393#comments
https://glamtheory.store/blogs/glam-school/how-to-avoid-the-makeup-devil-flashback?comment=125039509563#comments
https://appletonpackinggasket.com/blogs/news/tips-tricks-to-clean-and-organize-your-life-and-desk?comment=134118015053#comments
https://www.rigorer.store/blogs/articles/common-knee-supports-available-in-singapore-which-is-suitable-for-you?comment=130553774317#comments
https://www.runamics.com/blogs/the-concious-runner/starting-holes-with-cobwebs-a-call-to-action-from-runamics?comment=135135396105#comments
https://reyrgear.com/blogs/news/podcast-our-journey-and-mentality-on-gear?comment=133327356178#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-and-shipping-update-16?comment=127695945910#comments
https://minimalwastegrocery.com/blogs/minim-blog/zero-waste-hair-care-to-poo-or-not-to-poo?comment=135834173787#comments
https://sauercrowd.nl/blogs/gut-mind-journal-video/introduction-gut-mind-journal-with-mo?comment=135684882767#comments
https://www.elevatecoffee.com/blogs/elevate-coffee-blog/rude-awakening-k-cups?comment=131709861976#comments
https://www.eighthmain.com/blogs/eighth-main/christmas-wreath-making?comment=128509378720#comments
https://sigiskin.com/blogs/news/best-travel-skincare-routine?comment=133624430901#comments
https://kongbeerbong.com/blogs/news/1870-magazine-college-party-article?comment=133630001436#comments
https://gosciencecrazy.com/blogs/blog-science-crazy/how-ready-are-we-for-ready-player-one?comment=131112796388#comments
https://mevrouandco.com/blogs/news/milla-peertuin?comment=128926089368#comments
https://vani-t.com/blogs/news/farewell-ghostly-paleness-hello-bronze-beauty?comment=130601222317#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/harmony-week?comment=131820617927#comments
https://brandonsteiner.com/blogs/what-else/122123523-customer-service-how-do-you-know-what-you-dont-know?comment=126694949036#comments
https://www.iceandaslice.co.uk/blogs/news/a-juniper-berry-is-a-female-seed-cone?comment=128660013138#comments
https://crawford-denim.com/blogs/blog-posts/47452933-gunne-sax-dresses?comment=128953876535#Comments
https://enjoytheriderecords.com/blogs/what-were-listening-to/what-were-listening-to-april-2018?comment=130348318958#comments
https://ruthshouseinc.com/blogs/rh-blog/14069453-lowcountry-local-first-2014-chefs-potluck-middleton-place?comment=130007531590#comments
https://www.grunt.com/blogs/news/fear-of-marines-1?comment=125102063639#comments
https://cityfarmhouseantiques.com/blogs/news/collectible-folk-art-makes-for-great-conversation-pieces-to-display-in-the-home?comment=130007564358#comments
https://shitthatiknit.com/blogs/news/how-to-care-for-your-sh-t?comment=130349072622#comments
https://www.beckystallowtreasures.com/blogs/news/shredded-luffa-ground-coffee-creative-skincare-options?comment=131618242649#Comments
https://braveamerican.com/blogs/news/3-creative-ways-to-change-your-morning-cup-of-coffee
https://www.jackofalltradesclothing.com/blogs/news/8504347-who-doesnt-put-on-a-graphic-tee-at-some-point-during-the-week-nobody?comment=129901920421#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/east-end-flower-market-wins-s-as-best-florist-2020?comment=131821371591#comments
https://www.superchargeyourgut.com/blogs/news/meet-and-greet-with-lee-holmes-in-london?comment=133688164640#comments
https://theunchartedstudio.com/blogs/uncharted-goods/surfing-magazine-help-save-puerto-ricos-waves?comment=133630066972#comments
https://lisatibalditerramia.com/en/blogs/notizie-e-curiosita/scuola-al-via-da-oggi-nuove-regole-per-la-sicurezza-di-tutti?comment=134198231377#comments
https://elkshape.com/blogs/articles/building-a-garage-gym?comment=133508628795#comments
https://www.dotandlil.com/blogs/blog/86324804-new-things-and-places-to-be?comment=133641011479#comments
https://thetemplewolf.com/blogs/news/the-temple-wolf-gives-back?comment=135888273740#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128660406354#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134119031066#comments
https://paz-creations.com/blogs/blog/mothers-day-memorial-day-may-madness?comment=131005055228#comments
https://helloring.net/blogs/news/everything-you-need-to-know-about-sapphire-engagement-rings?comment=131074130144#comments
https://vickibrowndesigns.com/blogs/blog/gloucestershire-etsy-craft-party?comment=134025412895#comments
https://sofragrance.com/blogs/news/so-top-10-vegan-bath-body-products-all-under-10?comment=130696020187#comments
https://greenfoods.com/blogs/news/should-you-be-supplementing-with-collagen?comment=129957101731#comments
https://sneakerbinge.com/blogs/news/37523969-nick-young-2-chainz-shop-for-25k-jordans-most-expensivest-shit?comment=132169564381#comments
https://www.goldrushnuggetbucket.com/blogs/gold-panning-news/48004485-famous-folks-the-california-gold-rush?comment=126629085378#comments
https://www.jenni-smith.co.uk/blogs/news/90121153-my-very-first-trade-show-stitches-2016?comment=130501673031#comments
https://www.dropbottle.com/blogs/recipes/mint-cucumber-lemon-detox-water?comment=131110305890#comments
https://bradoria.com/blogs/blog/fashion-tricks-on-how-to-hide-your-fat?comment=131682238690#comments
https://www.baristaspace.com/blogs/baristaspace/stephen-hawking-the-scientific-superstar-leave-the-world?comment=130008023110#comments
https://www.macshop.com.sg/blogs/news/happy-2021?comment=130501705799#comments
https://trenaryhomebakery.com/blogs/history-series/stand-out-this-holiday-season-with-trenary-home-bakery?comment=126977147055#comments
https://vinoparaprincipiantes.com/blogs/podcast/episodio-04-sauvignon-blanc?comment=134719242342#comments
https://waywardgourmet.com/blogs/wayward-gourmet-blog/76557573-slow-cooker-buttery-indian-chicken-stew?comment=128926351512#comments
https://www.accessoryconcierge.com/blogs/news/14483101-nighttime-denim?comment=133670306099#comments
https://www.ultratribe.com/nl/blogs/news/the-mind-s-self-defense?comment=130864972031#comments
https://www.prevenbus.com/blog/2021/11/02/nouvelle-interview-france-3—-19-20-du-2-novembre-2021.html#commentaire
https://www.smallscalesseafood.com/blogs/recipes/halibut-with-morels-and-asparagus?comment=127175196853#comments
https://timelyandtimeless.com/blogs/blog/cuckoo-clocks-for-young-and-old?comment=135104037175#comments
https://blackmagicalchemy.com/blogs/blog/how-to-create-a-vegan-chaga-cheesecake?comment=130554790125#comments
https://dianeericson.com/blogs/journal/calling-in-2021-on-a-wing-n-a-prayer?comment=133755371836#comments
https://nikaukai.com/blogs/news/first-impressions-gofoil-gl140-vs-gl180?comment=137664921663#comments
https://vidavegana.mx/blogs/vida-vegana/torta-burrera-de-pan-vegana?comment=134119358746#comments
https://earthsideecobums.com.au/blogs/top-tips/does-size-matter?comment=130604007597#comments
https://monroehouseboutique.com/blogs/daily-life-of-a-retired-rn/our-kitchen-reno?comment=132015587558#comments
https://heavy-vinyl.com/blogs/live-gig-coverage/carolina-rebellion-friday-may-5-2017?comment=124844245142#comments
https://maisondemings.com/blogs/news/diy-carriage-doors?comment=134539608347#comments
https://physiclo.com/blogs/news/116063173-6-ways-to-get-creative-with-physiclo-instagram-contest?comment=134119751757#comments
https://www.lettingref.co.uk/blog/welcome-to-our-blog
https://www.bysashatattooing.com/blogs/news/top-tattoos-back-in-stock?comment=127785730114#comments
https://deltasurvivalist.com/blogs/news/12878885-how-to-survive-a-tornado?comment=125476569277#comments
https://www.chiclifestyle.com/blogs/news/how-to-prep-your-hair-and-use-a-wig-band?comment=129774157909#comments
https://www.hazaelgaray.com/blogs/blog/como-lidiar-con-el-rechazo?comment=130424242399#comments
https://anyonefortennis.sg/blogs/all-about-tennis/15735789-tip-of-the-week-coach-dave-why-should-my-child-use-coloured-tennis-balls-surely-the-sooner-they-get-to-full-pressure-yellow-balls-the-better-right-wrong?comment=124914237486#comments
https://www.yokentheam.com/blogs/yoke-theam/one-woman-show-alia-bastamam?comment=134933610782#comments
https://www.getyournailsdid.com/blogs/the-nail-files/d?comment=134021513530#comments
https://www.blackroll.com.au/blogs/exercises/workout-mobility-exercises?comment=118992961600#comments
https://www.noreciperequired.com/technique/10-steps-perfect-pork-chop?page=19#comment-343370
https://wildernessculture.com/blogs/adventures/5-adventure-spots-you-may-not-know-about?comment=133734498589#comments
https://bespokebinny.com/de/blogs/a-place-to-call-home/passport-to-africa?comment=131339616472#comments
https://jinmee.co.uk/blogs/news/skincare-products-sizing-guide-how-much-product-do-you-really-need?comment=132812341299#comments
https://www.tejariandco.com/blogs/blog/kale-apple-banana-smoothie?comment=133733941567#comments
https://www.babybento.com.au/blogs/product-review/bento-boxes-kids-will-love?comment=128661127250#comments
https://fikacoffee.com/blogs/fikablog/122639681-the-story-behind-the-red-winged-blackbird?comment=129473904819#comments
https://www.juntadeandalucia.es/averroes/centros-tic/41009937/helvia/bitacora/index.cgi
https://www.superfood-world.com/blogs/news/benefits-baobab-pre-post-workout-endurance?comment=129435599024#comments
https://www.moirabeauty.com/blogs/news/moira-prime-plus-primer-water?comment=127149834314#comments
http://www.tpso.moc.go.th/th/node/275?page=675#comment-3624685
https://tinysparkboutique.com/blogs/news/boundaries-and-balance?comment=129490878525#comments
https://punisherspb.com/blogs/news/the-gi-stealth-paintball-marker-a-better-gtek-160r?comment=126471078006#comments
https://www.wilkinswalk.com/blogs/blogger/nike-air-max-90-total-orange-photo-blue?comment=130362278052#comments
https://www.camelhug.com/blogs/camel-hair-clothing-fabric/74318595-100-sustainable-fibre?comment=125377249364#comments
https://www.kassausa.com/blogs/inspiration/diy-whiteboard-pantry-door?comment=131380052225#comments
http://fromscotland.org.uk/news-and-events/thank-you-from-malama-feeding-centre-trust
https://www.wunderkinco.com/blogs/news/alice-and-ames?comment=126744592564#comments
https://www.chatostudio.com/blogs/news/white-sand-dune-vietnam?comment=128015302817#comments
https://www.ezscreenprint.com/blogs/diy-screen-printing-at-home/christmas-silk-screen-stencils?comment=133987532905#comments
https://gardenersorchardandbakery.com/blogs/news/save-on-your-holiday-orders?comment=122830487597#comments
https://momentum-boards.com/blogs/news/hollow-wheel-manufacturing-shipping-update-8?comment=127696863414#comments
https://johnwind.com/blogs/gotta-have-it/102816513-gifts-for-dad?comment=132173922525#comments
https://margospatterns.com/blogs/news/the-big-project?comment=131323723854#comments
https://brandonsteiner.com/blogs/what-else/122123523-customer-service-how-do-you-know-what-you-dont-know?comment=126695932076#comments
https://www.shopthebyb.com/blogs/blog-the-byb-atom/outfit-inspo-casually-cropped?comment=133733974335#comments
https://johnwind.com/blogs/gotta-have-it/102816513-gifts-for-dad?comment=132173955293#comments
https://margospatterns.com/blogs/news/the-big-project?comment=131323756622#comments
https://devilmountaincoffee.com/blogs/blogs/cold-brew-recipe?comment=130555347181#comments
https://slipins.com/blogs/news/14868935-welcome-to-our-new-website-its-opening-day?comment=130783051929#comments
https://www.missrosesisterviolet.com/blogs/all/monday-motivation?comment=128739147862#comments
https://www.thekindstoreonline.co.uk/blogs/blog/the-period-product-tax-has-finally-been-scrapped-what-now?comment=132132831478#comments
https://www.iceandaslice.co.uk/blogs/news/a-juniper-berry-is-a-female-seed-cone?comment=128661160018#comments
https://petitpeony.com/blogs/news/69803395-petit-spook-out?comment=129794211923#comments
https://crawford-denim.com/blogs/blog-posts/47452933-gunne-sax-dresses?comment=128955613239#Comments
https://ruthshouseinc.com/blogs/rh-blog/14069453-lowcountry-local-first-2014-chefs-potluck-middleton-place?comment=130008350790#comments
https://merkaba.nz/blogs/hemp-blog/how-to-make-hemp-burgers-with-hemp-seeds?comment=137670099007#comments
https://houseofsarah14.com/blogs/health-business/join-longrich?comment=131084026103#comments
https://maivino.com/blogs/maivinopours/vogue-printed-this-crazy-wine-diet-in-the-70s?comment=130352742638#comments
https://www.dotandlil.com/blogs/blog/86324804-new-things-and-places-to-be?comment=133642617111#comments
https://lisatibalditerramia.com/en/blogs/notizie-e-curiosita/scuola-al-via-da-oggi-nuove-regole-per-la-sicurezza-di-tutti?comment=134199509329#comments
https://bohochicfiberco.com/blogs/news/87446145-handmade-wardrobe?comment=127786745922#comments
https://thetemplewolf.com/blogs/news/the-temple-wolf-gives-back?comment=135889813836#comments
https://www.shopsocialthreads.com/blogs/news/the-best-jean-shorts?comment=128662241362#comments
https://paz-creations.com/blogs/blog/mothers-day-memorial-day-may-madness?comment=131006169340#comments
https://abrazostyle.com/blogs/musings/81981569-adeles-latin-threads-trading-co-blogs?comment=134121160986#comments
https://helloring.net/blogs/news/everything-you-need-to-know-about-sapphire-engagement-rings?comment=131076292832#comments
https://vickibrowndesigns.com/blogs/blog/gloucestershire-etsy-craft-party?comment=134027378975#comments
https://savannahhayes.com/blogs/blog/artemis-design-co?comment=128413958313#comments
https://sneakerbinge.com/blogs/news/37523969-nick-young-2-chainz-shop-for-25k-jordans-most-expensivest-shit?comment=132181983453#comments
https://www.goldrushnuggetbucket.com/blogs/gold-panning-news/48004485-famous-folks-the-california-gold-rush?comment=126630953154#comments
https://trenaryhomebakery.com/blogs/history-series/stand-out-this-holiday-season-with-trenary-home-bakery?comment=126978326703#comments
https://zazu.cl/en/blogs/mundo-zazu/cuarentena-si-pero-con-estilo?comment=128194576453#comments
https://www.accessoryconcierge.com/blogs/news/14483101-nighttime-denim?comment=133670666547#comments
https://www.ultratribe.com/fr/blogs/news/the-mind-s-self-defense?comment=130866151679#comments
https://www.bysashatattooing.com/blogs/news/sashatattooing-giveaway?comment=127786844226#comments
https://naattumarundhukadai.com/blogs/news/%E0%AE%87%E0%AE%AF%E0%AE%B1%E0%AF%8D%E0%AE%95%E0%AF%88%E0%AE%AF%E0%AE%BF%E0%AE%A9%E0%AF%8D-%E0%AE%B5%E0%AE%AF%E0%AE%BE%E0%AE%95%E0%AE%B0%E0%AE%BE-%E0%AE%AE%E0%AF%81%E0%AE%B0%E0%AF%81%E0%AE%99%E0%AF%8D%E0%AE%95%E0%AF%88?comment=131257270530#comments
https://chicletzipline.com/blogs/news/los-suenos-marriott?comment=133727584553#comments
https://www.jakemax.com/blogs/news/test-blog?comment=125652041916#comments
https://sigiskin.com/blogs/news/de-puffing-your-eyes-with-gentle-gaze?comment=133625282869#comments
https://onechewthree.com.au/blogs/news/mindful-and-simplicity-gifting?comment=135285670192#comments
https://pseudolabs.com/blogs/in-pseudo/seen-in-pseudo-vol-58?comment=130479390962#comments
https://www.austinkeen.com/blogs/news/skimming-in-the-street?comment=126072291525#comments
https://nanasbabywear.co.za/blogs/behind-the-scenes/tea-party?comment=126771953737#comments
https://goteamstripes.com/blogs/the-ref-tribune/starting-with-a-handshake-the-perspective-of-a-youth-official?comment=129235812465#comments
https://www.kissedbythesunspiceco.com/blogs/blog/101370753-what-is-seasoned-sea-salt?comment=134157140269#comments
https://www.onevillagecoffee.com/blogs/ask-steve/brewing-a-chemex?comment=131262415081#comments
https://www.united-tees.com/blogs/united-tees-blog/8307948-american-legends?comment=129493794877#Comments
https://petitpeony.com/blogs/news/69803395-petit-spook-out?comment=129795588179#comments
https://eastendflowermarket.com.au/blogs/flower-blogs/why-you-should-treat-yourself-to-an-indoor-plant?comment=131824517319#comments
https://duluthlabs.com/blogs/weeklymolecule/styrene-and-polystyrene?comment=131113746530#comments
https://www.luckydipltd.co.uk/blogs/lucky-dip-blog/knives-and-fawkes-a-bonfire-feast?comment=127733596314#comments
https://pekesafety.com/blogs/news/when-to-change-your-filters-in-the-work-place?comment=133972951333#comments
https://www.spicyelement.com/blogs/recipe/bamboo-shoots-salad?comment=125778034884#comments
https://www.utzmarket.com/blogs/musings/guatemala-is-our-muse?comment=126697701548#comments
https://cherokeesoap.com/blogs/news/hallmark-holidays?comment=127816302673#comments
https://www.shopsocialthreads.com/blogs/news/maximum-impact-minimum-effort-our-best-dresses-for-summer?comment=128662274130#comments
https://shelbykregel.com/blogs/the-green-bus-us/whimsy?comment=130141126765#comments
https://www.oddpears.com/blogs/blog/119848451-amber-day?comment=130150629568#comments
https://wendigotea.com/blogs/news/16217141-its-eyes-have-opened?comment=134936658206#comments
https://www.pinkfreshstudio.com/blogs/card-making-challenges/april-2022-challenge?comment=130766307502#comments
https://www.smallscalesseafood.com/blogs/recipes/alaska-sablefish-marinated-with-acacia-honey?comment=127175655605#comments
https://renewskinsolutions.com/blogs/news/hair-loss-the-many-causes?comment=129357447326#comments
https://birdling.com/blogs/notes-from-the-nest/diaper-duty?comment=133898993942#comments
https://www.nundlenaturalskin.com.au/blogs/news/exceptional-value-family-skin-care-pack?comment=128079790138#Comments
https://goalkicksoccer.com/blogs/news/the-new-nemeziz-cleats-and-the-tango-shoe?comment=134704922944#comments
https://starpapermoon.com/blogs/news/7-tips-to-make-your-home-beautiful-for-ramadan-and-eid?comment=129477771443#comments
https://www.sportstakeoff.com/blogs/look-at-this/vlog-2-i-am-not-a-model-deante-battle?comment=135770702163#comments
https://hourloop.com/blogs/news/40945537-disney-villains-part-5-sing-sweet-nightingale?comment=128082837562#comments
https://hardtimesclothing.co.uk/blogs/news/black-friday-2016?comment=131300982999#comments
https://www.kstuned.com/blogs/knowledge/all-h-f-series-engines-have-the-same-deck-height?comment=133687869720#comments
https://www.fortnegrita.com/blogs/fort-negrita-blog/we-understood-the-assignment?comment=128095813707#comments
https://jophiel.com/blogs/style-news/132385283-how-does-a-fashion-trend-start?comment=132505567466#comments
https://battlebars.com/blogs/news/commanders-reading-list-pumpkin-flowers-by-matti-friedman?comment=131683385570#comments
https://jamhuriwear.com/blogs/jw-blog/africa-top-5-music-videos-week-43-nov-2018?comment=126978883759#comments
https://www.plantnmore.com/blogs/news/article?comment=132505600234#comments
https://jinmee.co.uk/blogs/news/the-3-second-rule-the-korean-skincare-trend-that-improves-your-hydration?comment=132818108467#comments
https://www.wildlandorganics.com/blogs/livelight/a-very-tiny-christmas?comment=130866577663#comments
https://www.gallantoro.com/blogs/gallantoro/why-men-want-war?comment=131264807145#comments
https://sectsshop.com/blogs/sects-blog/sects-showcase-hubert?comment=131264839913#Comments
https://www.skinnymetea.com.au/blogs/smtblog/how-to-keep-active-when-you-have-a-desk-job?comment=134126108749#comments
https://www.donnasrecipe.com/blogs/donnas-blog/difference-between-leave-in-conditioner-deep-conditioner?comment=126978949295#comments
https://www.sigeeka.com/blogs/articles/shakha-pola-loha-bangles?comment=133678563626#comments
https://easternoutfitter.com/blogs/blog/six-old-duck-hunting-tips-that-still-work?comment=127787794498#comments
https://maivino.com/blogs/maivinopours/vogue-printed-this-crazy-wine-diet-in-the-70s?comment=130354446574#comments
https://imageskincare.com.au/blogs/blog/how-to-turn-your-skincare-masks-into-halloween-masks?comment=130667610206#comments
http://seminar.eventnn.ru/redir.php?link=https://spo337.com/
http://svadba.eventnn.ru/redir.php?link=https://spo337.com/
http://www.eventnn.ru/catalog/actions/?a=redirect&link=https://spo337.com/
http://www.evrika41.ru/redirect?url=https://spo337.com/
http://expomodel.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.extrimdrive.ru/bitrix/rk.php?goto=https://spo337.com/
https://facto.ru/bitrix/rk.php?goto=https://spo337.com/
http://fallout3.ru/utils/ref.php?url=https://spo337.com/
http://fbsearch.ru/away.php?url=https://spo337.com/
https://fc-zenit.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.finanalis.ru/out.php?linkout=https://spo337.com/
https://flyd.ru/away.php?to=https://spo337.com/
http://forum-region.ru/forum/away.php?s=https://spo337.com/
http://forumdate.ru/redirect-to/?redirect=https://spo337.com/
https://fr-gtr.ru/go?https://spo337.com/
https://freediving.ru/bitrix/redirect.php?goto=https://spo337.com/
http://sevensport.ru/go.php?to=https://spo337.com/
https://www.sgvavia.ru/go?https://spo337.com/
http://shckp.ru/ext_link?url=https://spo337.com/
https://sherif-karter.ru/bitrix/redirect.php?goto=https://spo337.com/
https://shinglas.ru/bitrix/redirect.php?goto=https://spo337.com/
https://shtilniisad.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.shtrih-m.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.sibpsa.ru/bitrix/redirect.php?goto=https://spo337.com/
https://sibran.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.sibsiu.ru/bitrix/rk.php?goto=https://spo337.com/
http://simvol-veri.ru/xp/?goto=https://spo337.com/
https://www.sinetic.ru/bitrix/redirect.php?goto=https://spo337.com/
http://sintez-oka.ru/bitrix/redirect.php?goto=https://spo337.com/
https://lk.sistemagorod.ru/lk/away?to=https://spo337.com/
https://lk.sistemagorod.ru/lk/away?to=https://spo337.com/
https://skamata.ru/bitrix/redirect.php?goto=https://spo337.com/
https://www.skamata.ru/bitrix/redirect.php?event1=cafesreda&event2=&event3=&goto=https://spo337.com/
http://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
https://skibaza.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=https://spo337.com/
http://mias.skoda-avto.ru/bitrix/redirect.php?event1=news_out&event2=&goto=https://spo337.com/
http://slotex.ru/bitrix/redirect.php?goto=https://spo337.com/
https://smartservices.ru/bitrix/rk.php?goto=https://spo337.com/
http://smartsourcing.ru/link?https://spo337.com/
https://smolkassa.ru/?b=1&r=https://spo337.com/
http://rexband.top4cats.ru/scripts/redirect.php?url=https://spo337.com/
http://zvezda-kuril.top4cats.ru/scripts/redirect.php?url=https://spo337.com/
http://www.topkam.ru/gtu/?url=https://spo337.com/
https://torggrad.ru/bitrix/rk.php?goto=https://spo337.com/
http://torgi-rybinsk.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.totzyv.ru/cgi-bin/clicks/redirect.pl?ei=rgb_thai_tour&goto=https://spo337.com/
http://tpprt.ru/bitrix/rk.php?goto=https://spo337.com/
https://www.tral.ru/images/get.php?go=https://spo337.com/
http://www.trucktown.ru/go.php?https://spo337.com/
http://tsm.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.tsm.ru/bitrix/rk.php?goto=https://spo337.com/
https://www.tu-bryansk.ru/bitrix/redirect.php?goto=https://spo337.com/
https://turbazar.ru/url/index?url=https://spo337.com/
https://www.tutanetam.ru/redirect?url=https://spo337.com/
http://stanko.tw1.ru/redirect.php?url=https://spo337.com/
https://twilightrussia.ru/go?https://spo337.com/
https://u74.ru/away?to=https://spo337.com/
https://go.ucrazy.ru/?to=aHR0cHM6Ly93d3cuc3BpbnN1cGVyc2xvdC5jb20v
https://ulfishing.ru/forum/go.php?https://spo337.com/
https://underwood.ru/away.html?url=https://spo337.com/
https://unicom.ru/links.php?go=https://spo337.com/
http://www.unifin.ru/bitrix/redirect.php?goto=https://spo337.com/
https://uogorod.ru/feed/520?redirect=https://spo337.com/
https://www.urso.ru/action.redirect/url/aHR0cHM6Ly93d3cuc3BpbnN1cGVyc2xvdC5jb20v/dG9wX2lkPTQxNDk=
https://utmagazine.ru/r?url=https://spo337.com/
https://utmagazine.ru/r?url=https://spo337.com/
http://econom.uu.ru/?a[]=<a+href=https://spo337.com/
http://econom.uu.ru/?a[]=<a+href=https://spo337.com/
http://uvbnb.ru/go?https://spo337.com/
https://uziolog.ru/redirect?url=https://spo337.com/
https://www.businessinsider.com.au/?s=https://spo337.com/
https://www.ehow.com/search?q=https://spo337.com/
https://www.codeproject.com/search.aspx?q=https://spo337.com/
https://www.javaworld.com/search?query=https://spo337.com/
https://www.meetup.com/find/?keywords=https://spo337.com/
https://www.familylife.com/?s=https://spo337.com/
https://thefamilydinnerproject.org/?s=https://spo337.com/
https://www.ufs.ac.za/Search-Results?indexCatalogue=All-Sites-Search-Index&searchQuery=https://https://spo337.com/
https://www.glamour.com/search?q=HTTPS%3A%2F%2Fhttps://spo337.com/
https://www.bloomberg.com/search?query=https://https://spo337.com/
https://www.chalmers.se/en/search/Pages/default.aspx?q=https://spo337.com/
https://en-urban.tau.ac.il/tau/search?keys=https://https://spo337.com/
https://www.eaie.org/search.html?queryStr=https://spo337.com/
https://www.co.monterey.ca.us/how-do-i/search?q=https://spo337.com/
https://www.handbook.fca.org.uk/handbook?site-search=https://spo337.com/
https://iconic.ftn.uns.ac.rs/?s=https://spo337.com/
https://mixxmix.com/product/search.html?banner_action=&keyword=https://spo337.com/
https://link.springer.com/search?query=https://spo337.com/
https://www.sciencedirect.com/search?qs=https://spo337.com/
https://www.nature.com/search?q=https://spo337.com/
https://www.sapo.pt/pesquisa/web/tudo?q=https://spo337.com/
https://videos.sapo.pt/search.html?word=https://spo337.com/
https://www.nbcnews.com/search/?q=https://spo337.com/
https://discover.hubpages.com/search?query=https://spo337.com/
https://search.sheffield.ac.uk/s/search.html?query=https://spo337.com/
https://news.abs-cbn.com/special-pages/search?q=https://spo337.com/#gsc.tab=0&gsc.q=https://spo337.com/
https://www.microsoft.com/nl-nl/search/explore?q=https://spo337.com/
https://en.wikipedia.org/w/index.php?search=https://spo337.com/
https://www.istockphoto.com/nl/search/2/image?
family=creative&phrase=https://spo337.com/
https://github.com/search?q=https://spo337.com/
https://www.youtube.com/results?search_query=https://spo337.com/
https://play.google.com/store/search?q=https://spo337.com/
https://www.globo.com/busca/?q=https://spo337.com/
https://www.hugedomains.com/domain_search.cfm?domain_name=https://spo337.com/
https://www.reuters.com/site-search/?query=https://spo337.com/
https://www.brandbucket.com/search?q=https://spo337.com/
https://www.weebly.com/domains?search=https://spo337.com/
https://www.gmanetwork.com/news/#/search;query=https://spo337.com/
https://edex.adobe.com/search?q=https://spo337.com/
https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=www.healthit.gov&query=https://spo337.com/
https://www.tumblr.com/search/https:https://www.businessinsider.com.au/?s=https://spo337.com/
https://www.ehow.com/search?q=https://spo337.com/
https://www.codeproject.com/search.aspx?q=https://spo337.com/
https://www.javaworld.com/search?query=https://spo337.com/
https://www.meetup.com/find/?keywords=https://spo337.com///spo337.com/
https://www.deviantart.com/search?q=https://spo337.com/
https://domains.lycos.com/search/?search=https://spo337.com/
https://www.instructables.com/howto/https://spo337.com/
https://discover.hubpages.com/search?query=https://spo337.com/
https://www.soup.io/?s=https://spo337.com/
https://startupxplore.com/en/search?q=https://spo337.com/
https://www.mywot.com/scorecard/https://spo337.com/
https://www.designspiration.com/search/saves/?q=https://spo337.com/
https://www.isixsigma.com/qsearch/?sphrase=https://spo337.com/
https://www.bloglovin.com/search?q=https://spo337.com/&search_term=https://spo337.com/
https://casa.abril.com.br/?s=https://spo337.com/
https://blogs.ubc.ca/mannatcheema/?s=https://spo337.com/&submit=Search
https://trove.nla.gov.au/search/category/websites?keyword=https://spo337.com/
https://edukite.org/?s=https://spo337.com/&post_type=course
https://www.australiantraveller.com/search/?search_term=https://spo337.com/
https://www.coupondunia.in/blog/?s=https://spo337.com/
https://jbf4093j.videomarketingplatform.co/search/perform?search=https://spo337.com/
https://ga.videomarketingplatform.co/search/perform?search=https://spo337.com/
https://www.apple.com/nz/search/https%3A-https://spo337.com/
https://www.reddit.com/search/?q=https://spo337.com/
https://observer.com/?s=https://spo337.com/
https://gektor-nsk.ru/bitrix/redirect.php?goto=https://spo337.com/
https://getfaster.ru/bitrix/rk.php?goto=https://spo337.com/
http://gfaq.ru/go?https://spo337.com/
http://ggeek.ru/bitrix/redirect.php?goto=https://spo337.com/
http://azt.ggeek.ru/azt-zbt.php?p=ggeek&backurl=https://spo337.com/
http://www.gigatran.ru/go?url=https://spo337.com/
https://gituha.ru/root/redirect.php?https://spo337.com/
https://glavkniga.ru/away.php?to=https://spo337.com/
https://glazev.ru/redirect?url=https://spo337.com/
https://globalmedia51.ru/bitrix/redirect.php?goto=https://spo337.com/
http://www.go2golf.ru/link/redirect?link=https://spo337.com/
https://godsvoice.ru/redirect?url=https://spo337.com/
https://golden-resort.ru/out.php?out=https://spo337.com/
https://goldfish.ru/bitrix/rk.php?goto=https://spo337.com/
http://avalon.gondor.ru/away.php?link=https://spo337.com/
https://good-surf.ru/r.php?g=https://spo337.com/
https://www.gooddoor.ru/bitrix/rk.php?goto=https://spo337.com/
https://clients1.google.ad/url?q=https://spo337.com/
https://cse.google.ad/url?q=https://spo337.com/
https://images.google.ad/url?q=https://spo337.com/
https://maps.google.ad/url?q=https://spo337.com/
https://www.google.ad/url?q=https://spo337.com/
https://emaratyah.ae/new-redirect.php?w=https://spo337.com/
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=https://spo337.com/
http://rafco.ae/container.asp?url=https://spo337.com/
http://for-css.ucoz.ae/go?https://spo337.com/
https://clients1.google.com.af/url?q=https://spo337.com/
https://cse.google.com.af/url?q=https://spo337.com/
https://images.google.com.af/url?q=https://spo337.com/
http://toolbarqueries.google.com.af/url?sa=t&url=https://spo337.com/
https://www.google.com.af/url?q=https://spo337.com/
https://www.snek.ai/redirect?url=https://spo337.com/
http://www.torrent.ai/lt/redirect.php?url=https://spo337.com/
http://avto.al/az/home/redirect?carId=1639612&url=https://spo337.com/
https://clients1.google.al/url?q=https://spo337.com/
https://cse.google.al/url?q=https://spo337.com/
https://images.google.al/url?q=https://spo337.com/
https://images.google.al/url?q=https://spo337.com/
http://toolbarqueries.google.al/url?q=https://spo337.com/
https://www.google.al/url?q=https://spo337.com/
http://tido.al/vazhdo.php?url=https://spo337.com/
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=https://spo337.com/
https://oxleys.app/friends.php?q=https://spo337.com/
http://www.ain.com.ar/openpop.php?url=https://spo337.com/
http://webfeeds.brookings.edu/%7E/t/0/0/%7Ewww.https://spo337.com/
https://www.weebly.com/domains?search=spo337.com
https://www.gmanetwork.com/news/#/search;query=spo337.com
https://edex.adobe.com/search?q=spo337.com
https://search.usa.gov/search?utf8=%E2%9C%93&affiliate=www.healthit.gov&query=spo337.com
https://www.tumblr.com/search/spo337.com/
https://www.deviantart.com/search?q=spo337.com/
https://domains.lycos.com/search/?search=spo337.com/
https://www.instructables.com/howto/spo337.com/
https://discover.hubpages.com/search?query=spo337.com/
https://www.imp.mx/salto.php?va=https://spo337.com/
https://splash.hume.vic.gov.a u/analytics/outbound?url=https://spo337.com/
http://www.tupassi.pr.gov.br/enquete/enquete.php?id_cliente=1027&url=https://spo337.com/
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=https://spo337.com/
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2012011201&return=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2014062701&return=https://spo337.com/
https://rspcb.safety.fhwa.dot.gov/pageRedirect.aspx?RedirectedURL=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2014062701&return=https://spo337.com/
https://splash.hume.vic.gov.au/analytics/outbound?url=https://spo337.com/
https://www.wrasb.gov.tw/opennews/opennews01_detail.aspx?nno=2012011201&return=https://spo337.com/
http://www.tupassi.pr.gov.br/enquete/enquete.php?id_cliente=1027&url=https://spo337.com/
https://www.fhwa.dot.gov/reauthorization/reauexit.cfm?link=https://spo337.com/
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://spo337.com/
https://clubs.london.edu/click?uid=24ce7c96-76b4-11e6-8631-0050569d64d5&r=https://spo337.com/
https://www.nbc.edu/videoplayer.php?u=https://spo337.com/
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=https://spo337.com/
http://rs.rikkyo.ac.jp/rs/error/ApplicationError.aspx?TopURL=https://spo337.com/
https://publishing.brookes.ac.uk/?URL=https://spo337.com/
https://toolbarqueries.google.co.vi/url?q=https://spo337.com/
http://jclick.koreadaily.com/JAgent1.dll?3*16*https://spo337.com/
http://my.51edu.cc/link.php?url=https://spo337.com/
https://mybrawlstats.com/redirect.php?lang=german&urlReceived=https://spo337.com/
http://www.peterblum.com/DES/DateAndTime.aspx?Returnurl=https://spo337.com/
http://m.17ll.com/apply/tourl/?url=https://spo337.com/
https://arxip.com/bitrix/rk.php?goto=https://spo337.com/
https://fpess.scu.ac.ir/en/web/cow/display/-/asset_publisher/6j7CwiyKWPfS/document/id/1307867?redirect=https://spo337.com/
https://www.autocar.co.nz/Redirect.aspx?destination=https://spo337.com/
https://track.effiliation.com/servlet/effi.redir?id_compteur=13215059&url=https://spo337.com/
http://ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=https://spo337.com/
https://www.usjournal.com/go.php?campusID=190&url=https://spo337.com/
https://www.winnipegfreepress.com/s?action=doLogout&rurl=https://spo337.com/
https://www.kyoto-good.jp/ad.php?url=https://spo337.com/
https://analytics.bluekai.com/site/16231?phint=event=click&phint=campaign=BRAND-TAB&phint=platform=search&done=https://spo337.com/
https://temptationsaga.com/buy.php?url=https://spo337.com/&store=Books-A-Million
https://socialmart.ru/redirect.php?url=https://spo337.com/
https://www.interpals.net/url_redirect.php?href=https://spo337.com/
https://toolbarqueries.google.co.zw/url?q=https://spo337.com/
https://www.xfdq123.com/url.aspx?url=https://spo337.com/
http://www.bshare.cn/share?url=https://spo337.com/
https://www.nanotech-now.com/redir.cgi?dest=https://spo337.com/
https://sukhoverkhov.ru/wp-content/plugins/wp-js-external-link-info/redirect.php?blog=&url=https://spo337.com/
https://toolbarqueries.google.gm/url?q=https://spo337.com/
https://www.cosmeticchoice.com.au/index.php?route=banners/banners/clickthru&banner_id=159&expiration_setting=2&impressions=0&link=https://spo337.com/
https://toolbarqueries.google.ae/url?sa=i&url=https://spo337.com/
https://www.bausch.in/redirect/?url=https://spo337.com/
https://www.bausch.com.ph/redirect/?url=https://spo337.com/
http://www.wittstock.chemie.uni-oldenburg.de/agef/link_extern.html?link=https://spo337.com/
https://uk.kindofbook.com/redirect.php/?red=https://spo337.com/
https://kakaku-navi.net/items/detail.php?url=https://spo337.com/
https://www.viidii.info/?https://spo337.com/
https://www.cricbattle.com/register.aspx?returnurl=https://spo337.com/
https://www.havanautos.com/dtcspot/public/banner_redirect.aspx?idca=343&ids=86839669&cp=167&idcli=0&ci=1&p=https://spo337.com/
https://linkerload.com/url/go.php?url=https://spo337.com/
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=https://spo337.com/
https://aquajeux.com/produits/controle-et-gestion-d-eau/activation-electronique/pado-329?r=https://spo337.com/
http://www.howtotrainyourdragon.co.nz/notice.php?url=https://spo337.com/
https://www.bausch.com.tw/redirect/?url=https://spo337.com/
http://www.shippingchina.com/pagead.php?id=RW4uU2hpcC5tYWluLjE=&tourl=https://spo337.com/
https://toolbarqueries.google.com.tw/url?q=https://spo337.com/
http://janeyleegrace.worldsecuresystems.com/redirect.aspx?destination=https://spo337.com/
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=https://spo337.com/
https://www.sigel.de/en_gb/Weiterempfehlen?url=https://spo337.com/
https://www.apegs.ca/Portal/Pages/sign-up-member?returnUrl=https://spo337.com/
https://namathis.com/verpagina_header.php?pagina=https://spo337.com/
https://api.mymosey.com/forward?url=https://spo337.com/&vendor=KLOOK
https://g0.more.game.tw/ogames.php?l=210&t=280&w=910&h=750&s=https://spo337.com/
https://www.lionscup.dk/?side_unique=4fb6493f-b9cf-11e0-8802-a9051d81306c&s_id=30&s_d_id=64&go=https://spo337.com/
https://toolbarqueries.google.dk/url?q=https://spo337.com/
http://aps.sn/spip.php?page=clic&id_publicite=366&id_banniere=6&from=/actualites/sports/lutte/article/modou-lo-lac-de-guiers-2-l-autre-enjeu&redirect=https://spo337.com/
https://pse5-esd5.aadnc-aandc.gc.ca/pubcbw/publication/display_file.aspx?link=https://spo337.com/
http://kreepost.com/go/?https://spo337.com/
https://www.1upfun.com/redirect.aspx?url=https://spo337.com/
http://mailer.dt.co.kr/bin/checker?mode=5&module=11&mailidx=4275&dmidx=0&emidx=0&service=0&etime=20080227100001&seqidx=18963&objidx=16&url=https://spo337.com/
https://www.matchesfashion.com/us/affiliate?url=https://spo337.com/
https://im.tonghopdeal.net/pic.php?q=https://spo337.com/
https://toolbarqueries.google.com.bn/url?q=https://spo337.com/
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=https://spo337.com/
https://leadertoday.org/topframe2014.php?goto=https://spo337.com/
https://spb-medcom.ru/redirect.php?https://spo337.com/
https://www.info-realty.ru/bitrix/rk.php?goto=https://spo337.com/
http://www.sermemole.com/public/serbook/redirect.php?url=https://spo337.com/
https://www.hradycz.cz/redir.php?b=445&t=https://spo337.com/
https://heaven.porn/te3/out.php?u=https://spo337.com/
https://toolbarqueries.google.com.ar/url?sa=i&url=https://spo337.com/
http://prlog.ru/backlink/https://spo337.com/
https://www.ntnu.edu.tw/frame.php?url=https://spo337.com/
http://park3.wakwak.com/~yadoryuo/cgi-bin/click3/click3.cgi?cnt=chalet-main&url=https://spo337.com/
https://adminstation.ru/verification/?url=https://spo337.com/
https://toolbarqueries.google.bg/url?q=https://spo337.com/
https://weblib.lib.umt.edu/redirect/proxyselect.php?url=//https://spo337.com/
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=https://spo337.com/
http://www.jtrchina.com/m2c/2/s_date0.jsp?tree_id=0&sdate=2020-03-09&url=https://spo337.com/
http://weekly.chosun.com/protect_weekly/redirect.asp?url=https://spo337.com/
http://www.paladiny.ru/go.php?url=https://spo337.com/
https://toolbarqueries.google.com.ng/url?sa=i&url=https://spo337.com/
https://maps.google.cv/url?q=https://spo337.com/
https://www.datum.tv/ra.asp?url=https://spo337.com/
https://vssillc.asureforce.net/redirect.aspx?redirecturl=https://spo337.com/
http://2ch-ranking.net/redirect.php?url=https://spo337.com/
https://www.google.mk/url?q=https://spo337.com/
https://mycounter.com.ua/go.php?https://spo337.com/
http://db.studyincanada.ca/forwarder.php?f=https://spo337.com/
https://live.warthunder.com/away/?to=https://spo337.com/
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=https://spo337.com/
http://www.yoger.com.cn/union/transfer.php?uID=10125&url=https://spo337.com/
http://www.016788.com/gourl.asp?url=https://spo337.com/
https://maps.google.bt/url?q=https://spo337.com/
https://guru.sanook.com/?URL=https://spo337.com/
https://www.badaweb.com/detall.php?uid=20000530190000-002&control=hoQChn9YtFJwg&idioma=0&keyword=P%E0ginaPrincipaldeBW&cat=&ciutat=5&url=https://spo337.com/
https://tool.365jz.com/alexa/https://spo337.com/
https://www.juina.mt.gov.br/atalhoconta.php?id=7&link=https://spo337.com/
https://www.bb.com.tr/util/change-language.php?lng=EN&url=https://spo337.com/
http://www.01caijing.com/go.htm?url=https://spo337.com/