Naphthalene: Main Ingredient In Mothballs!
Naphthalene is a white, volatile, solid polycyclic hydrocarbon with a strong mothball odor.Naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.
Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride, but is also used in moth repellents. It is also produced when things burn, so naphthalene is found in cigarette smoke, car exhaust, and smoke from forest fires. Exposure to naphthalene is associated with hemolytic anemia, damage to the liver and neurological system, cataracts and retinal hemorrhage. Naphthalene is reasonably anticipated to be a human carcinogen and may be associated with an increased risk of developing laryngeal and colorectal cancer.
What does Naphthalene look like in Chemistry?
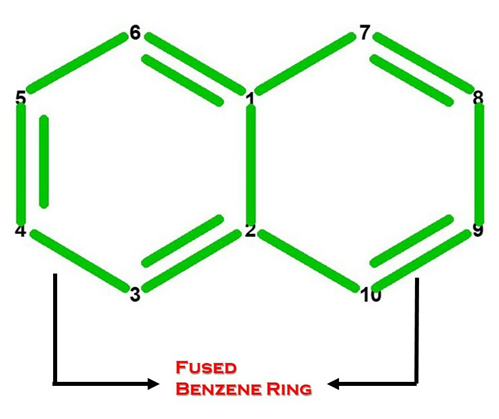
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Naphthalene! You’ll need:
-
10 Carbon Atoms
-
8 Hydrogen atoms
-
8 Small connectors (compact small bonds for hydrogen)
-
6 Medium Connectors
-
10 Long connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building With Our Benzene Portion (Ring A).
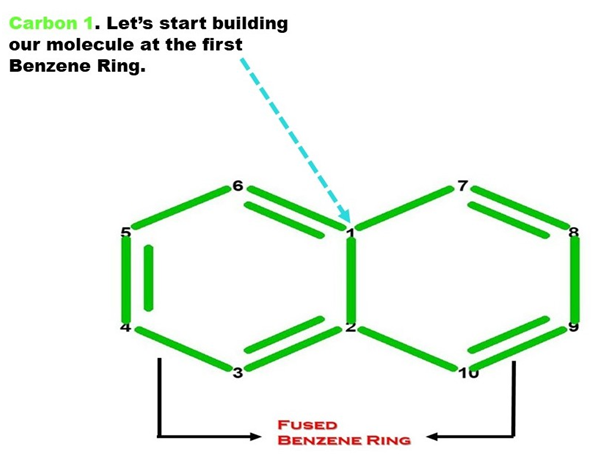
Note: We build this portion in a clockwise direction, starting with Carbon 1.
Let’s start!
Steps:
-
1

1. Get a carbon atom (Carbon 1) then attach another carbon (Carbon 2) below it using a medium connector.
-
2

2. Attach a carbonatom(Carbon 3)to Carbon 2using two long connectors. Place a hydrogen atom to Carbon 3 using a small connector.
-
3

3. Get another carbon atom(Carbon 4) then attach this toCarbon 3 using a medium connector. Add a hydrogen atom to this carbon using a small connector
-
4

4. Then, attach another carbon atom (Carbon 5) to Carbon 4 using 2 long connectors. Then, add a hydrogen atom to this carbon.
-
5

5. Attach one carbon atom (Carbon 6) toCarbon 5 using 1 medium connector. Using a small connector, add a hydrogen atom to this carbon.
-
6

6. Join(Carbon 6) andCarbon 1 togetherusing 2 long connectors.(
-

Yay! We've just built our First Benzene (Ring A) Portion!
Note: Let’s now build the Second Benzene (Ring B) portion of our Naphthalene molecule. We build this portion in a clockwise direction, starting with Carbon 7.
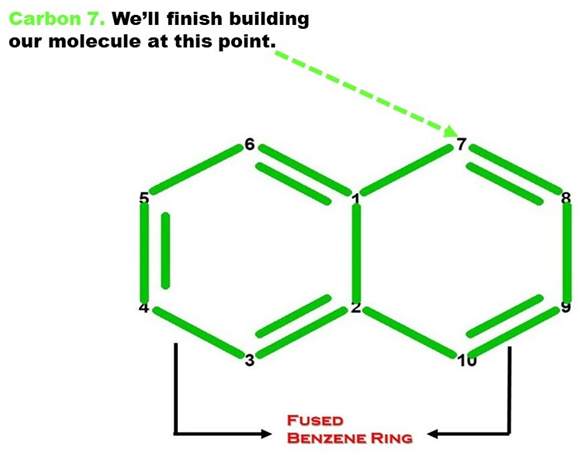
Let’s continue building!
Steps:
-
1

1. Get one carbon atom (Carbon 7) then attach this to Carbon 1(of Ring A) using a medium connector. Then, place a hydrogen atom on carbon 7 using a small connector.
-
2

2. Get another carbon atom (Carbon 8) then attach thisto Carbon 7using two long connectors. Then, add one hydrogen atom on carbon 8 using a small connector.
-
3

3. Add another carbon atom (Carbon 9) then attach this to Carbon 8using a medium connector. Place one hydrogen atom on carbon 9 using a small connector.
-
4

4. Get another Carbon atom (Carbon 10) then attach this to Carbon 9using two long connectors.Use a short connector to attach a hydrogen atom to Carbon 10.
-
5

5. Using a medium connector, attach Carbon 10to Carbon 2(of Ring A).


















http://slkjfdf.net/ – Olasan Ikiosof zyk.zizw.duluthlabs.com.vsd.zf http://slkjfdf.net/
http://slkjfdf.net/ – Aqovumek Opuyow act.cjwl.duluthlabs.com.jdi.ef http://slkjfdf.net/
http://slkjfdf.net/ – Ekamefuc Aqopihee mus.zgtx.duluthlabs.com.tgz.mx http://slkjfdf.net/