Glycerol: A Natural Moisturizer!
Glycerol, sometimes referred to as glycerin, is a sugar alcohol used in a variety of personal care products, including toothpaste, hair conditioner, cosmetics, and moisturizers.
It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in all lipids known as triglycerides. It is widely used in the food industry as a sweetener and humectant and in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water.
What does Glycerol look like in Chemistry?
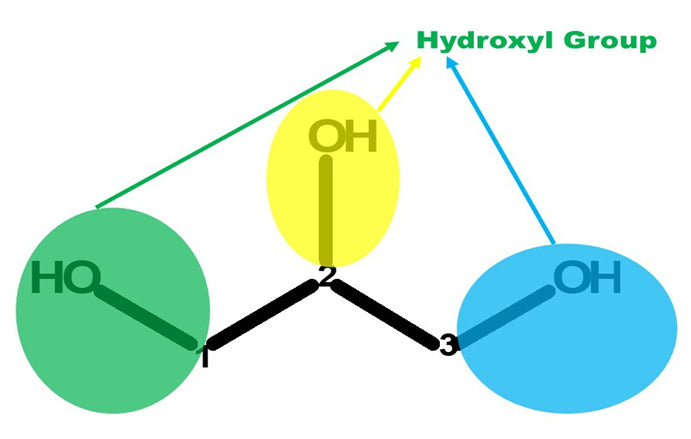
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Benzoic Acid! You’ll need:
-
3 Carbon Atoms
-
3 Oxygen atoms
-
8 Hydrogen atoms
-
8Small connectors (compact small bonds for hydrogen)
-
5 Medium Connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building With Our First Hydroxyl Group.
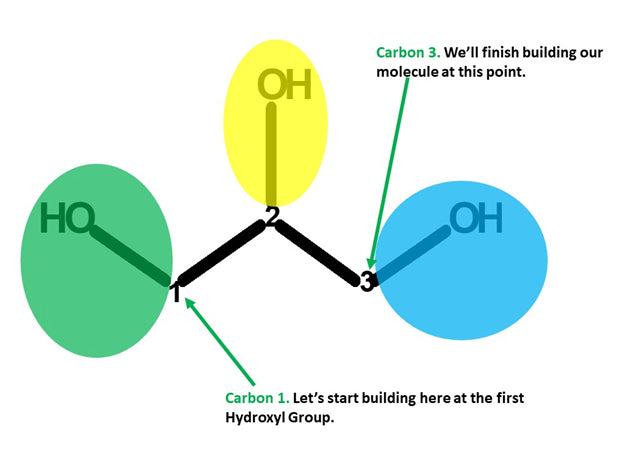
Note: We build this portion in a clockwise direction, starting with Carbon 1 and end with Carbon 3.
Let’s start!
Steps:
-
1
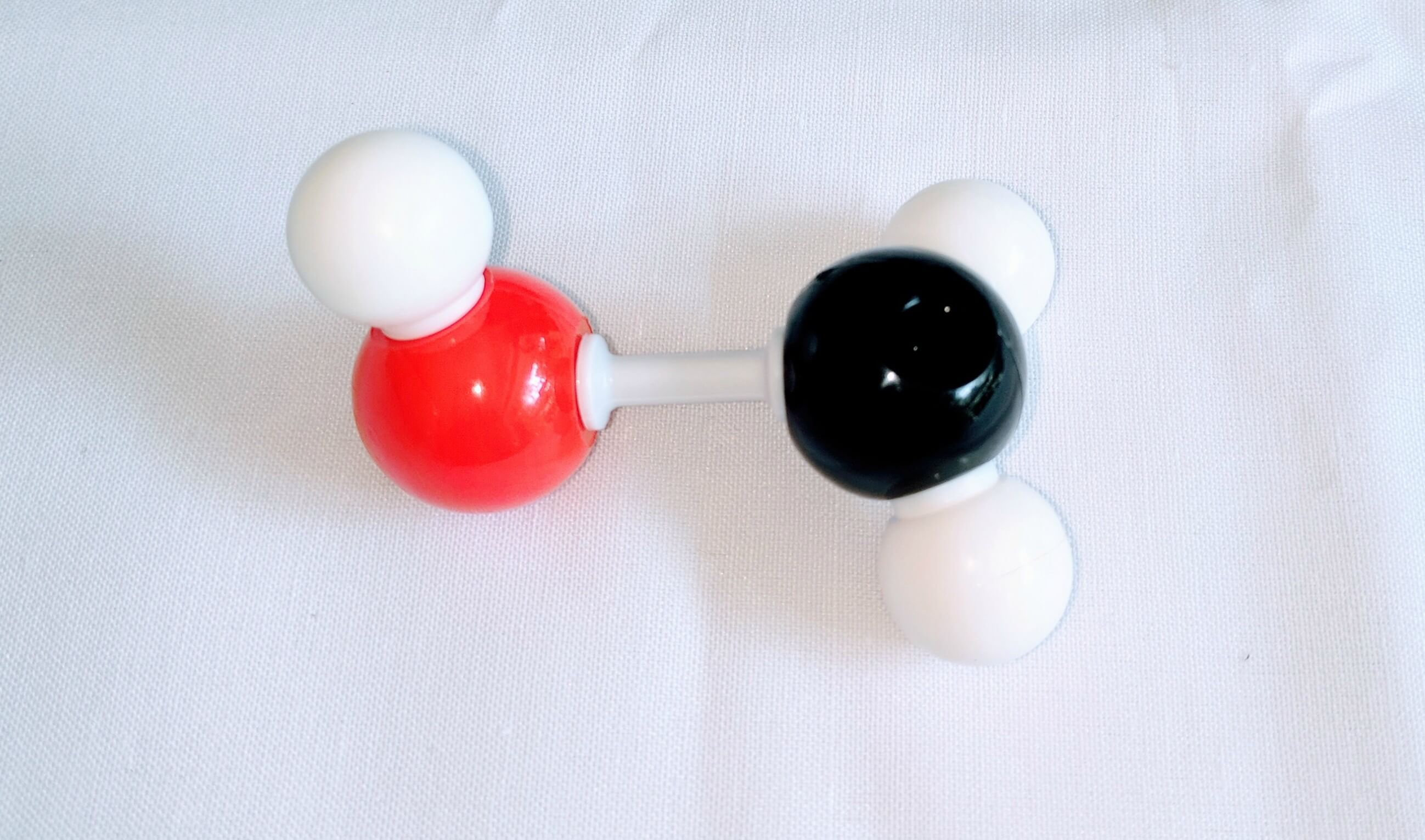
1. Get a carbon atom (Carbon 1) then attach two Hydrogen atoms to Carbon 1using two small connectors. Then use a medium connector and small connector to attach a hydroxyl group.
-
2
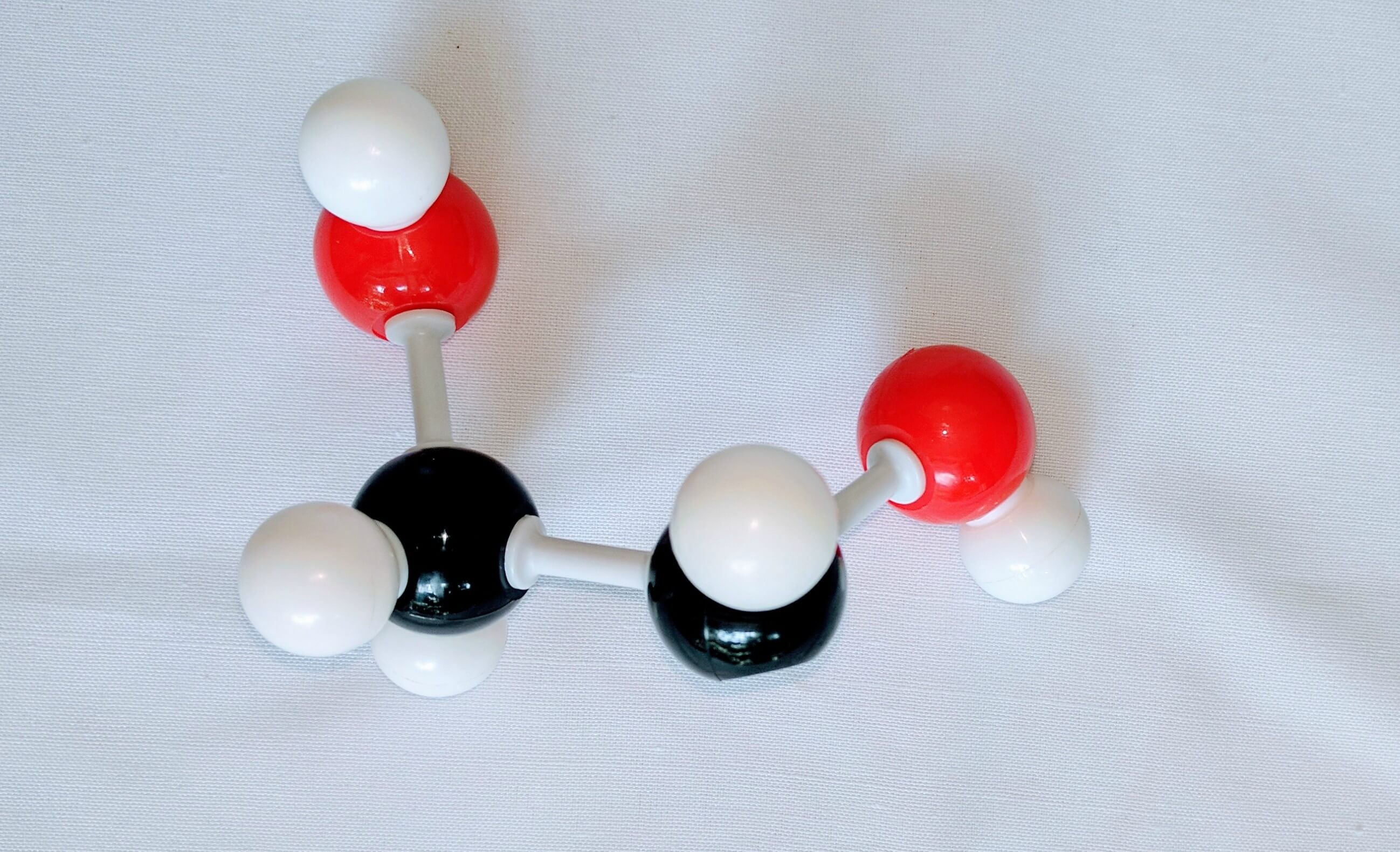
2. Get another carbon atom (Carbon 2)then attach this toCarbon 1using a medium connector. Then, using a small connector attach a Hydrogen atom below. The using a medium connector and a short connector attach a hydroxyl group to Carbon 2.
-
3
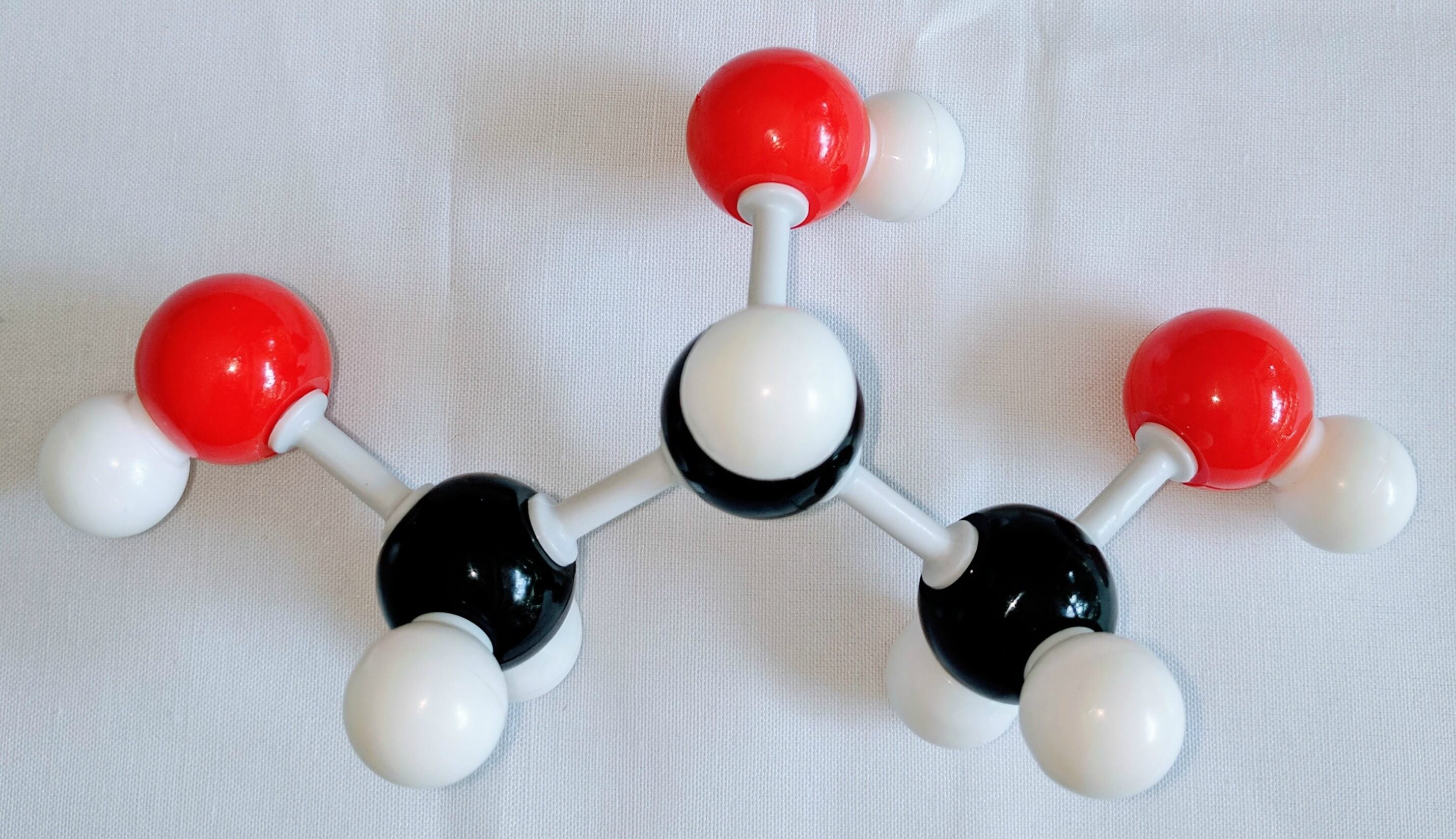
3. Using another carbon atom Carbon 3and attach it to Carbon 2using a medium connector. Then, place two Hydrogen atoms on Carbon 3using two small connectors.Finally, use a medium connector attach an Oxygen atom to Carbon 3. Place a Hydrogen atom on the Oxygen atom.










http://mewkid.net/when-is-xuxlya3/ – Amoxicillin 500 Mg Amoxicillin 500 Mg wtv.fpxb.duluthlabs.com.uwm.cp http://mewkid.net/when-is-xuxlya3/