URUSHIOL: The Poison Present in Poison Ivy
Poison ivy, also known as the “Eastern Poison Ivy”, is a plant native to Eastern North America. When touched, the secretions present in the leaves causes a condition called contact dermatitis, which is characterized with a severe, itchy and painful inflammation of the skin. The toxic principle present in the secretions is Urushiol and is being produced in certain parts of plants. With its slightly nonvolatile nature, urushiol is easily carried by different media such as clothing, gardening tools, soil, shoes, or even through smoke coming from the burned Poison Ivy plant.
How does Urushiol look like in Chemistry?

Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Urushiol! You’ll need:
- 21 Carbon atoms
- 2 Oxygen atoms
- 36 Hydrogen atoms
- 9 Small connectors (compact small bonds for hydrogen)
- 6 Medium Connectors
- 8 Long connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
We will build Urushiol in a clockwise direction, starting with Carbon 1!
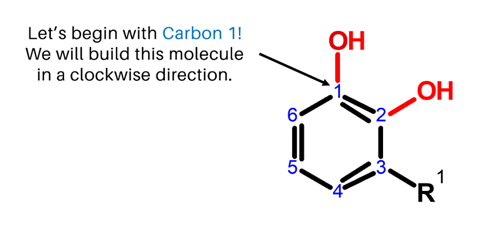
Steps:
-
1

1. Get one carbon atom (Carbon 1) then, attach an Oxygen atom to it using a medium connector. Add 1 hydrogen atom to this oxygen using 1 small connector.
-
2

2. Then, get another carbon atom (Carbon 2) and attach this to Carbon 1 using 2 long connectors.
-
3

3. Attach 1 oxygen atom Carbon 2 using 1 medium connector. Then place 1 hydrogen atom on this oxygen using 1 small connector
-
4

4. Next, get one carbon atom (Carbon 3) then attach this below Carbon 2 using a medium connector.
-
5

5. Then, get another carbon atom (Carbon 4) then attach this to Carbon 3 using 2 long connectors. Place 1 hydrogen atom on Carbon 4 using a small connector.
-
6

6. Grab one Carbon atom (Carbon 5) and attach this next to Carbon 4 using a medium connector. Likewise, place one hydrogen atom on Carbon 5 using a small connector.
-
7

7. Then, get the last carbon atom (Carbon 6) then attach this to Carbon 5 using 2 long connectors. Add one hydrogen atom on Carbon 6 using 1 small connector.
-
8

8. Finally, join Carbon 6 and Carbon 1 together using 1 medium connector.
We now have our Urushiol ring. Let’s try building its R substituent.
Have your 15 Carbon atoms and 31 Hydrogen atoms ready, and let’s start off with Carbon 1 of the alkyl chain!
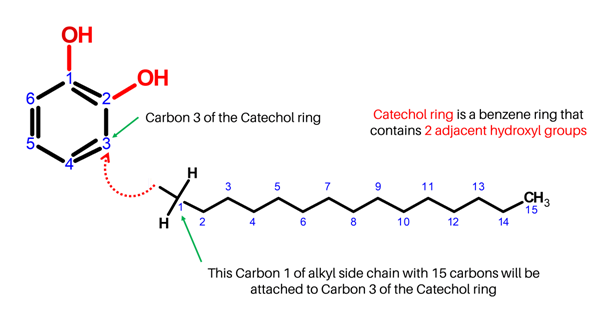
-
1

1. Get one carbon atom (Carbon 1) then, attach this to Carbon 3 of the benzene ring using a medium connector. Attach 2 hydrogen atoms to it with your 2 small connectors.
-
2

2. Get another Carbon atom (Carbon 2) then attach this next to Carbon 1 (of the alkyl chain) using a medium connector. Add 2 hydrogens to Carbon 2 using 2 small connectors.
-
3

3. Repeat step 2 for Carbons 3 to 14. Make sure to attach the succeeding carbons next to each other.
-
4

4. Finally, get the last Carbon atom (Carbon 15) then connect this to Carbon 14 of the alkyl chain using a medium connector. Add 3 hydrogen atoms to Carbon 15 using 3 small connectors.



















yhMWkRgnFuXTs
aBeXRfmDpjUTqvC
qoMIePLnW
HrIyjLxasZvpOlJ
FZvmbiUOPXwLIS