Styrene: The Building Block to Foam and Plastic!
Styrene, also known as ethenylbenzene, vinylbenzene, and phenylethene. Is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid that evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Polystyrene is the molecule behind plastics and foam. It is clear, hard, and rather brittle.
Styrene, a component of polystyrene is a colorless, toxic liquid with a strong aromatic odor. It is used to make rubbers, polymers and copolymers.
Styrene is found in alcoholic beverages, it is also present in cranberry, bilberry, currants, grapes, vinegar, parsley, milk and dairy products, whisky, cocoa, coffee, tea, roasted filberts and peanuts. Styrene is a flavoring ingredient.
What does Styrene look like in Chemistry?

Let’s Get Building!
Using your Student Molecular Model Set from Duluth Labs let’s create Styrene! You’ll need:
-
8 Carbon Atoms
-
8 Hydrogen atoms
-
8 Small connectors (compact small bonds for hydrogen)
-
4 Medium Connectors
-
8 Long connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building With Our Phenol Portion.
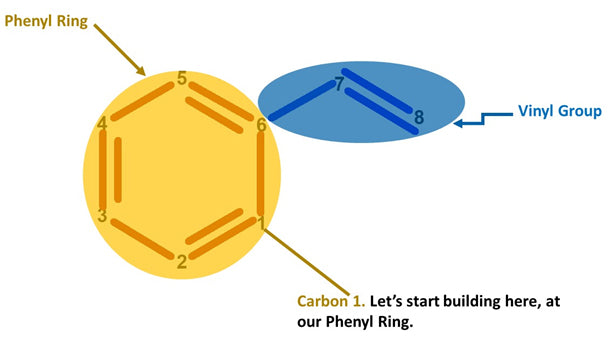
Note: We build this portion in a clockwise direction, starting with Carbon 1.
Let’s start!
Steps:
-
1

1. Get a carbon atom (Carbon 1) then attach another carbon (Carbon 2) to it using 2 long connectors. Add a hydrogen atom to Carbon 1 using a small connector
-
2

2. Attach another carbon atom (Carbon 3) to Carbon 2using a medium connector. Place a hydrogen atom on Carbon 2 using a small connector
-
3

3. Get another carbon atom (Carbon 4)then attach this to Carbon 3 using 2 long connectors. Using a small connector attach a hydrogen atom to carbon 3
-
4

4. Then, attach another carbon atom (Carbon 5) to Carbon 4 using a medium connector, then, attach a hydrogen atom to carbon 5 using a small connector.
-
5

5. Attach one carbon atom (Carbon 6)toCarbon 5 using 2 long connectors and then attach a hydrogen atom to Carbon 6 using a small connector.
-
6
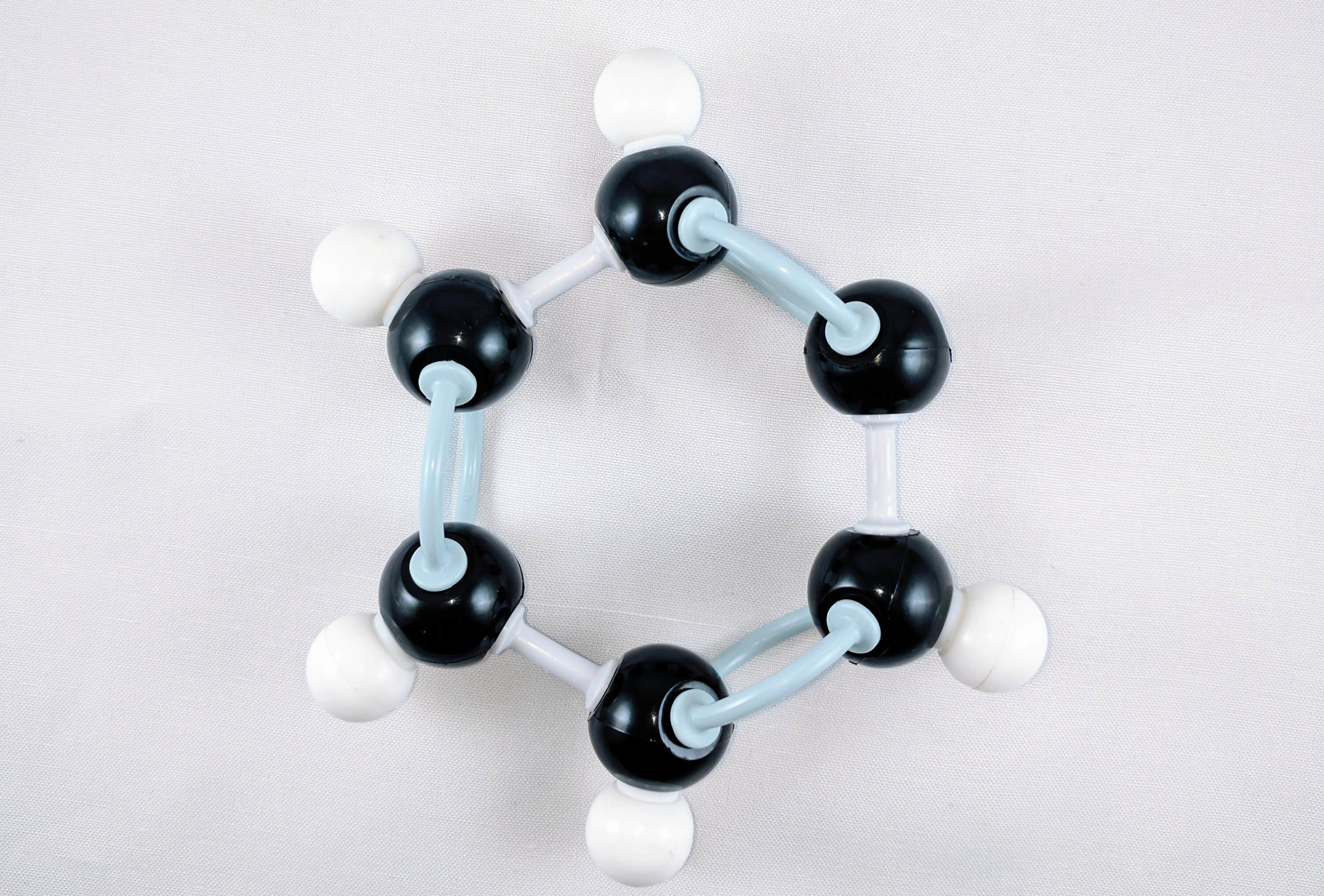
6. Join Carbon 6and Carbon 1together using a medium connector.
-

Yay! We've just built our Phenyl Portion!
Note: Let’s now build the Vinyl portion of our Styrene molecule. We build this portion starting with Carbon 7.

Let’s continue building!
Steps:
-
7

7. Get one carbon atom (Carbon 7) then attach this to Carbon 6 (of the Phenyl portion) using a medium connector. Then, place one hydrogen atom on carbon 7 using a small connector.
-
8
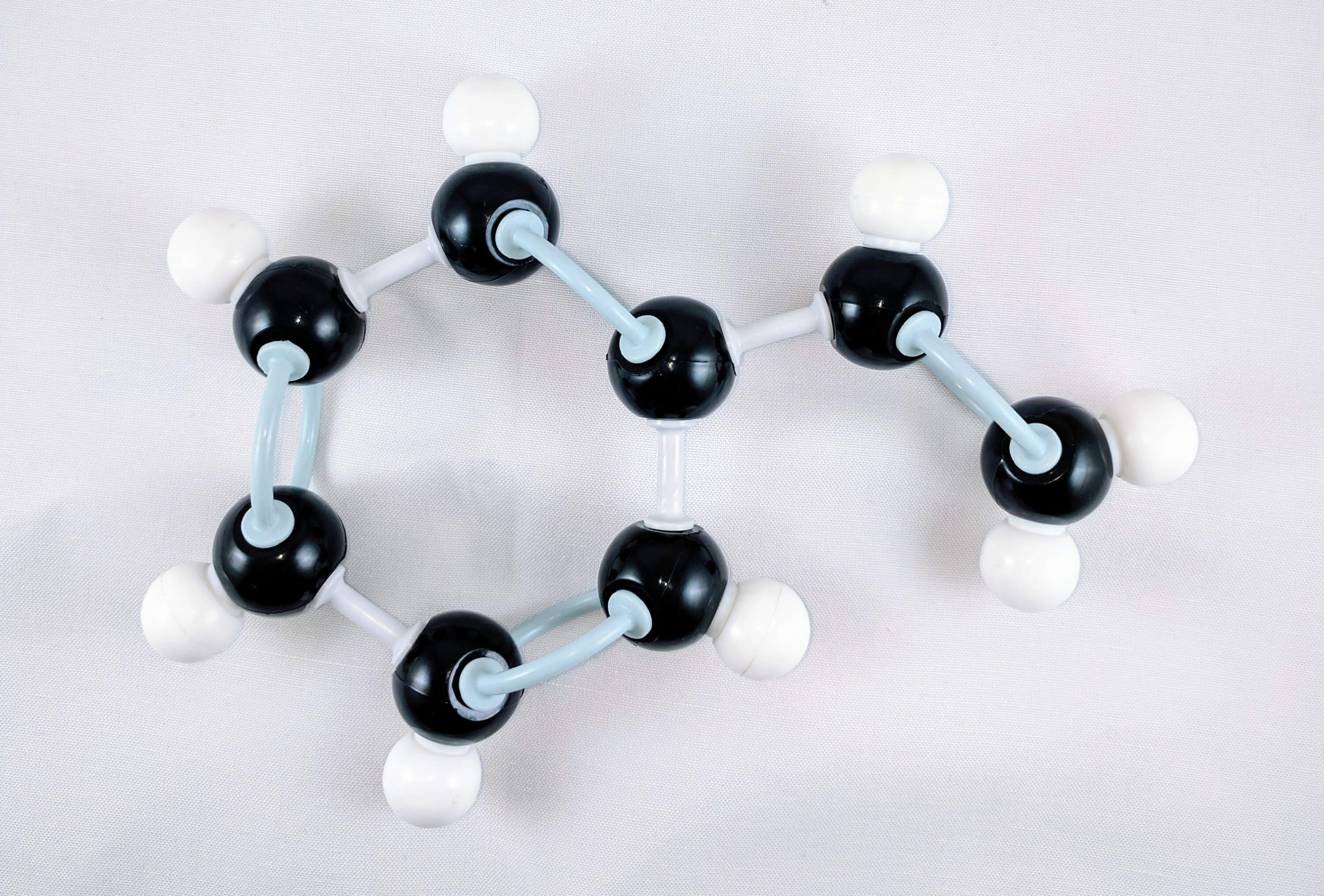
8. Get another carbon atom (Carbon 8) and use 2 long connectors to attach Carbon 8 to Carbon 7. Then, add two hydrogen atoms to carbon 8 using two small connectors.
-

Yay! We've just built our Vinyl Portion!















카지노사이트
슬롯사이트
온라인카지노
카지노주소
카지노검증사이트
안전한카지노사이트
슬롯카지노
바카라게임
카지노추천
비바카지노
퀸즈슬롯
카지노
바카라
안전한 바카라사이트
온라인슬롯
카지노사이트
바카라
바카라사이트
파라오카지노
제왕카지노
mgm카지노
더킹카지노
코인카지노
솔레어카지노
카지노게임
마이크로게이밍
아시아게이밍
타이산게이밍
오리엔탈게임
에볼루션게임
드래곤타이거
드림게이밍
비보게이밍
카지노사이트
슬롯사이트
온라인카지노
카지노주소
카지노검증사이트
안전한카지노사이트
슬롯카지노
바카라게임
카지노추천
비바카지노
퀸즈슬롯
카지노
바카라
안전한 바카라사이트
온라인슬롯
카지노사이트
바카라
바카라사이트
파라오카지노
제왕카지노
mgm카지노
더킹카지노
코인카지노
솔레어카지노
카지노게임
마이크로게이밍
아시아게이밍
타이산게이밍
오리엔탈게임
에볼루션게임
드래곤타이거
드림게이밍
비보게이밍
https://youube.me/
https://gamja888.com/
https://instagrme.com/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
온라인바카라
카지노사이트
바카라사이트
인터넷카지노
바카라게임사이트
퀸즈슬롯
카지노주소
비바카지노
카지노추천
카지노게임
온라인카지노사이트
카지노
바카라
온라인카지노
카지노게임사이트
카지노검증사이트
로얄카지노계열
슬롯머신사이트
맥스카지노
바카라게임사이트
카심바코리아 카지노
모바일카지노
실시간바카라
라이브카지노
온라인슬롯
바카라 이기는방법
안전카지노사이트
우리카지노사이트
샌즈카지노주소
바카라 게임규칙
바카라 게임방법
온라인바카라
카지노사이트
바카라사이트
인터넷카지노
바카라게임사이트
퀸즈슬롯
카지노주소
비바카지노
카지노추천
카지노게임
온라인카지노사이트
카지노
바카라
온라인카지노
카지노게임사이트
카지노검증사이트
로얄카지노계열
슬롯머신사이트
맥스카지노
바카라게임사이트
카심바코리아 카지노
모바일카지노
실시간바카라
라이브카지노
온라인슬롯
바카라 이기는방법
안전카지노사이트
우리카지노사이트
샌즈카지노주소
바카라 게임규칙
바카라 게임방법
https://gamja888.com/
https://instagrme.com/
https://youubbe.me/
https://Instagrm.me/
https://Instagrme.net/
https://internetgame.me/
https://instagrme.live/
https://naverom.me
https://facebokom.me
otizjess6@gmail.com 09/16/22
https://bit.ly/36En2s8+
카지노사이트 https://ce-top10.com/
바카라사이트 https://ce-top10.com/
온라인카지노 https://ce-top10.com/
온라인바카라 https://ce-top10.com/
온라인슬롯사이트 https://ce-top10.com/
카지노사이트게임 https://ce-top10.com/
카지노사이트검증 https://ce-top10.com/
카지노사이트추천 https://ce-top10.com/
안전카지노사이트 https://ce-top10.com/
안전카지노사이트도메인 https://ce-top10.com/
안전한 카지노사이트 추천 https://ce-top10.com/
바카라사이트게임 https://ce-top10.com/
바카라사이트검증 https://ce-top10.com/
바카라사이트추천 https://ce-top10.com/
안전바카라사이트 https://ce-top10.com/
안전바카라사이트도메인 https://ce-top10.com/
안전한 바카라사이트 추천 https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.sn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.sh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.rw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ru/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.pt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ps/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.pl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.nl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ms/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.mg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.me/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.md/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.li/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.la/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.kz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.kg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.jo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.je/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.it/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.iq/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ht/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.hn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.gp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.gm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.gl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.gg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ge/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.fr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.fm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.dz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.dj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.cz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.vn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ua/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.tw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.sv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.sg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.qa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.py/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.pk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ph/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.pe/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.pa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.om/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ni/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ng/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.na/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.mz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.mx/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.mt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ly/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.lb/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.kw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.kh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.jm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.gi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.gh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.fj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.et/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.cy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.cu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.co/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.bz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.br/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.bo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.bn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.bh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.bd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.au/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.ag/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.com.af/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.uk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.tz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.ma/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.ls/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.jp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.in/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.cm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ci/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ch/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.cd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.cat/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ca/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.by/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.bs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.bi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.bf/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.be/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ba/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.az/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.as/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.am/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.al/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://www.google.ad/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://plus.google.com/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.tn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.sn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.sh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.rw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ru/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.pt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.pl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.nl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ms/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.mg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.li/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.la/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.kz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.kg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.jo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.je/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.it/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.iq/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ht/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.hn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.gm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.gl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.gg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ge/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.fr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.fm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.dz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.dj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.cz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ua/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.tw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.sv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.sg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.qa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.py/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ph/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.pe/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.pa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.om/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ni/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ng/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.na/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.mz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.mx/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.mt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ly/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.lb/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.kw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.kh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.jm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.gi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.gh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.fj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.et/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.cu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.co/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.bz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.br/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.bo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.bn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.bh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.bd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.au/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.com.ag/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.uk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.tz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.ls/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.jp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.in/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.cm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ci/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ch/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.cd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.cat/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ca/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.by/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.bs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.bi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.bf/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.be/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ba/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.as/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://maps.google.ad/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.tn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.sn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.sh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.rw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ru/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.pt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ps/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.pl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.nl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ms/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.mg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.me/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.md/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.li/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.la/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.kz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.kg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.jo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.je/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.it/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.iq/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ht/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.hn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.gp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.gm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.gl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.gg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ge/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.fr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.fm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.dz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.dm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.dj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.vn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.vc/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ua/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.tw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.sv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.sg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.qa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.py/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.pk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ph/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.pe/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.pa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.om/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.np/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ni/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ng/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.na/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.mz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.mx/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.mt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ly/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.lb/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.kw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.kh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.jm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.gi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.gh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.fj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.et/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.cy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.cu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.co/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.bz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.br/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.bo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.bn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.bh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.bd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.au/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.ag/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.com.af/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.zm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.za/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.uz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.uk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.tz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.th/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ma/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ls/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.kr/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.jp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.in/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.id/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.cr/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.ck/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ci/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ch/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.cat/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ca/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.by/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.bs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.bi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.bf/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.be/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ba/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ba/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.az/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.as/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.am/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.al/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ae/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://images.google.ad/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.vg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.to/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.tn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.tm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.sn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.sm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.sh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.sc/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.rw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.pt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ps/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.mw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ms/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.mn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.md/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.kz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.kg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.jo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ht/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.gm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.gl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.gg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ge/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.fm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.es/url?sa=i&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.dm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.dj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.vn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ua/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.sg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.pk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ph/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.pa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.om/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.np/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ng/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.mt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ly/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.lb/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.kw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.jm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.hk/url?sa=i&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.gi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.fj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.et/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.cu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.co/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.bz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.bh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.bd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.au/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.com.ag/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.zm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.uz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.jp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.id/url?sa=i&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.ck/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.cm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ci/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ch/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ch/url?sa=i&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.cg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.cd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.by/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.bs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.bi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.be/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.be/url?sa=i&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ba/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.az/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.am/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://cse.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.vg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.to/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.tn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.tm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.sn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.sm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.sh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.sc/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.rw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.pt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ps/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.mw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ms/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.mn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.md/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.kz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.kg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.jo/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ie/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ht/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.gm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.gl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.gg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ge/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.fm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.es/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.dm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.dj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.vn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.vc/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ua/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.tr/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.sg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.qa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.py/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.pk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ph/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.pa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.np/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ng/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.mt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ly/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.lb/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.kw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.jm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.gi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.fj/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.et/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.co/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.co/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.bz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.bh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.bd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.com.ag/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.zm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.uz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.ck/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.cm/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.cl/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ci/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ch/url?q=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.cg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.cd/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.by/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.bs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.bi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.be/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ba/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.az/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.am/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
https://clients1.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.sk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.si/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.se/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ru/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.rs/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ro/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.pl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.no/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.nl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.lv/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.lu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.lt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.lk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.it/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.is/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ie/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.hu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.hr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.gr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.fr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.fi/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.es/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ee/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.dk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.de/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.cz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.vn/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.uy/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.tw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.tr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.sa/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.py/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.pr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.pk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.pe/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.my/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.hk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.gt/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.gh/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.eg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.ec/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.do/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.cu/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.br/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.au/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.com.ar/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.za/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.ve/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.uk/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.ug/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.th/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.nz/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.kr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.ke/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.jp/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.in/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.il/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.id/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.cr/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.co.bw/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.cl/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ch/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ca/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.bg/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.at/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
http://toolbarqueries.google.ae/url?sa=t&url=https://teenagegambling.blogspot.com/2022/09/do-you-understand-trade-off-between.html
WhiskeyPeak77@gmail.com 9/15/22
https://opviewer.com/
http://www.erotikplatz.at/redirect.php?id=939&mode=fuhrer&url=https://opviewer.com
http://www.imsnet.at/LangChange.aspx?uri=https://opviewer.com
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=https://opviewer.com
http://gs.matzendorf.at/includes/linkaufruf.asp?art=kapitel&link=https://opviewer.com
http://www.nuttenzone.at/jump.php?url=https://opviewer.com
https://cms.oeav-events.at/wGlobal/nessyEDVapps/layout/fancybox.php?link=https://opviewer.com
https://www.oebb.at/nightjet_newsletter/tc/xxxx?url=https://opviewer.com
https://www.gardensonline.com.au/Global/Players/YouTube.aspx?VideoURL=https://opviewer.com
http://www2.golflink.com.au/out.aspx?frm=gglcmicrosite&target=https://opviewer.com
http://www2.golflink.com.au/out.aspx?frm=logo&target=https://opviewer.com
https://www.golfselect.com.au/redirect?activityType_cd=WEB-LINK&course_id=2568&tgturl=https://opviewer.com
https://www.malcolmturnbull.com.au/?URL=https://opviewer.com
http://march-hare.com.au/library/default.asp?PP=/library/toc/lib-12.xml&tocPath=&URL=https://https://opviewer.com
https://www.oliverhume.com.au/enquiry/thank-you/?redirectTo=https://opviewer.com
http://www.parents-guide-illawarra.com.au/Redirect.aspx?destination=https://https://opviewer.com
https://ramset.com.au/Document/Url/?url=https://opviewer.com
https://ramset.com.au/document/url/?url=https://opviewer.com
http://rubyconnection.com.au/umbraco/newsletterstudio/tracking/trackclick.aspx?url=https://opviewer.com
http://southburnett.com.au/movies/movie.php?url=https://opviewer.com
https://www.vicsport.com.au/analytics/outbound?url=https://opviewer.com
https://www.vwwatercooled.com.au/forums/redirect-to/?redirect=https://https://opviewer.com
http://clients3.weblink.com.au/clients/aluminalimited/priceframe1.aspx?link=https://opviewer.com
https://maps.google.lt/url?sa=t&url=https://opviewer.com
https://ref.gamer.com.tw/redir.php?url=https://opviewer.com
https://images.google.com.sa/url?sa=t&url=https://opviewer.com
https://maps.google.com.sa/url?sa=t&url=https://opviewer.com
https://www.google.com.sa/url?sa=t&url=https://opviewer.com
https://images.google.hr/url?sa=t&url=https://opviewer.com
https://www.google.hr/url?sa=t&url=https://opviewer.com
https://maps.google.hr/url?sa=t&url=https://opviewer.com
https://images.google.com.pe/url?sa=t&url=https://opviewer.com
https://www.google.com.pe/url?sa=t&url=https://opviewer.com
https://maps.google.ae/url?sa=t&url=https://opviewer.com
https://images.google.ae/url?sa=t&url=https://opviewer.com
https://www.google.ae/url?sa=t&url=https://opviewer.com
https://www.google.co.ve/url?sa=t&url=https://opviewer.com
https://maps.google.co.ve/url?sa=t&url=https://opviewer.com
https://images.google.co.ve/url?sa=t&url=https://opviewer.com
http://onlinemanuals.txdot.gov/help/urlstatusgo.html?url=https://opviewer.com
https://www.google.com.pk/url?sa=t&url=https://opviewer.com
https://images.google.com.pk/url?sa=t&url=https://opviewer.com
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=https://opviewer.com
https://www.google.com.eg/url?sa=t&url=https://opviewer.com
https://maps.google.com.eg/url?sa=t&url=https://opviewer.com
https://images.google.com.eg/url?sa=t&url=https://opviewer.com
https://www.google.si/url?sa=t&url=https://opviewer.com
https://maps.google.si/url?sa=t&url=https://opviewer.com
https://images.google.si/url?sa=t&url=https://opviewer.com
http://www.pickyourown.org/articles.php?NAME=Visit+Us&URL=https://opviewer.com
https://maps.google.lv/url?sa=t&url=https://opviewer.com
https://www.google.lv/url?sa=t&url=https://opviewer.com
https://images.google.lv/url?sa=t&url=https://opviewer.com
https://community.cypress.com/t5/custom/page/page-id/ExternalRedirect?url=https://opviewer.com
https://www.google.ee/url?sa=t&url=https://opviewer.com
https://cms.oeav-events.at/wGlobal/nessyEDVapps/layout/fancybox.php?link=https://opviewer.com
https://www.oebb.at/nightjet_newsletter/tc/xxxx?url=https://opviewer.com
https://www.gardensonline.com.au/Global/Players/YouTube.aspx?VideoURL=https://opviewer.com
http://www2.golflink.com.au/out.aspx?frm=gglcmicrosite&target=https://opviewer.com
http://www2.golflink.com.au/out.aspx?frm=logo&target=https://opviewer.com
https://www.golfselect.com.au/redirect?activityType_cd=WEB-LINK&courseid=2568&tgturl=https://opviewer.com
https://www.malcolmturnbull.com.au/?URL=https://opviewer.com
http://march-hare.com.au/library/default.asp?PP=/library/toc/lib-12.xml&tocPath=&URL=https://https://opviewer.com
https://www.oliverhume.com.au/enquiry/thank-you/?redirectTo=https://opviewer.com
http://www.parents-guide-illawarra.com.au/Redirect.aspx?destination=https://https://opviewer.com
https://ramset.com.au/Document/Url/?url=https://opviewer.com
https://ramset.com.au/document/url/?url=https://opviewer.com
http://rubyconnection.com.au/umbraco/newsletterstudio/tracking/trackclick.aspx?url=https://opviewer.com
http://southburnett.com.au/movies/movie.php?url=https://opviewer.com
https://www.vicsport.com.au/analytics/outbound?url=https://opviewer.com
https://www.vwwatercooled.com.au/forums/redirect-to/?redirect=https://https://opviewer.com
http://clients3.weblink.com.au/clients/aluminalimited/priceframe1.aspx?link=https://opviewer.com
https://clients1.google.ad/url?q=https://opviewer.com
https://cse.google.ad/url?q=https://opviewer.com
https://images.google.ad/url?q=https://opviewer.com
https://maps.google.ad/url?q=https://opviewer.com
https://www.google.ad/url?q=https://opviewer.com
https://emaratyah.ae/new-redirect.php?w=https://opviewer.com
http://mbrf.ae/knowledgeaward/language/ar/?redirecturl=https://opviewer.com
http://rafco.ae/container.asp?url=https://opviewer.com
http://for-css.ucoz.ae/go?https://opviewer.com
https://clients1.google.com.af/url?q=https://opviewer.com
https://cse.google.com.af/url?q=https://opviewer.com
https://images.google.com.af/url?q=https://opviewer.com
http://toolbarqueries.google.com.af/url?sa=t&url=https://opviewer.com
https://www.google.com.af/url?q=https://opviewer.com
https://www.snek.ai/redirect?url=https://opviewer.com
http://www.torrent.ai/lt/redirect.php?url=https://opviewer.com
http://avto.al/az/home/redirect?carId=1639612&url=https://opviewer.com
https://clients1.google.al/url?q=https://opviewer.com
https://cse.google.al/url?q=https://opviewer.com
https://images.google.al/url?q=https://opviewer.com
https://images.google.al/url?q=https://opviewer.com
http://toolbarqueries.google.al/url?q=https://opviewer.com
https://www.google.al/url?q=https://opviewer.com
http://tido.al/vazhdo.php?url=https://opviewer.com
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=https://opviewer.com
https://oxleys.app/friends.php?q=https://opviewer.com
http://www.ain.com.ar/openpop.php?url=https://opviewer.com
http://www.ain.com.ar/openpop.php?url=https://opviewer.com
https://www.google.nl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ms/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.mg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.me/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.md/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.lv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.lu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.lt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.lk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.li/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.la/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.kz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.kg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.jo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.je/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.it/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.is/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.iq/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ie/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.hu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ht/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.hr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.hn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.gr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.gp/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.gm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.gl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.gg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ge/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.fr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.fm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.fi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.es/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ee/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.dz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.dk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.dj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.de/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.cz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.vn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.uy/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ua/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.tw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.tr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.sv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.sg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.sa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.qa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.py/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.pr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.pk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ph/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.pe/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.pa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.om/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ni/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ng/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.na/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.mz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.my/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.mx/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.mt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ly/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.lb/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.kw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.kh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.jm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.hk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.gt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.gi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.gh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.fj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.et/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.eg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ec/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.do/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.cy/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.cu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.co/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.bz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.br/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.bo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.bn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.bh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.bd/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.au/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ar/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.ag/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.com.af/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.za/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.ve/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.uk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.ug/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.tz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.th/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.nz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.ma/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.ls/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.kr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.ke/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.jp/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.in/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.il/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.id/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.cr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.co.bw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.cm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.cl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ci/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ch/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.cd/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.cat/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ca/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.by/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.bs/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.bi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.bg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.bf/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.be/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ba/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.az/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.at/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.as/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.am/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.al/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ae/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://www.google.ad/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://plus.google.com/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.tn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.sn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.sk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.si/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.sh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.se/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.rw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ru/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.rs/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ro/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.pt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.pl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.no/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.nl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ms/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.mg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.lv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.lu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.lt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.lk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.li/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.la/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.kz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.kg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.jo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.je/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.it/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.is/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.iq/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ie/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.hu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ht/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.hr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.hn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.gr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.gm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.gl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.gg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ge/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.fr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.fm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.fi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.es/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ee/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.dz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.dk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.dj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.de/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.cz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.uy/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ua/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.tw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.tr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.sv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.sg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.sa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.qa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.py/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.pr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ph/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.pe/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.pa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.om/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ni/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ng/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.na/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.mz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.my/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.mx/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.mt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ly/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.lb/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.kw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.kh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.jm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.hk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.gt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.gi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.gh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.fj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.et/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.eg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ec/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.do/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.cu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.co/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.bz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.br/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.bo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.bn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.bh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.bd/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.au/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ar/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.com.ag/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.za/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.ve/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.uk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.ug/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.tz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.th/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.nz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.ls/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.kr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.ke/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.jp/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.in/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.il/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.id/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.cr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.co.bw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.cm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.cl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ci/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ch/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.cd/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.cat/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ca/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.by/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.bs/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.bi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.bg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.bf/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.be/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ba/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.at/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.as/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ae/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://maps.google.ad/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.tn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.sn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.sk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.si/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.sh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.se/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.rw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ru/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.rs/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ro/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.pt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ps/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.pl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.no/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.nl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ms/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.mg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.me/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.md/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.lv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.lu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.lt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.lk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.li/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.la/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.kz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.kg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.jo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.je/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.it/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.is/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.iq/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ie/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.hu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ht/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.hr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.hn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.gr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.gp/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.gm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.gl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.gg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ge/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.fr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.fm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.fi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.es/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ee/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.dz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.dm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.dk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.dj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.de/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.cz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.vn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.vc/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.uy/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ua/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.tw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.tr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.sv/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.sg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.sa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.qa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.py/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.pr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.pk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ph/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.pe/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.pa/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.om/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.np/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ni/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ng/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.na/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.mz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.my/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.mx/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.mt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ly/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.lb/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.kw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.kh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.jm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.hk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.gt/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.gi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.gh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.fj/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.et/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.eg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ec/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.do/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.cy/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.cu/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.co/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.bz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.br/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.bo/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.bn/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.bh/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.bd/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.au/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ar/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.ag/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.com.af/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.zm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.za/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.za/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ve/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.uz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.uk/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ug/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.tz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.th/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.th/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.nz/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ma/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ls/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.kr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.kr/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ke/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.jp/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.in/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.il/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.id/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.id/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.cr/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.cr/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.ck/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.co.bw/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.cm/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.cl/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ci/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ch/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.cg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.cd/url?sa?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ca/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.by/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.bs/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.bi/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.bg/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.bf/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.be/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ba/url?sa=t&url=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
https://images.google.ba/url?q=https://xenomini1.wixsite.com/calmingtouchmassage/post/loosening-up-head-and-scalp-massage-in-sydney
otizjess6@gmail.com 09/03/22
https://bit.ly/36En2s8+
카지노사이트 https://ce-top10.com/
바카라사이트 https://ce-top10.com/
온라인카지노 https://ce-top10.com/
온라인바카라 https://ce-top10.com/
온라인슬롯사이트 https://ce-top10.com/
카지노사이트게임 https://ce-top10.com/
카지노사이트검증 https://ce-top10.com/
카지노사이트추천 https://ce-top10.com/
안전카지노사이트 https://ce-top10.com/
안전카지노사이트도메인 https://ce-top10.com/
안전한 카지노사이트 추천 https://ce-top10.com/
바카라사이트게임 https://ce-top10.com/
바카라사이트검증 https://ce-top10.com/
바카라사이트추천 https://ce-top10.com/
안전바카라사이트 https://ce-top10.com/
안전바카라사이트도메인 https://ce-top10.com/
안전한 바카라사이트 추천 https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://ce-top10.com/
https://www.google.tn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.sn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.sh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.rw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ru/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.pt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ps/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.pl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.nl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ms/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.mg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.me/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.md/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.li/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.la/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.kz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.kg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.jo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.je/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.it/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.iq/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ht/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.hn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.gp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.gm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.gl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.gg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ge/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.fr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.fm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.dz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.dj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.cz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.vn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ua/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.tw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.sv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.sg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.qa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.py/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.pk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ph/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.pe/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.pa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.om/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ni/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ng/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.na/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.mz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.mx/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.mt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ly/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.lb/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.kw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.kh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.jm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.gi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.gh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.fj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.et/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.cy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.cu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.co/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.bz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.br/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.bo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.bn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.bh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.bd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.au/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.ag/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.com.af/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.uk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.tz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.ma/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.ls/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.jp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.in/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.cm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ci/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ch/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.cd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.cat/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ca/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.by/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.bs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.bi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.bf/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.be/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ba/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.az/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.as/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.am/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.al/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://www.google.ad/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://plus.google.com/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.tn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.sn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.sh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.rw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ru/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.pt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.pl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.nl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ms/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.mg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.li/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.la/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.kz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.kg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.jo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.je/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.it/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.iq/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ht/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.hn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.gm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.gl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.gg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ge/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.fr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.fm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.dz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.dj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.cz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ua/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.tw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.sv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.sg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.qa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.py/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ph/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.pe/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.pa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.om/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ni/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ng/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.na/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.mz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.mx/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.mt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ly/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.lb/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.kw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.kh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.jm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.gi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.gh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.fj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.et/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.cu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.co/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.bz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.br/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.bo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.bn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.bh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.bd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.au/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.com.ag/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.uk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.tz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.ls/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.jp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.in/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.cm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ci/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ch/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.cd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.cat/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ca/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.by/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.bs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.bi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.bf/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.be/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ba/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.as/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://maps.google.ad/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.tn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.sn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.sh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.rw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ru/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.pt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ps/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.pl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.nl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ms/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.mg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.me/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.md/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.li/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.la/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.kz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.kg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.jo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.je/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.it/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.iq/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ht/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.hn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.gp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.gm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.gl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.gg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ge/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.fr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.fm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.dz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.dm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.dj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.vn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.vc/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ua/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.tw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.sv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.sg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.qa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.py/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.pk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ph/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.pe/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.pa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.om/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.np/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ni/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ng/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.na/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.mz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.mx/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.mt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ly/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.lb/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.kw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.kh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.jm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.gi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.gh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.fj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.et/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.cy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.cu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.co/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.bz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.br/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.bo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.bn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.bh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.bd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.au/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.ag/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.com.af/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.zm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.za/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.uz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.uk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.tz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.th/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ma/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ls/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.kr/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.jp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.in/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.id/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.cr/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.ck/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ci/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ch/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.cat/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ca/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.by/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.bs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.bi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.bf/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.be/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ba/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ba/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.az/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.as/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.am/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.al/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ae/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://images.google.ad/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.vg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.to/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.tn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.tm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.sn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.sm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.sh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.sc/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.rw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.pt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ps/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.mw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ms/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.mn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.md/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.kz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.kg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.jo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ht/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.gm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.gl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.gg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ge/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.fm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.es/url?sa=i&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.dm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.dj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.vn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ua/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.sg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.pk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ph/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.pa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.om/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.np/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ng/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.mt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ly/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.lb/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.kw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.jm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.hk/url?sa=i&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.gi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.fj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.et/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.cu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.co/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.bz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.bh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.bd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.au/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.com.ag/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.zm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.uz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.jp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.id/url?sa=i&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.ck/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.cm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ci/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ch/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ch/url?sa=i&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.cg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.cd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.by/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.bs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.bi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.be/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.be/url?sa=i&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ba/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.az/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.am/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://cse.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.vg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.to/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.tn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.tm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.sn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.sm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.sh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.sc/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.rw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.pt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ps/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.mw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ms/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.mn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.md/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.kz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.kg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.jo/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ie/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ht/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.gm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.gl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.gg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ge/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.fm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.es/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.dm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.dj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.vn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.vc/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ua/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.tr/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.sg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.qa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.py/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.pk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ph/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.pa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.np/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ng/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.mt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ly/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.lb/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.kw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.jm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.gi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.fj/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.et/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.co/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.co/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.bz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.bh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.bd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.com.ag/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.zm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.uz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.ck/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.cm/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.cl/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ci/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ch/url?q=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.cg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.cd/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.by/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.bs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.bi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.be/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ba/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.az/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.am/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
https://clients1.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.sk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.si/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.se/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ru/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.rs/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ro/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.pl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.no/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.nl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.lv/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.lu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.lt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.lk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.it/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.is/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ie/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.hu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.hr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.gr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.fr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.fi/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.es/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ee/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.dk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.de/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.cz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.vn/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.uy/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.tw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.tr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.sa/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.py/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.pr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.pk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.pe/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.my/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.hk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.gt/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.gh/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.eg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.ec/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.do/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.cu/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.br/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.au/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.com.ar/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.za/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.ve/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.uk/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.ug/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.th/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.nz/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.kr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.ke/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.jp/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.in/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.il/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.id/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.cr/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.co.bw/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.cl/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ch/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ca/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.bg/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.at/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines
http://toolbarqueries.google.ae/url?sa=t&url=https://terry36sao.wixsite.com/expertgambling/post/6-tips-for-winning-at-online-slot-machines