ISOPROPANOL: The all-around disinfectant
Without a doubt, Isopropanol (or chemically known as isopropyl alcohol) is one of the most well-known chemicals that has been a part of our lives. Whenever we get some minor cuts or wounds, it’s never a strange thing to ask for an isopropyl alcohol. But how this small molecule does its job is simply amazing. Generally, the antibacterial properties of alcohol increase with increased length of alkyl chain increases (maximum number of carbons is 6) and decreases with increased branching. With its relatively favorable length and small branch, isopropyl alcohol can reach the inner portions of bacterial cell, and effectively denatures proteins of the cell membranes. In fact, this molecule exhibits more activity against diarrhea causing agents such as E. coli and S. aureus. Hence, it’s always a good practice to disinfect your hands with hand soap or isopropyl alcohol before eating.
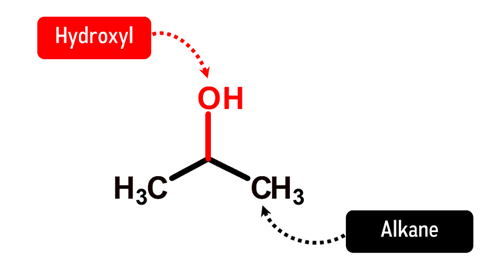
How does Isopropanol look like in Chemistry?
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Isopropanol! You’ll need:
- 3 Carbon atoms
- 1 Oxygen atoms
- 8 Hydrogen atoms
- 8 Small connectors (compact small bonds for hydrogen)
- 3 Medium Connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start off With Carbon 1!
Steps:
-
1

1. Get one carbon atom(Carbon 1)add 3 hydrogen atoms to it using 3 small connectors.
-
2

2. Then, get another carbon atom (Carbon 2)and attach this to Carbon 1 using 1 medium connector. Place a hydrogen atom on Carbon 2 using a small connector.
-
3
3.Grab an Oxygen atom then attach this to Carbon 2 using a medium connector.
-
4

4. Get one carbon atom (Carbon 3) then attach this to Carbon 2 using 1 medium connector. Add 3 hydrogen atoms to Carbon 3 using 3 small connectors.











VipyDeruMlBH
LUfYzCwA
XYZWuGfvxzEnp
aepEmuQW
TLcaseUnSfPkAZIw