GERANIOL: Drives Pests Away
Geraniol is a colorless liquid with a combined sweet and citrus odor naturally found in rose extracts. This main ingredient of rose oil makes it capable to protect itself from insects. Geraniol undergoes an extensive process of extraction and refinement, but only a small amount is employed in insect repellant formulations.
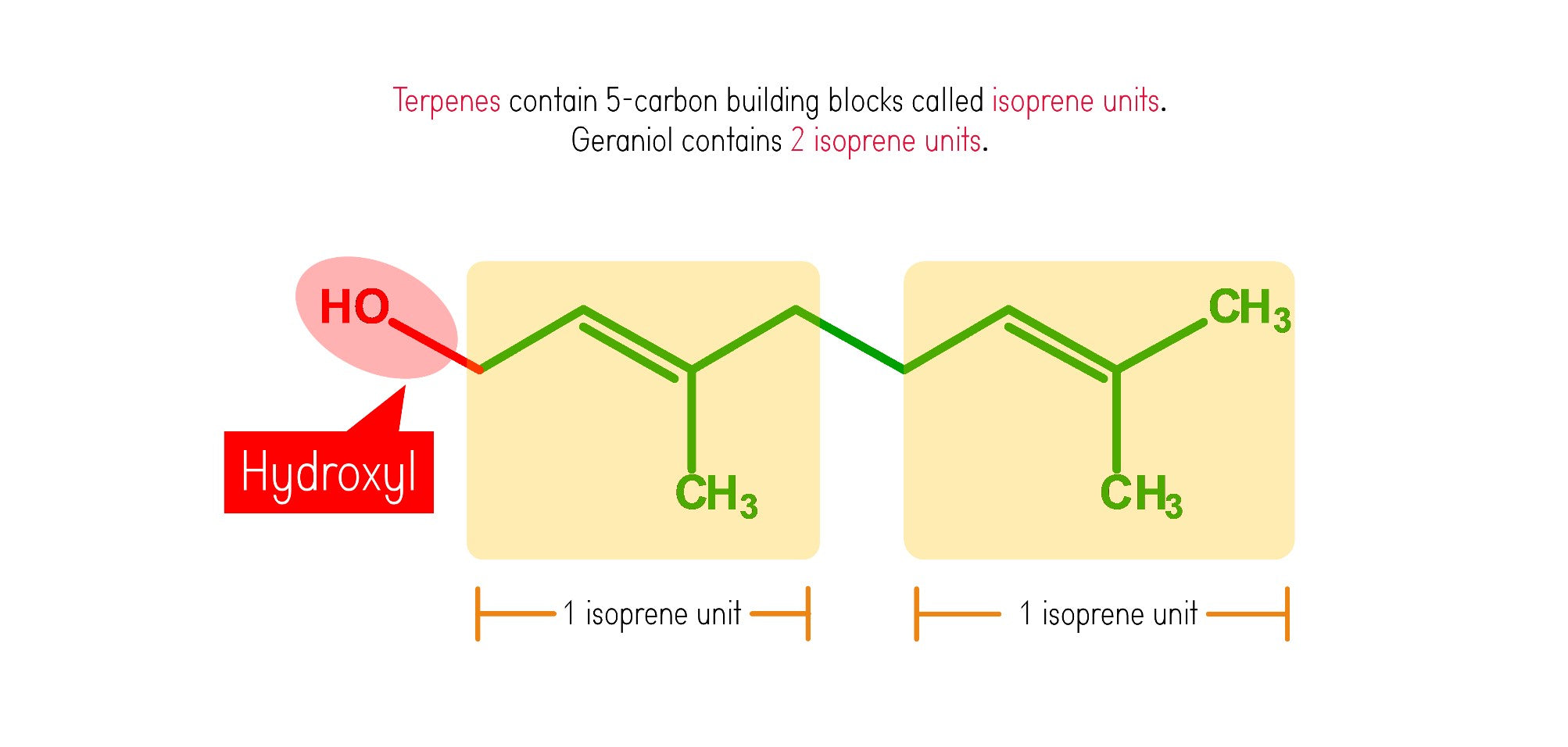
Geraniol belongs to a chemical subclass called terpineols. Terpineols are terpenes that contains a hydroxyl group, and is therefore an alcohol. Most plant oils contain terpenes, which is made up of five-carbon building blocks called isoprenes.
Terpenes are the compounds responsible for the unique scent of most plants. Geraniol is also abundant in some plants such as lemongrass.
How does Geraniol look like in Chemistry?
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Geraniol! You’ll need:
- 10 Carbon atoms
- 1 Oxygen atoms
- 18 Hydrogen atoms
- 18 Small connectors (compact small bonds for hydrogen)
- 8 Medium Connectors
- 4 Long connectors
- Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
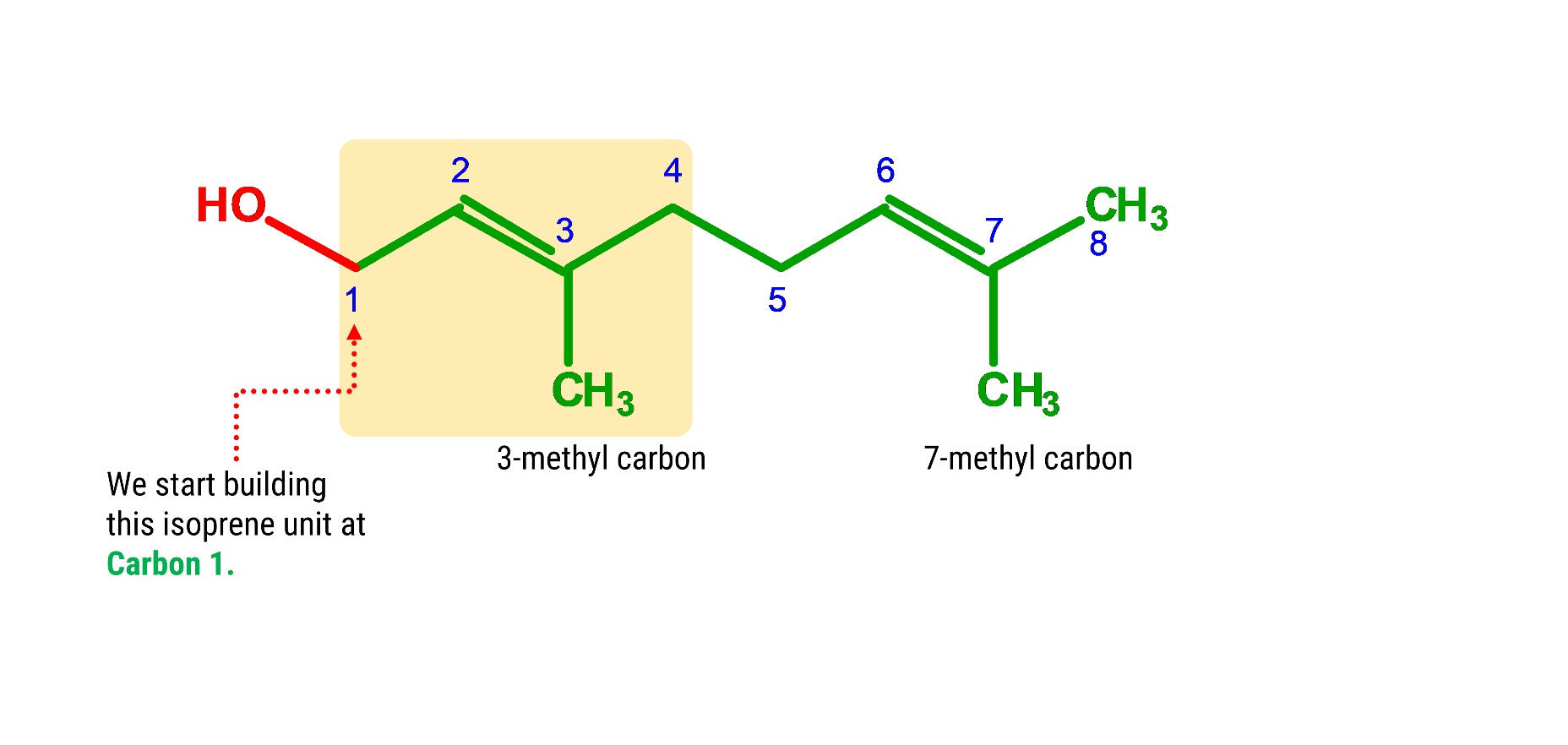
Let’s build our first isoprene unit starting off with Carbon 1!
Steps:
-
1

1. Get one carbon atom (Carbon 1)then, attach 2 hydrogen atom to it using 2 small connectors.
-
2

2. Attachan Oxygen atomto Carbon 1 using a medium connectors.Then, place a hydrogen atom to it using a small connector.
-
3

3. Grab another carbon atom (Carbon 2) and attach this to Carbon 1 using 1 medium connector. Add 2 hydrogen atoms on Carbon 2 using 2 small connectors.
-
4

4. Attach one carbon atom Carbon 2 using 2 long connectors.
-
5

5. Get another carbon atom (3-methyl carbon) and attach this to Carbon 3 using a medium connector. Add 3 hydrogens to 3-methyl carbon using 3 short connectors.
-
6

6. Then, get another carbon (Carbon 4) and attach this to Carbon 3 using a medium connector. Place 2 hydrogen atoms on this carbon using 2 small connectors.
-
7

7. Yay! We’re now done with the first isoprene unit.
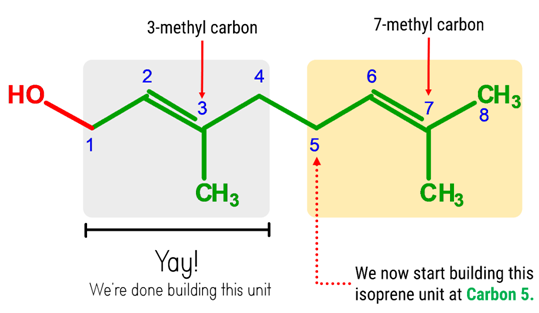
Let’s now build the second isoprene unit, starting at Carbon 5.
Steps:
-
1

1. Get one carbon atom (Carbon 5)then, attach 2 hydrogen atom to it using 2 small connectors.
-
2

2. Grab another carbon atom (Carbon 6) and attach this to Carbon 5 using 1 medium connector.Add a 1 hydrogen atom on Carbon 2 using 1 small connector.
-
3

3. Attach one carbon atom (Carbon 7) to Carbon 6 using 2 long connectors.
-
4

4. Get another carbon atom (7-methyl carbon) and attach this to Carbon 7 using 2 medium connectors.Add 3 hydrogens to 7-methyl carbon using 3 short connectors
-
5

5. Then, get the last carbon atom(Carbon 8) and attach this toCarbon 7 using a medium connector. Place 3 hydrogen atoms on this carbon using 3 small connectors.
-
6

6. Using a medium connector, join (Carbon 4) 4 of the first isoprene unit to Carbon 5 of the second isoprene unit.



















https://github.com/Wimonporn22/Phupakdee22/issues/1
https://github.com/Kaewmala00/Weerasak00/issues/1
https://github.com/Thammarat00/Kommak00/issues/1
https://github.com/Jongjanya00/Nannapin00/issues/1
https://github.com/Jantrapa00/Pitiwat00/issues/1
https://github.com/Wongwarang00/Bongkoch00/issues/1
https://github.com/Anupongprasit00/Chokchai00/issues/1
https://github.com/Jirapat00/Panupon00/issues/1
https://github.com/Chaiyasak00/Siladee00/issues/1
https://github.com/Petcharat00/Chayon00/issues/1
https://github.com/kaina12345678999/kaina123/issues/2
https://github.com/Bunyaporn22/Nannapin22/issues/1
https://github.com/Neeraya22/Chokchai22/issues/1
https://github.com/Sitapat22/Chidchon22/issues/1
https://github.com/Prisana22/Rassameechot22/issues/1
https://github.com/Sunisorn22/Marika22/issues/1
https://cccv.to/eahs9yrtp7c2msn
https://cccv.to/zt4uee9n1oh
https://cccv.to/s96pp
https://cccv.to/9akehs2bzjpn8vs
https://cccv.to/9wkea1qvfj4r
https://cccv.to/2z7ncimc
https://cccv.to/ekqhbawy12wkj
https://cccv.to/ocixvvjzj2b76i
https://cccv.to/saq3p9s3jguky
https://cccv.to/pzr5ovy2e28w3
https://cccv.to/7awe9zidp1
https://cccv.to/ytz4du5saq
https://cccv.to/jqxagk7po
https://cccv.to/j4sx23ru5c4
https://cccv.to/cpvyst65
https://cccv.to/9td1zmtbkc
https://cccv.to/us6unvdy7
https://cccv.to/7jy97a8yd6rdadyj
https://cccv.to/x8esschtr
https://cccv.to/3mv9vcgny
https://cccv.to/vdo98z9
https://cccv.to/wi8w9wwc5
https://cccv.to/g1kn1d52
https://cccv.to/faudfqtub133pse
https://cccv.to/51cy22c
https://cccv.to/advpidfpyw
https://cccv.to/ph91edxw6h18g
https://cccv.to/g38zgpm595jaku
https://cccv.to/7sk4m7bhx
https://cccv.to/gqjvt5t17ci44ce
https://cccv.to/58agxb
https://github.com/Suwankaruna23/Kiatsakul23/issues/1
https://github.com/Chayud56/Pattanadech23/issues/1
https://github.com/Tangtip339/Reongrit23/issues/1
https://github.com/Prangsub23/Suchart23/issues/1
https://cccv.to/s57vr1xid
https://cccv.to/2cvecr
https://cccv.to/3s48ja3z
https://cccv.to/24c8iop
https://www.bitsdujour.com/profiles/fX7Y6D
https://www.bitsdujour.com/profiles/tQtja8
https://github.com/dmpe/R/issues/16
https://github.com/adamdruppe/arsd/issues/482
https://github.com/Vithun235/Senasakul235/issues/1
https://github.com/Santipipak235/Nathayu235/issues/1
https://github.com/Sarayut235/Wannasiri235/issues/1
https://github.com/udlbook/udlbook/issues/275
https://github.com/selfteaching/the-craft-of-selfteaching/issues/1204
https://github.com/LeeJunHyun/Image_Segmentation/issues/99
https://cccv.to/muwkrw
https://cccv.to/pvyh3m5cqfgw8os
https://cccv.to/idzcy98zqkgb7f
https://cccv.to/7guc2znqtc
https://cccv.to/9euxx
https://cccv.to/bywg2769mxebdgch
https://cccv.to/gjhupwmy4q
https://cccv.to/p1xn1
https://cccv.to/ht7jejo
https://www.bitsdujour.com/profiles/ffPEOy
https://telegra.ph/JOP-DIAMOND168-03-06-2
https://telegra.ph/JOP-DIAMOND168-03-06
https://www.bitsdujour.com/profiles/IRIIGX
https://telegra.ph/DIAMOND168-JOP-03-05
https://telegra.ph/SLOT-PG-JOP-DIAMOND168-03-05
https://cccv.to/zhbjyr95cqkpdh
https://cccv.to/7mkryare9i
https://cccv.to/n6xhq3v7sj
https://cccv.to/m9etzq4eywgiwi
https://cccv.to/os12cmn1xi
https://github.com/voidint/g/issues/153
https://github.com/mit-pdos/xv6-riscv/issues/326
https://github.com/mit-pdos/xv6-riscv/issues/327
https://github.com/xiesuichao/KLineView/issues/36
https://github.com/nicklockwood/iCarousel/issues/912
https://www.bitsdujour.com/profiles/S2UpOu
https://telegra.ph/JOP-DIAMOND168-03-08
https://cccv.to/npm7whxmvemf
https://cccv.to/1sqsr
https://cccv.to/o2if6wu1evk13jh
https://cccv.to/qk48vvkaq8cyh5
https://cccv.to/5s637pcr72d
https://cccv.to/yjcrjjp5bx
https://cccv.to/c9equ1
https://cccv.to/zsf62
https://cccv.to/jjq1fi41
https://github.com/supercrabtree/k/issues/126
https://github.com/AllenDowney/ThinkDSP/issues/118
https://github.com/MiRO92/uYou-for-YouTube/issues/514
https://github.com/jarun/nnn/issues/2009
https://github.com/mit-pdos/xv6-riscv/issues/328
https://github.com/adamdruppe/arsd/issues/485
https://github.com/zquestz/s/issues/189
https://github.com/nicklockwood/iCarousel/issues/914
https://github.com/p-org/P/issues/826
https://github.com/DAMDAMme11225566/Natoo/issues/1
https://github.com/MAVKMAVK11111111111142/Mack1259/issues/1
https://github.com/sggsdfsdfdsfds6745123/NAgoo168/issues/1
https://github.com/DIAMOND168N/Mavk/issues/1
https://github.com/11DIAMOND168/Mac/issues/1
https://github.com/22DIAMOND168/Mack/issues/1
https://github.com/DIAMOND168MM/Mac/issues/1
https://github.com/4DIAMOND168/Phan/issues/1
https://github.com/5DIAMOND168/May/issues/1
https://github.com/3DIAMOND168/Muay/issues/1
https://github.com/6DIAMOND168/Mack/issues/1
https://github.com/2DIAMOND168/Mack/issues/1
https://github.com/1DIAMOND168/Mack/issues/1
https://github.com/12DIAMOND168/Mack/issues/1
https://github.com/16DIAMOND168/Mack/issues/1
https://github.com/8DIAMOND168/Mack/issues/1
https://github.com/7DIAMOND168/Mack1/issues/1
https://cccv.to/ccni8bzzwvedmm
https://cccv.to/tmx63jm4dzhgt5
https://cccv.to/97n8u9q
https://cccv.to/uxdfjj8cdj3px5
https://cccv.to/eb9mg
https://cccv.to/dm1tayz19kwk2j
https://cccv.to/z7p9xcwnbgnmjqf
https://cccv.to/fpexade65i1tysp
https://cccv.to/kiyivv8
https://cccv.to/rc51d39bd
https://cccv.to/24ntippanfgz4
https://cccv.to/tuemv
https://cccv.to/t4p67et99n73nxw
https://cccv.to/679njdk477os9
https://cccv.to/xyripq8
https://cccv.to/dzzyfr5
https://cccv.to/st5qozh5fwkt2w
https://cccv.to/i6gq3unvqcf
https://github.com/Wattanasaksakul1/Kanjanee-/issues/1
https://github.com/Reongsamai154/Thammapak-/issues/1
https://github.com/Tanasombat/Kiatsakul-/issues/1
https://github.com/Wiwatthanasak/Ronnaporn-/issues/1
https://github.com/Supannapakin/Chompunut-/issues/1
https://github.com/antaraprasert/Patanapreecha-/issues/1
https://github.com/Patamadecha/Nichakarn-/issues/1
https://github.com/1Chalermkwansiri/Prapol-/issues/1
https://github.com/Jeerawongsa/Kiatwittaya-/issues/1
https://github.com/Bannawan/Weerachai-/issues/1
https://cccv.to/317dk8zdt19mg8yh
https://cccv.to/zch5uw3fm
https://cccv.to/m12rbakx48
https://cccv.to/jx3f12o4orbot
https://cccv.to/37xuk6
https://cccv.to/7b1tp738jvhz7u
https://cccv.to/92j92wjxuusfjw
https://cccv.to/fzcor7wtxqj1u
https://cccv.to/uezudxbstufpt
https://cccv.to/bp1mofi9n
https://cccv.to/h93tzqjgg3h7
https://cccv.to/hv4dzxdrpc5eyj
https://cccv.to/nm8wc24zxbeot
https://cccv.to/ygzu87esb9yr3y
https://cccv.to/n6hz9g4p48
https://cccv.to/wkc37k1grf4fvq
https://cccv.to/82vp429vvtkvi7ir
https://github.com/ethereumbook/ethereumbook/issues/1231
https://github.com/adamdruppe/arsd/issues/481
https://github.com/adamdruppe/arsd/issues/480
https://github.com/zserge/o/issues/8
https://github.com/adamdruppe/arsd/issues/484
https://github.com/Usani2541/ASS4_SEC1_603021736-0/issues/1
https://github.com/adamdruppe/arsd/issues/483
https://cccv.to/edv86
https://cccv.to/n89jm76fpg1vjms
https://cccv.to/kwwy5k
https://cccv.to/zrcpgsz7m
https://cccv.to/c5i15ins6
https://cccv.to/1e11gzuq4j6z
https://github.com/kaina134556666666666/kaina123/issues/3
https://github.com/kaina12345678999/kaina123/issues/3
https://github.com/fgjfdjnfdfh32656/Sujinda65652/issues/3
https://github.com/johnkim7268202s/Kochaporn56263/issues/3
https://github.com/Neeraya/DIAMOND/issues/2
https://github.com/MACMACMAC112255/DiAMOND/issues/1
https://github.com/Jansawangwong/DIAMOND/issues/1
https://github.com/Neeraya/DIAMOND/issues/1
https://github.com/MAVKMAVK11111111111142/Mack1259/issues/1
https://github.com/sggsdfsdfdsfds6745123/NAgoo168/issues/1
http://www.cityofrosedalems.com/
http://cityofrosedalems.com/
https://cityofrosedalems.com/
http://www.cityofrosedalems.com/
안전해외배팅사이트 https://cityofrosedalems.com/
해외 스포츠 배팅 사이트 https://cityofrosedalems.com/
해외배팅사이트 먹튀검증 https://cityofrosedalems.com/
안전배팅사이트 https://cityofrosedalems.com/
안전 온라인 카지노 https://cityofrosedalems.com/
해외 온라인 카지노 https://cityofrosedalems.com/
유럽 온라인 카지노 https://cityofrosedalems.com/
안전 온라인 바카라 https://cityofrosedalems.com/
해외 스포츠 배팅 안전주소 https://cityofrosedalems.com/
해외 스포츠 배팅 먹튀검증 https://cityofrosedalems.com/
안전 해외 카지노사이트 https://cityofrosedalems.com/
안전 해외 바카라사이트 https://cityofrosedalems.com/
헤라카지노 https://cityofrosedalems.com/헤라카지노/
아시안커넥트 https://ac-tm66.net/
에볼루션 카지노 https://cityofrosedalems.com/에볼루션카지노/
247벳코리아 https://cityofrosedalems.com/247벳코리아/
쿨카지노 https://cityofrosedalems.com/쿨카지노/
뉴헤븐카지노 https://cityofrosedalems.com/뉴헤븐카지노/
파라오카지노 https://cityofrosedalems.com/파라오카지노/
맥스벳 https://ac-tm66.net/
비티아이 스포츠 https://ac-tm66.net/
스보벳 https://ac-tm66.net/
1xbet https://cityofrosedalems.com/247벳코리아/
피나클 https://ac-tm66.net/
https://gamma.app/public/247-652nia2b0wmcbkc
https://gamma.app/public/247-6jcr5hfz3zo7hto
https://gamma.app/public/-n3szrlsehdr6wt3
https://sebsauvage.net/paste/?8c54344c01775406#XeaJJXo/6gTGsWMOAgoezJjCtAo7R/wLiaMhWSvxmu0=
https://sites.google.com/view/newhc/%ED%99%88
https://sites.google.com/view/newhc/
https://sites.google.com/view/newhc/%20
http://sites.google.com/view/newhc
https://sites.google.com/view/newhc/
https://sites.google.com/view/newhc/%2F
http://sites.google.com/view/newhc/%20
http://sites.google.com/view/newhc/
http://www.sites.google.com/view/newhc/
https://www.sites.google.com/view/newhc/
http://www.sites.google.com/view/newhc/%20
https://www.sites.google.com/view/newhc/%20
http://www.sites.google.com/view/newhc/%2F
https://www.sites.google.com/view/newhc/%2F
https://sites.google.com/view/247betkor/%ED%99%88
https://sites.google.com/view/247betkor/
https://sites.google.com/view/247betkor/%20
http://sites.google.com/view/247betkor
https://sites.google.com/view/247betkor/
https://sites.google.com/view/247betkor/%2F
http://sites.google.com/view/247betkor/%20
http://sites.google.com/view/247betkor/
http://www.sites.google.com/view/247betkor/
https://www.sites.google.com/view/247betkor/
http://www.sites.google.com/view/247betkor/%20
https://www.sites.google.com/view/247betkor/%20
http://www.sites.google.com/view/247betkor/%2F
https://www.sites.google.com/view/247betkor/%2F
https://sites.google.com/view/coolcasin0/%ED%99%88
https://sites.google.com/view/coolcasin0/
https://sites.google.com/view/coolcasin0/%20
http://sites.google.com/view/coolcasin0
https://sites.google.com/view/coolcasin0/
https://sites.google.com/view/coolcasin0/%2F
http://sites.google.com/view/coolcasin0/%20
http://sites.google.com/view/coolcasin0/
http://www.sites.google.com/view/coolcasin0/
https://www.sites.google.com/view/coolcasin0/
http://www.sites.google.com/view/coolcasin0/%20
https://www.sites.google.com/view/coolcasin0/%20
http://www.sites.google.com/view/coolcasin0/%2F
https://www.sites.google.com/view/coolcasin0/%2F
https://sites.google.com/view/herac/%ED%99%88
https://sites.google.com/view/herac/
https://sites.google.com/view/herac/%20
http://sites.google.com/view/herac
https://sites.google.com/view/herac/
https://sites.google.com/view/herac/%2F
http://sites.google.com/view/herac/%20
http://sites.google.com/view/herac/
http://www.sites.google.com/view/herac/
https://www.sites.google.com/view/herac/
http://www.sites.google.com/view/herac/%20
https://www.sites.google.com/view/herac/%20
http://www.sites.google.com/view/herac/%2F
https://www.sites.google.com/view/herac/%2F
https://codrose8.blogspot.com/
https://codtulip1.blogspot.com/
https://codlily3.blogspot.com/
https://codorchid00.blogspot.com/
https://codlotus5.blogspot.com/
https://codrose8.mystrikingly.com/
https://codtulip1.mystrikingly.com/
https://codlily3.mystrikingly.com/
https://codorchid00.mystrikingly.com/
https://codlotus5.mystrikingly.com/
codrose8.wordpress.com/
codtulip1.wordpress.com/
codorchid00.wordpress.com/
codlotus5.wordpress.com/
https://adamscottybest.wixsite.com/asianconnect
https://sites.google.com/view/maxbet7/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/btisports/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/sbobet10/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/asianconect/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/pinbet88/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://docs.google.com/drawings/d/1Rw9uy_zVHH-HFMfC6zzDogmKWEX4QfRJKouLbBVDX9Q/
https://docs.google.com/drawings/d/1jfDjmepvqsFmjddMk9ExSpiucLuLCooG25nHYJJv32E/
https://docs.google.com/drawings/d/1aOosLvI9NmB14qzndI73GAlLEbqLMx9ch9DKc66TIRE/
https://docs.google.com/drawings/d/1iw_h50dwb711A3vZJdapzQeqNjP8qSSAzOLTq1txjX4/
https://docs.google.com/drawings/d/1UlCrDTUNaz-oNqO7oehLZVMbQML_uu3mTVwxeOAUAno/
https://duckduckgo.com/?va=p&t=hc&q=https%3A%2F%2Fcityofrosedalems.com%2F&ia=web
https://www.google.com/search?q=https%3A%2F%2Fcityofrosedalems.com%2F&sca_esv=569194044&hl=ko&sxsrf=AM9HkKmEN6znojH7Kxw4Q8vGgB5hN2__yg%3A1702917062922&ei=xnOAZcruN5r2seMPx6u7cA&ved=0ahUKEwjK6fe0tJmDAxUae2wGHcfVDg4Q4dUDCBA&uact=5&oq=https%3A%2F%2Fcityofrosedalems.com%2F&gs_lp=Egxnd3Mtd2l6LXNlcnAiHWh0dHBzOi8vY2l0eW9mcm9zZWRhbGVtcy5jb20vMgQQIxgnSKhaUMlDWMlDcAJ4AJABAJgBUqABUqoBATG4AQPIAQD4AQL4AQHiAwQYASBBiAYB&sclient=gws-wiz-serp
https://www.google.com/search?q=site%3Acityofrosedalems.com%2F&sca_esv=569194044&hl=ko&sxsrf=AM9HkKkQ5LwIAxKBzwN5nI5szVREfrLPxw%3A1702966192183&ei=sDOBZajZCq_i4-EPh8mf8Aw&ved=0ahUKEwio8cu365qDAxUv8TgGHYfkB84Q4dUDCBA&uact=5&oq=site%3Acityofrosedalems.com%2F&gs_lp=Egxnd3Mtd2l6LXNlcnAiGnNpdGU6Y2l0eW9mcm9zZWRhbGVtcy5jb20vSI5vUIlNWPZYcAV4AJABAJgBY6ABpAOqAQE1uAEDyAEA-AEB4gMEGAEgQYgGAQ&sclient=gws-wiz-serp#ip=1
https://www.google.com/search?q=https%3A%2F%2Fcityofrosedalems.com%2F&tbm=isch&ved=2ahUKEwja_6Tb9JqDAxX1e2wGHStbAbEQ2-cCegQIABAA&oq=https%3A%2F%2Fcityofrosedalems.com%2F&gs_lcp=CgNpbWcQAzIECCMQJ1DVNljVNmCTP2gAcAB4AIABkQGIAaIDkgEDMC4zmAEAoAEBqgELZ3dzLXdpei1pbWfAAQE&sclient=img&ei=aj2BZdqiOvX3seMPq7aFiAs&bih=991&biw=2048
https://www.bing.com/search?q=cityofrosedalems.com%2F&form=QBLH&sp=-1&lq=0&pq=cityofrosedalems.com%2F&sc=3-21&qs=n&sk=&cvid=D93E7BBA539348C9B1981797A2C693C0&ghsh=0&ghacc=0&ghpl=
https://ph.search.yahoo.com/yhs/search?hspart=bc&hsimp=yhs-fresh&type=jjdeaf&p=cityofrosedalems.com%2F
mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=https://cityofrosedalems.com/
materinstvo.ru/forward?link=https://cityofrosedalems.com/
http://computer-chess.org/lib/exe/fetch.php?media=https://cityofrosedalems.com/
https://enchantedcottageshop.com/shop/trigger.php?r_link=https://cityofrosedalems.com/
https://buist-keatch.org/sphider/include/click_counter.php?url=https://cityofrosedalems.com/
www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=https://cityofrosedalems.com/
rel.chubu-gu.ac.jp/soumokuji/cgi-bin/go.cgi?https://cityofrosedalems.com/
fallout3.ru/utils/ref.php?url=https://cityofrosedalems.com/
www.veloxbox.us/link/?h=https://cityofrosedalems.com/
www.adult-plus.com/ys/rank.php?mode=link&id=592&url=https://cityofrosedalems.com/
mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=https://cityofrosedalems.com/
sparktime.justclick.ru/lms/api-login/?hash=MO18szcRUQdzpT%2FrstSCW5K8Gz6ts1NvTJLVa34vf1A%3D&authBhvr=1&email=videotrend24%40mail.ru&expire=1585462818&lms%5BrememberMe%5D=1&targetPath=https://cityofrosedalems.com/
http://vcteens.com/cgi-bin/at3/out.cgi?trade=https://cityofrosedalems.com/
www.bquest.org/Links/Redirect.aspx?ID=164&url=https://cityofrosedalems.com/
www.stipendije.info/phpAdsNew/adclick.php?bannerid=129&zoneid=1&source=&dest=https://cityofrosedalems.com/
today.od.ua/redirect.php?url=https://cityofrosedalems.com/
search.kcm.co.kr/jump.php?url=https://cityofrosedalems.com/
laskma.megastart-slot.ru/redirect/?g=https://cityofrosedalems.com/
www.uktrademarkregistration.co.uk/JumpTo.aspx?url=https://cityofrosedalems.com/
www.mir-stalkera.ru/go?https://cityofrosedalems.com/
duhocphap.edu.vn/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
www.millerovo161.ru/go?https://cityofrosedalems.com/
www.naturaltranssexuals.com/cgi-bin/a2/out.cgi?id=97&l=toplist&u=https://cityofrosedalems.com/
https://amanaimages.com/lsgate/?lstid=pM6b0jdQgVM-Y9ibFgTe6Zv1N0oD2nYuMA&lsurl=https://cityofrosedalems.com/
planszowkiap.pl/trigger.php?r_link=https://cityofrosedalems.com/
http://covenantpeoplesministry.org/cpm/wp/sermons/?show&url=https://cityofrosedalems.com/
www.sporteasy.net/redirect/?url=https://cityofrosedalems.com/
mightypeople.asia/link.php?id=M0ZGNHFISkd2bFh0RmlwSFU4bDN4QT09&destination=https://cityofrosedalems.com/
www.chinaleatheroid.com/redirect.php?url=https://cityofrosedalems.com/
i.s0580.cn/module/adsview/content/?action=click&bid=5&aid=163&url=https://cityofrosedalems.com/&variable=&source=https%3A%2F%2Fcutepix.info%2Fsex%2Friley-reyes.php
jsv3.recruitics.com/redirect?rx_cid=506&rx_jobId=39569207&rx_url=https://cityofrosedalems.com/
https://jobinplanet.com/away?link=https://cityofrosedalems.com/
www.supermoto8.com/sidebanner/62?href=https://cityofrosedalems.com/
presse.toyota.dk/login.aspx?returnurl=https://cityofrosedalems.com/
http://www.youtube.de/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.com/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.es/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ca/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.nl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.pl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ch/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.be/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.se/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.dk/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.pt/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.no/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.gr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.cl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.at/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.bg/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.sk/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.rs/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.lt/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.si/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.hr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ee/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.lu/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.tn/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co.ke/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co.cr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.kz/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.cat/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ge/redirect?event=channel_description&q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?event=channel_description&q=https://cityofrosedalems.com/&gl=ml
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=DE
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=IT
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=AR
https://www.youtube.at/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ch/redirect?q=https://cityofrosedalems.com/
https://www.youtube.fr/redirect?q=https://cityofrosedalems.com/
https://www.youtube.es/redirect?q=https://cityofrosedalems.com/
https://www.youtube.jp/redirect?q=https://cityofrosedalems.com/
https://www.youtube.co.uk/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ru/redirect?q=https://cityofrosedalems.com/
https://www.youtube.pl/redirect?q=https://cityofrosedalems.com/
https://www.youtube.gr/redirect?q=https://cityofrosedalems.com/
https://www.youtube.nl/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ca/redirect?q=https://cityofrosedalems.com/
https://www.youtube.cz/redirect?q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=AU
https://www.youtube.com.tw/redirect?q=https://cityofrosedalems.com/
golfpark.jp/banner/counter.aspx?url=https://cityofrosedalems.com/
www.mexicolore.co.uk/click.php?url=https://cityofrosedalems.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=https://cityofrosedalems.com/
www.invisalign-doctor.in/api/redirect?url=https://cityofrosedalems.com/
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=https://cityofrosedalems.com/
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=https://cityofrosedalems.com/
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=https://cityofrosedalems.com/
cipresso.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=https://cityofrosedalems.com/
www.beeicons.com/redirect.php?site=https://cityofrosedalems.com/
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=https://cityofrosedalems.com/
www.joeshouse.org/booking?link=https://cityofrosedalems.com/&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=https://cityofrosedalems.com/
http://okane-antena.com/redirect/index/fid100269/?u=https://cityofrosedalems.com/
www.gmwebsite.com/web/redirect.asp?url=https://cityofrosedalems.com/
swra.backagent.net/ext/rdr/?https://cityofrosedalems.com/
namiotle.pl/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
www.wqketang.com/logout?goto=https://cityofrosedalems.com/
access.bridges.com/externalRedirector.do?url=https://cityofrosedalems.com/
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://cityofrosedalems.com/
bot.buymeapie.com/recipe?url=https://cityofrosedalems.com/
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://cityofrosedalems.com/
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=https://cityofrosedalems.com/
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://cityofrosedalems.com/
www.knet-web.net/m/pRedirect.php?uID=2&iID=259&iURL=https://cityofrosedalems.com/
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44__zoneid=1__cb=7743e8d201__oadest=https://cityofrosedalems.com/ ?
www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=https://cityofrosedalems.com/
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2__bannerid=40__zoneid=16__cb=fc1d72225c__oadest=https://cityofrosedalems.com/
psylive.ru/success.aspx?id=0&goto=https://cityofrosedalems.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=https://cityofrosedalems.com/
www.mybunnies.net/te3/out.php?u=https://cityofrosedalems.com/
lubaczowskie.pl/rdir/?l=https://cityofrosedalems.com/&lid=1315
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=https://cityofrosedalems.com/
www.wave24.net/cgi-bin/linkrank/out.cgi?id=106248&cg=1&url=https://cityofrosedalems.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
e.ourger.com/?c=scene&a=link&id=47154371&url=https://cityofrosedalems.com/
zh-hk.guitarians.com/home/redirect/ubid/1015?r=https://cityofrosedalems.com/
www.mastercleaningsupply.com/trigger.php?r_link=https://cityofrosedalems.com/
www.cumshoter.com/cgi-bin/at3/out.cgi?id=98&tag=top&trade=https://cityofrosedalems.com/
shp.hu/hpc_uj/click.php?ml=5&url=https://cityofrosedalems.com/
https://indonesianmma.com/modules/mod_jw_srfr/redir.php?url=https://cityofrosedalems.com/
www.dive-international.net/places/redirect.php?b=797&web=www.https://cityofrosedalems.com/
www.themza.com/redirect.php?r=https://cityofrosedalems.com/
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://https://cityofrosedalems.com/
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=https://cityofrosedalems.com/
spb-medcom.ru/redirect.php?https://cityofrosedalems.com/
forest.ru/links.php?go=https://cityofrosedalems.com/
reefcentral.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=https://cityofrosedalems.com/
https://trackdaytoday.com/redirect-out?url=https://cityofrosedalems.com/
https://bigjobslittlejobs.com/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=4__zoneid=4__cb=68dbdae1d1__oadest=https://cityofrosedalems.com/
www.obertaeva.com/include/get.php?go=https://cityofrosedalems.com/
https://studiohire.com/admin-web-tel-process.php?memberid=4638&indentifier=weburl&websitelinkhitnumber=7&telnumberhitnumber=0&websiteurl=https://cityofrosedalems.com/
www.quanmama.com/t/goto.aspx?url=https://cityofrosedalems.com/
quartiernetz-friesenberg.ch/links-go.php?to=https://cityofrosedalems.com/
http://anorexicpornmovies.com/cgi-bin/atc/out.cgi?id=20&u=https://cityofrosedalems.com/
www.maultalk.com/url.php?to=https://cityofrosedalems.com/
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=https://cityofrosedalems.com/
www.e-expo.net/category/click_url.html?url=https://cityofrosedalems.com/
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=https://cityofrosedalems.com/
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=https://cityofrosedalems.com/
http://getdatasheet.com/url.php?url=https://cityofrosedalems.com/
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://cityofrosedalems.com/
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=https://cityofrosedalems.com/
www.aldolarcher.com/tools/esstat/esdown.asp?File=https://cityofrosedalems.com/
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&no_chat=1&domain=https://cityofrosedalems.com/
cs.payeasy.com.tw/click?url=https://cityofrosedalems.com/
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=https://cityofrosedalems.com/
http://tswzjs.com/go.asp?url=https://cityofrosedalems.com/
asstomouth.guru/out.php?url=https://cityofrosedalems.com/
advtest.exibart.com/adv/adv.php?id_banner=7201&link=https://cityofrosedalems.com/
https://thairesidents.com/l.php?b=85&p=2,5&l=https://cityofrosedalems.com/
www.latestnigeriannews.com/link_channel.php?channel=https://cityofrosedalems.com/
www.haogaoyao.com/proad/default.aspx?url=https://cityofrosedalems.com/
globalmedia51.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
citysafari.nl/Home/setCulture?language=en&returnUrl=https://cityofrosedalems.com/
es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
lorena-kuhni.kz/redirect?link=https://cityofrosedalems.com/
www.webshoptrustmark.fr/Change/en?returnUrl=https://cityofrosedalems.com/
https://processon.com/setting/locale?language=zh&back=https://cityofrosedalems.com/
c.yam.com/srh/wsh/r.c?https://cityofrosedalems.com/
www.jolletorget.no/J/l.php?l=https://cityofrosedalems.com/
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=https://cityofrosedalems.com/
www.moonbbs.com/dm/dmlink.php?dmurl=https://cityofrosedalems.com/
www.sparetimeteaching.dk/forward.php?link=https://cityofrosedalems.com/
https://paspn.net/default.asp?p=90&gmaction=40&linkid=52&linkurl=https://cityofrosedalems.com/
www.dialogportal.com/Services/Forward.aspx?link=https://cityofrosedalems.com/
www.poddebiczak.pl/?action=set-desktop&url=https://cityofrosedalems.com/
ant53.ru/file/link.php?url=https://cityofrosedalems.com/
www.docin.com/jsp_cn/mobile/tip/android_v1.jsp?forward=https://cityofrosedalems.com/
old.magictower.ru/cgi-bin/redir/redir.pl?https://cityofrosedalems.com/
go.flx1.com/click?id=1&m=11&pl=113&dmcm=16782&euid=16603484876&out=https://cityofrosedalems.com/
moba-hgh.de/link2http.php?href=https://cityofrosedalems.com/
https://gazetablic.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=34__zoneid=26__cb=0e0dfef92b__oadest=https://cityofrosedalems.com/
watchvideo.co/go.php?url=https://cityofrosedalems.com/
www.todoku.info/gpt/rank.cgi?mode=link&id=29649&url=https://cityofrosedalems.com/
www.fotochki.com/redirect.php?go=https://cityofrosedalems.com/
bayerwald.tips/plugins/bannerverwaltung/bannerredirect.php?bannerid=1&url=https://cityofrosedalems.com/
ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=https://cityofrosedalems.com/
www.widgetinfo.net/read.php?sym=FRA_LM&url=https://cityofrosedalems.com/
arctic.nyheter24.se/rdb/nyheter24_eed6ad4b451f2fb8193922f832bc91ed/5?url=https://cityofrosedalems.com/
www.sdam-snimu.ru/redirect.php?url=https://cityofrosedalems.com/
school.mosreg.ru/soc/moderation/abuse.aspx?link=https://cityofrosedalems.com/
affiliates.kanojotoys.com/affiliate/scripts/click.php?a_aid=widdi77&desturl=https://cityofrosedalems.com/
www.gotomypctech.com/affiliates/scripts/click.php?a_aid=ed983915&a_bid=&desturl=https://cityofrosedalems.com/
www.my-sms.ru/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/&rel=external
www.genderpsychology.com/https://cityofrosedalems.com/
http://chtbl.com/track/118167/https://cityofrosedalems.com/
www.360wichita.com/amp-banner-tracking?adid=192059&url=https://cityofrosedalems.com/
www.tsv-bad-blankenburg.de/cms/page/mod/url/url.php?eid=16&urlpf=https://cityofrosedalems.com/
https://fishki.net/click?https://cityofrosedalems.com/
https://zerlong.com/bitrix/redirect.php?goto=https://cityofrosedalems.com/
catalog.flexcom.ru/go?z=36047&i=55&u=https://cityofrosedalems.com/
www.wellvit.nl/response/forward/c1e41491e30c5af3c20f80a2af44e440.php?link=0&target=https://cityofrosedalems.com/
www.ident.de/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=76__zoneid=2__cb=8a18c95a9e__oadest=https://cityofrosedalems.com/
www.fertilab.net/background_manager.aspx?ajxName=link_banner&id_banner=50&url=https://cityofrosedalems.com/
shop-uk.fmworld.com/Queue/Index?url=https://cityofrosedalems.com/
www.weddinginlove.com/redirect/?url=https://cityofrosedalems.com/
nieuws.rvent.nl/bitmailer/statistics/mailstatclick/42261?link=https://cityofrosedalems.com/
https://jobanticipation.com/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=jobanticipation.com
www.xsbaseball.com/tracker/index.html?t=ad&pool_id=3&ad_id=5&url=https://cityofrosedalems.com/
eos.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
b.sm.su/click.php?bannerid=56&zoneid=10&source=&dest=https://cityofrosedalems.com/
https://bethlehem-alive.com/abnrs/countguideclicks.cfm?targeturl=https://cityofrosedalems.com/&businessid=29579
www.bookmark-favoriten.com/?goto=https://cityofrosedalems.com/
shop.yuliyababich.eu/RU/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/
www.depmode.com/go.php?https://cityofrosedalems.com/
https://urgankardesler.com/anasayfa/yonlen?link=https://cityofrosedalems.com/
www.metalindex.ru/netcat/modules/redir/?&site=https://cityofrosedalems.com/
www.rprofi.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://darudar.org/external/?link=https://cityofrosedalems.com/
www.postsabuy.com/autopost4/page/generate/?link=https://cityofrosedalems.com/&list=PL9d7lAncfCDSkF4UPyhzO59Uh8cOoD-8q&fb_node=942812362464093&picture&name=%E0%B9%82%E0%B8%9B%E0%B8%A3%E0%B9%81%E0%B8%81%E0%B8%A3%E0%B8%A1%E0%B9%82%E0%B8%9E%E0%B8%AA%E0%B8%82%E0%B8%B2%E0%B8%A2%E0%B8%AA%E0%B8%B4%E0%B8%99%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%AD%E0%B8%AD%E0%B8%99%E0%B9%84%E0%B8%A5%E0%B8%99%E0%B9%8C+&caption=%E0%B9%80%E0%B8%A5%E0%B8%82%E0%B8%B2%E0%B8%AA%E0%B9%88%E0%B8%A7%E0%B8%99%E0%B8%95%E0%B8%B1%E0%B8%A7+%E0%B8%97%E0%B8%B5%E0%B8%84%E0%B8%B8%E0%B8%93%E0%B8%A5%E0%B8%B7%E0%B8%A1%E0%B9%84%E0%B8%A1%E0%B9%88%E0%B8%A5%E0%B8%87+Line+%40postsabuy&description=%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%B2%E0%B8%96%E0%B8%B9%E0%B8%81%E0%B8%97%E0%B8%B5%E0%B9%88%E0%B8%AA%E0%B8%B8%E0%B8%94%E0%B9%83%E0%B8%99+3+%E0%B9%82%E0%B8%A5%E0%B8%81+%E0%B8%AD%E0%B8%B4%E0%B8%AD%E0%B8%B4
www.perimeter.org/track.pdf?url=https://cityofrosedalems.com/
forum.darievna.ru/go.php?https://cityofrosedalems.com/
techlab.rarus.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.spiritualforums.com/vb/redir.php?link=https://cityofrosedalems.com/
www.review-mag.com/cdn/www/delivery/view.php?ct=1&oaparams=2__bannerid=268__zoneid=1__cb=8c1317f219__oadest=https://cityofrosedalems.com/
belantara.or.id/lang/s/ID?url=https://cityofrosedalems.com/
ele-market.ru/consumer.php?url=https://cityofrosedalems.com/
https://www.хорошие-сайты.рф/r.php?r=https://cityofrosedalems.com/
https://jobsflagger.com/jobclick/?RedirectURL=https://cityofrosedalems.com/
https://gpoltava.com/away/?go=https://cityofrosedalems.com/
www.cardexchange.com/index.php/tools/packages/tony_mailing_list/services/?mode=link&mlm=62&mlu=0&u=2&url=https://cityofrosedalems.com/
services.nfpa.org/Authentication/GetSSOSession.aspx?return=https://cityofrosedalems.com/
http://spaceup.org/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
yarko-zhivi.ru/redirect?url=https://cityofrosedalems.com/
365sekretov.ru/redirect.php?action=url&goto=https://cityofrosedalems.com/%20
www.sgdrivingtest.com/redirect.php?page=https://cityofrosedalems.com/
www.jxren.com/news/link/link.asp?id=7&url=https://cityofrosedalems.com/
particularcareers.co.uk/jobclick/?RedirectURL=https://cityofrosedalems.com/
https://bsaonline.com/MunicipalDirectory/SelectUnit?unitId=411&returnUrl=https://cityofrosedalems.com/&sitetransition=true
www.elit-apartament.ru/go?https://cityofrosedalems.com/
sendai.japansf.net/rank.cgi?mode=link&id=1216&url=https://cityofrosedalems.com/
www.bmwfanatics.ru/goto.php?l=https://cityofrosedalems.com/
www.saabsportugal.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=https://cityofrosedalems.com/
cms.sive.it/Jump.aspx?gotourl=https://cityofrosedalems.com/
largusladaclub.ru/go/url=https:/https://cityofrosedalems.com/
https://kekeeimpex.com/Home/ChangeCurrency?urls=https://cityofrosedalems.com/&cCode=GBP&cRate=77.86247
mycounter.com.ua/go.php?https://cityofrosedalems.com/
l2base.su/go?https://cityofrosedalems.com/
https://freeseotool.org/url/?q=https://cityofrosedalems.com/
www.duomodicagliari.it/reg_link.php?link_ext=https://cityofrosedalems.com/&prov=1
assine.hostnet.com.br/cadastro/?rep=17&url=https://cityofrosedalems.com/
www.dvnlp.de/profile/gruppe/redirect/5?url=https://cityofrosedalems.com/
http://hotmaturegirlfriends.com/cl.php?porno=MzB4MzB4NjEw&url=https://cityofrosedalems.com/
www.smkn5pontianak.sch.id/redirect/?alamat=https://cityofrosedalems.com/
www.cheapdealuk.co.uk/go.php?url=https://cityofrosedalems.com/
bilometro.brksedu.com.br/tracking?url=https://cityofrosedalems.com/&zorigem=hotsite-blackfriday
www.omschweiz.ch/select-your-country?publicUrl=https://cityofrosedalems.com/
https://anacolle.net/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
www.cubamusic.com/Home/ChangeLanguage?lang=es-ES&returnUrl=https://cityofrosedalems.com/
expoclub.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.deypenburgschecourant.nl/reklame/www/delivery/ck.php?oaparams=2__bannerid=44__zoneid=11__cb=078c2a52ea__oadest=https://cityofrosedalems.com/
tramplintk.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
cnc.extranet.gencat.cat/treball_cnc/AppJava/FileDownload.do?pdf=https://cityofrosedalems.com/&codi_cnv=9998045
www.gamecollections.co.uk/search/redirect.php?retailer=127&deeplink=https://cityofrosedalems.com/
bearcong.no1.sexy/hobby-delicious/rank.cgi?mode=link&id=19&url=https://cityofrosedalems.com/
dawnofwar.org.ru/go?https://cityofrosedalems.com/
www.surinenglish.com/backend/conectar.php?url=https://cityofrosedalems.com/
www.reference-cannabis.com/interface/sortie.php?adresse=https://cityofrosedalems.com/
login.0×69416d.co.uk/sso/logout?tenantId=tnl&gotoUrl=https://cityofrosedalems.com/&domain=0×69416d.co.uk
blog.link-usa.jp/emi?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://damki.net/go/?https://cityofrosedalems.com/
opensesame.wellymulia.zaxaa.com/b/66851136?s=1&redir=https://cityofrosedalems.com/
ism3.infinityprosports.com/ismdata/2009100601/std-sitebuilder/sites/200901/www/en/tracker/index.html?t=ad&pool_id=1&ad_id=112&url=https://cityofrosedalems.com/
apps.cancaonova.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=149__zoneid=20__cb=87d2c6208d__oadest=https://cityofrosedalems.com/
www.lespritjardin.be/?advp_click_bimage_id=19&url=https://cityofrosedalems.com/&shortcode_id=10
www.dresscircle-net.com/psr/rank.cgi?mode=link&id=14&url=https://cityofrosedalems.com/
www.counterwelt.com/charts/click.php?user=14137&link=https://cityofrosedalems.com/
fms.csonlineschool.com.au/changecurrency/1?returnurl=https://cityofrosedalems.com/
www.v-archive.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.jagat.co.jp/analysis/analysis.php?url=https://cityofrosedalems.com/
https://vse-doski.com/redirect/?go=https://cityofrosedalems.com/
www.karatetournaments.net/link7.asp?LRURL=https://cityofrosedalems.com/&LRTYP=O
simracing.su/go/?https://cityofrosedalems.com/
click.cheshi.com/go.php?proid=218&clickid=1393306648&url=https://cityofrosedalems.com/
fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=https://cityofrosedalems.com/
flypoet.toptenticketing.com/index.php?url=https://cityofrosedalems.com/
www.horsesmouth.com/LinkTrack.aspx?u=https://cityofrosedalems.com/
d-click.fiemg.com.br/u/18081/131/75411/137_0/82cb7/?url=https://cityofrosedalems.com/
www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=https://cityofrosedalems.com/
www.packmage.net/uc/goto/?url=https://cityofrosedalems.com/
my.9991.com/login_for_index_0327.php?action=logout&forward=https://cityofrosedalems.com/
www.sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=986&redirect=https://cityofrosedalems.com/
www.naturum.co.jp/ad/linkshare/?siteID=p_L785d6UQY-V4Fh4Rxs7wNzOPgtzv95Tg&lsurl=https://cityofrosedalems.com/
www.d-e-a.eu/newsletter/redirect.php?link=https://cityofrosedalems.com/
www.bom.ai/goweburl?go=https://cityofrosedalems.com/
enews2.sfera.net/newsletter/redirect.php?id=sabricattani@gmail.com_0000006566_144&link=https://cityofrosedalems.com/
underwater.com.au/redirect_url/id/7509/?redirect=https://cityofrosedalems.com/
https://eqsoftwares.com/languages/setlanguage?languagesign=en&redirect=https://cityofrosedalems.com/
www.jagdambasarees.com/Home/ChangeCurrency?urls=https://cityofrosedalems.com/&cCode=MYR&cRate=14.554
www.priegeltje.nl/gastenboek/go.php?url=https://cityofrosedalems.com/
www.serie-a.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.rz114.cn/url.html?url=https://cityofrosedalems.com/
www.greatdealsindia.com/redirects/infibeam.aspx?url=https://cityofrosedalems.com/
rs.345kei.net/rank.php?id=37&mode=link&url=https://cityofrosedalems.com/
webapp.jgz.la/?c=scene&a=link&id=8665466&url=https://cityofrosedalems.com/
www.erotiikkalelut.com/url.php?link=https://cityofrosedalems.com/
vicsport.com.au/analytics/outbound?url=https://cityofrosedalems.com/
https://fachowiec.com/zliczanie-bannera?id=24&url=https://cityofrosedalems.com/
ieea.ir/includes/change_lang.php?lang=en&goto=https://cityofrosedalems.com/
scribe.mmonline.io/click?evt_nm=Clicked+Registration+Completion&evt_typ=clickEmail&app_id=m4marry&eml_sub=Registration+Successful&usr_did=4348702&cpg_sc=NA&cpg_md=email&cpg_nm=&cpg_cnt=&cpg_tm=NA&link_txt=Live+Chat&em_type=Notification&url=https://cityofrosedalems.com/
polo-v1.feathr.co/v1/analytics/crumb?flvr=email_link_click&rdr=https://cityofrosedalems.com/
sintesi.provincia.mantova.it/portale/LinkClick.aspx?link=https://cityofrosedalems.com/
https://careerchivy.com/jobclick/?RedirectURL=https://cityofrosedalems.com/
shinsekai.type.org/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
https://maned.com/scripts/lm/lm.php?tk=CQkJZWNuZXdzQGluZm90b2RheS5jb20JW05ld3NdIE1FSSBBbm5vdW5jZXMgUGFydG5lcnNoaXAgV2l0aCBUd2l4bCBNZWRpYQkxNjcyCVBSIE1lZGlhIENvbnRhY3RzCTI1OQljbGljawl5ZXMJbm8=&url=https://cityofrosedalems.com/
www.etaigou.com/turn2.php?ad_id=276&link=https://cityofrosedalems.com/
crewroom.alpa.org/SAFETY/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=12872
www.tagirov.org/out.php?url=https://cityofrosedalems.com/
startlist.club/MSF/Language/Set?languageIsoCode=en&returnUrl=https://cityofrosedalems.com/
www.tetsumania.net/search/rank.cgi?mode=link&id=947&url=https://cityofrosedalems.com/
https://imperial-info.net/link?idp=125&url=https://cityofrosedalems.com/
www.brainlanguage-sa.com/setcookie.php?lang=en&file=https://cityofrosedalems.com/
www.asensetranslations.com/modules/babel/redirect.php?newlang=en_US&newurl=https://cityofrosedalems.com/
gameshock.jeez.jp/rank.cgi?mode=link&id=307&url=https://cityofrosedalems.com/
https://tripyar.com/go.php?https://cityofrosedalems.com/
www.floridafilmofficeinc.com/?goto=https://cityofrosedalems.com/
tsvc1.teachiworld.com/bin/checker?mode=4&module=11&mailidx=19130&dmidx=0&emidx=0&service=0&cidx=&etime=20120328060000&seqidx=3&objidx=22&encoding=0&url=https://cityofrosedalems.com/
rechner.atikon.at/lbg.at/newsletter/linktracking?subscriber=&delivery=38116&url=https://cityofrosedalems.com/
www.pcreducator.com/Common/SSO.aspx?returnUrl=https://cityofrosedalems.com/
go.eniro.dk/lg/ni/cat-2611/http:/https://cityofrosedalems.com/
www.fisherly.com/redirect?type=website&ref=listing_detail&url=https://cityofrosedalems.com/
bio-pack.ru/bitrix/redirect.php?goto=http://https://cityofrosedalems.com/
fid.com.ua/redirect/?go=https://cityofrosedalems.com/
www.modernipanelak.cz/?b=618282165&redirect=https://cityofrosedalems.com/
h5.hbifeng.com/index.php?c=scene&a=link&id=14240604&url=https://cityofrosedalems.com/
www.bkdc.ru/bitrix/redirect.php?event1=news_out&event2=32reg.roszdravnadzor.ru/&event3=A0A0B5A09180D0%A09582A0BBA1A085%D0E2A084D0D1C2D0%A085+A0A0B5A182B0A0%C2D0D0D096+A1A0BBA0B180D0%A09795+A0A0B0A09582A1%D1D0D0D0A182B5+A0A091A08695A0%D1D0A6A185A0A085%D0D1D0D082A1A085%D0D0D1D0A095B1A0%C2D0D0D091&goto=https://cityofrosedalems.com/
record.affiliatelounge.com/WS-jvV39_rv4IdwksK4s0mNd7ZgqdRLk/7/?deeplink=https://cityofrosedalems.com/
d-click.artenaescola.org.br/u/3806/290/32826/1416_0/53052/?url=https://cityofrosedalems.com/
www.morroccoaffiliate.com/aff.php?id=883&url=https://cityofrosedalems.com/
www.omegon.eu/de/?r=https://cityofrosedalems.com/
www.flooble.com/cgi-bin/clicker.pl?id=grabbadl&url=https://cityofrosedalems.com/
www.sportsbook.ag/ctr/acctmgt/pl/openLink.ctr?ctrPage=https://cityofrosedalems.com/
www.ra2d.com/directory/redirect.asp?id=596&url=https://cityofrosedalems.com/
www.anorexicnudes.net/cgi-bin/atc/out.cgi?u=https://cityofrosedalems.com/
www.zjjiajiao.com.cn/ad/adredir.asp?url=https://cityofrosedalems.com/
https://hakobo.com/wp/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
t.agrantsem.com/tt.aspx?cus=216&eid=1&p=216-2-71016b553a1fa2c9.3b14d1d7ea8d5f86&d=https://cityofrosedalems.com/
moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.cheek.co.jp/location/location.php?id=keibaseminar&url=https://cityofrosedalems.com/
www.quantixtickets3.com/php-bin-8/kill_session_and_redirect.php?redirect=https://cityofrosedalems.com/
www.photokonkurs.com/cgi-bin/out.cgi?url=https://cityofrosedalems.com/
https://www.nbda.org/?URL=https://cityofrosedalems.com/
https://www.thislife.net/cgi-bin/webcams/out.cgi?id=playgirl&url=https://cityofrosedalems.com/
https://www.dans-web.nu/klick.php?url=https://cityofrosedalems.com/
https://voobrajulya.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.resnichka.ru/partner/go.php?https://cityofrosedalems.com/
www.nafta-him.com/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.kyoto-osaka.com/search/rank.cgi?mode=link&id=9143&url=https://cityofrosedalems.com/
www.findingfarm.com/redir?url=https://cityofrosedalems.com/
www.fairpoint.net/~jensen1242/gbook/go.php?url=https://cityofrosedalems.com/
www.cnainterpreta.it/redirect.asp?url=https://cityofrosedalems.com/
red.ribbon.to/~zkcsearch/zkc-search/rank.cgi?mode=link&id=156&url=https://cityofrosedalems.com/
iphoneapp.impact.co.th/i/r.php?u=https://cityofrosedalems.com/
akademik.tkyd.org/Home/SetCulture?culture=en-US&returnUrl=https://cityofrosedalems.com/
www.rencai8.com/web/jump_to_ad_url.php?id=642&url=https://cityofrosedalems.com/
http://crackstv.com/redirect.php?bnn=CabeceraHyundai&url=https://cityofrosedalems.com/
https://www.baby22.com.tw/Web/turn.php?ad_id=160&link=https://cityofrosedalems.com/
https://www.kichink.com/home/issafari?uri=https://cityofrosedalems.com/
https://blogranking.fc2.com/out.php?id=1032500&url=https://cityofrosedalems.com/
5cfxm.hxrs6.servertrust.com/v/affiliate/setCookie.asp?catId=1180&return=https://cityofrosedalems.com/
http://3dcreature.com/cgi-bin/at3/out.cgi?id=187&trade=https://cityofrosedalems.com/
https://nowlifestyle.com/redir.php?k=9a4e080456dabe5eebc8863cde7b1b48&url=https://cityofrosedalems.com/
http://slipknot1.info/go.php?url=https://cityofrosedalems.com/
www.acutenet.co.jp/cgi-bin/lcount/lcounter.cgi?link=https://cityofrosedalems.com/
http://news-matome.com/method.php?method=1&url=https://cityofrosedalems.com/
https://lifecollection.top/site/gourl?url=https://cityofrosedalems.com/
https://www.toscanapiu.com/web/lang.php?lang=DEU&oldlang=ENG&url=https://cityofrosedalems.com/
speakrus.ru/links.php?go=https://cityofrosedalems.com/
https://edcommunity.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=https://cityofrosedalems.com/
https://www.sainttropeztourisme.com/en/bannieres/redirection/index.html?id=649&lien=https://cityofrosedalems.com/
www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
www.interracialhall.com/cgi-bin/atx/out.cgi?trade=https://cityofrosedalems.com/
https://www.tumimusic.com/link.php?url=https://cityofrosedalems.com/
www.glorioustronics.com/redirect.php?link=https://cityofrosedalems.com/
mail.resen.gov.mk/redir.hsp?url=https://cityofrosedalems.com/
https://www.pompengids.net/followlink.php?id=495&link=https://cityofrosedalems.com/
https://www.hirforras.net/scripts/redir.php?url=https://cityofrosedalems.com/
https://lens-club.ru/link?go=https://cityofrosedalems.com/
https://t.raptorsmartadvisor.com/.lty?url=https://cityofrosedalems.com/&loyalty_id=14481&member_id=b01bbee6-4592-4345-a0ee-5d71ed6f1929
https://evoautoshop.com/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://beautycottageshop.com/change.php?lang=cn&url=https://cityofrosedalems.com/
http://1000love.net/lovelove/link.php?url=https://cityofrosedalems.com/
https://www.ffw-ellar.de/ref.php?url=https://cityofrosedalems.com/
https://www.contactlenshouse.com/currency.asp?c=CAD&r=https://cityofrosedalems.com/
https://www.bartaz.lt/wp-content/plugins/clikstats/ck.php?Ck_id=70&Ck_lnk=https://cityofrosedalems.com/
https://sogo.i2i.jp/link_go.php?url=https://cityofrosedalems.com/
https://ceb.bg/catalog/statistic/?id=61&location=https://cityofrosedalems.com/
https://malehealth.ie/redirect/?age=40&part=waist&illness=obesity&refer=https://cityofrosedalems.com/
https://magicode.me/affiliate/go?url=https://cityofrosedalems.com/
forum.marillion.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
https://jobregistry.net/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=jobregistry.net&rgp_m=title13&et=4495
https://www.goatzz.com/adredirect.aspx?adType=SiteAd&ItemID=9595&ReturnURL=https://cityofrosedalems.com/
https://experts.richdadworld.com/assets/shared/php/noabp.php?oaparams=2bannerid=664zoneid=5cb=0902f987cboadest=https://cityofrosedalems.com/
https://miyagi.lawyer-search.tv/details/linkchk.aspx?type=o&url=https://cityofrosedalems.com/
https://www.snwebcastcenter.com/event/page/count_download_time.php?url=https://cityofrosedalems.com/
https://go.onelink.me/v1xd?pid=Patch&c=Mobile%20Footer&af_web_dp=https://cityofrosedalems.com/
https://www.weather.net/cgi-bin/redir?https://cityofrosedalems.com/
https://gaggedtop.com/cgi-bin/top/out.cgi?ses=sBZFnVYGjF&id=206&url=https://cityofrosedalems.com/
https://e-bike-test.net/wp-content/plugins/AND-AntiBounce/redirector.php?url=https://cityofrosedalems.com/
https://www.morhipo.com/shared/partnercookie?k=gort&url=https://cityofrosedalems.com/
https://www.luckyplants.com/cgi-bin/toplist/out.cgi?id=rmontero&url=https://cityofrosedalems.com/
https://webankety.cz/dalsi.aspx?site=https://cityofrosedalems.com/
https://runkeeper.com/apps/authorize?redirect_uri=https://cityofrosedalems.com/
https://www.emiratesvoice.com/footer/comment_like_dislike_ajax/?code=like&commentid=127&redirect=https://cityofrosedalems.com/
https://envios.uces.edu.ar/control/click.mod.php?id_envio=8147&email=gramariani@gmail.com&url=https://cityofrosedalems.com/
https://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=https://cityofrosedalems.com/
https://ulfishing.ru/forum/go.php?https://cityofrosedalems.com/
https://www.ab-search.com/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
https://www.studyrama.be/tracking.php?origine=ficheform5683&lien=http://https://cityofrosedalems.com/
https://shop.merchtable.com/users/authorize?return_url=https://cityofrosedalems.com/
https://nudewwedivas.forumcommunity.net/m/ext.php?url=https://cityofrosedalems.com/
https://cztt.ru/redir.php?url=https://cityofrosedalems.com/
https://ageoutloud.gms.sg/visit.php?item=54&uri=https://cityofrosedalems.com/
https://multimedia.inrap.fr/redirect.php?li=287&R=https://cityofrosedalems.com/
http://mientaynet.com/advclick.php?o=textlink&u=15&l=https://cityofrosedalems.com/
https://atlanticleague.com/tracker/index.html?t=ad&pool_id=11&ad_id=5&url=https://cityofrosedalems.com/
https://www.castellodivezio.it/lingua.php?lingua=EN&url=https://cityofrosedalems.com/
https://www.podcastone.com/site/rd?satype=40&said=4&aaid=email&camid=-4999600036534929178&url=https://cityofrosedalems.com/
https://www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://cityofrosedalems.com/
https://monarchbeachmembers.play18.com/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/
https://wayi.com.tw/wayi_center.aspx?flag=banner&url=https://cityofrosedalems.com/&idno=443
https://d.agkn.com/pixel/2389/?che=2979434297&col=22204979,1565515,238211572,435508400,111277757&l1=https://cityofrosedalems.com/
https://mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=https://cityofrosedalems.com/
https://bemidji.bigdealsmedia.net/include/sort.php?return_url=https://cityofrosedalems.com/&sort=a:3:{s:9:%E2%80%9Ddirection%E2%80%9D;s:3:%E2%80%9DASC%E2%80%9D;s:5:%E2%80%9Dfield%E2%80%9D;s:15:%E2%80%9DItems.PriceList%E2%80%9D;s:5:%E2%80%9Dlabel%E2%80%9D;s:9:%E2%80%9Dvalue_asc%E2%80%9D;}
https://hslda.org/content/a/LinkTracker.aspx?id=4015475&appeal=385&package=36&uri=https://cityofrosedalems.com/
https://www.sign-in-china.com/newsletter/statistics.php?type=mail2url&bs=88&i=114854&url=https://cityofrosedalems.com/
https://panarmenian.net/eng/tofv?tourl=https://cityofrosedalems.com/
https://www.kvinfo.dk/visit.php?linkType=2&linkValue=https://cityofrosedalems.com/
https://lavoro.provincia.como.it/portale/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=935
https://www.dodeley.com/?action=show_ad&url=https://cityofrosedalems.com/
https://www.ignicaodigital.com.br/affiliate/?idev_id=270&u=https://cityofrosedalems.com/
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=https://cityofrosedalems.com/
https://m.wedkuje.pl/mobile/redirect.php?redir=https://cityofrosedalems.com/
https://area51.to/go/out.php?s=100&l=site&u=https://cityofrosedalems.com/
https://games4ever.3dn.ru/go?https://cityofrosedalems.com/
https://de.reasonable.shop/SetCurrency.aspx?currency=CNY&returnurl=https://cityofrosedalems.com/
https://www.pcbheaven.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
https://mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=https://cityofrosedalems.com/
https://www.shop-bell.com/out.php?id=kibocase&category=ladies&url=https://cityofrosedalems.com/
https://m.addthis.com/live/redirect/?url=https://cityofrosedalems.com/
https://www.pcreducator.com/Common/SSO.aspx?returnUrl=https://cityofrosedalems.com/
https://horsesmouth.com/LinkTrack.aspx?u=https://cityofrosedalems.com/
https://soom.cz/projects/get2mail/redir.php?id=c2e52da9ad&url=https://cityofrosedalems.com/
https://www.iaai.com/VehicleInspection/InspectionProvidersUrl?name=AA%20Transit%20Pros%20Inspection%20Service&url=https://cityofrosedalems.com/
https://cingjing.fun-taiwan.com/AdRedirector.aspx?padid=303&target=https://cityofrosedalems.com/
https://rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://cityofrosedalems.com/
https://campaign.unitwise.com/click?emid=31452&emsid=ee720b9f-a315-47ce-9552-fd5ee4c1c5fa&url=https://cityofrosedalems.com/
https://www.swipeclock.com/sc/cookie.asp?sitealias=79419397&redirect=https://cityofrosedalems.com/
https://www.gutscheinaffe.de/wp-content/plugins/AND-AntiBounce/redirector.php?url=https://cityofrosedalems.com/
https://home.uceusa.com/Redirect.aspx?r=https://cityofrosedalems.com/
https://www.seankenney.com/include/jump.php?num=https://cityofrosedalems.com/
https://runningcheese.com/go?url=https://cityofrosedalems.com/
http://chtbl.com/track/118167/https://cityofrosedalems.com/
https://m.17ll.com/apply/tourl/?url=https://cityofrosedalems.com/
https://crewroom.alpa.org/SAFETY/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=12872
https://rs.businesscommunity.it/snap.php?u=https://cityofrosedalems.com/
http://dstats.net/redir.php?url=https://cityofrosedalems.com/
https://www.aiac.world/pdf/October-December2015Issue?pdf_url=https://cityofrosedalems.com/
https://union.591.com.tw/stats/event/redirect?e=eyJpdiI6IjdUd1B5Z2FPTmNWQzBmZk1LblR2R0E9PSIsInZhbHVlIjoiQTI4TnVKMzdjMkxrUjcrSWlkcXdzbjRQeGRtZ0ZGbXdNSWxkSkVieENwNjQ1cHF5aDZmWmFobU92ZGVyUk5jRTlxVnI2TG5pb0dJVHZSUUlHcXFTbGo3UDliYWU5UE5MSjlMY0xOQnFmbVRQSFNoZDRGd2dqVDZXZEU4WFoyajJ0S0JITlQ2XC9SXC9jRklPekdmcnFGb09vRllqNHVtTHlYT284ZmN3d0ozOHFkclRYYnU5UlY2NTFXSGRheW5SbGxJb3BmYjQ2Mm9TWUFCTEJuXC9iT25nYkg4QXpOd2pHVlBWTWxWXC91aWRQMVhKQmVJXC9qMW9IdlZaVVlBdWlCYW4rS0JualhSMElFeVZYN3NnUW1qcUdxcWUrSlFROFhKbWttdkdvMUJ3aWVRa2I3MVV5TXpER3doa2ZuekFWNWd3OGpuQ1VSczFDREVKaklaUks0TTRIY2pUeXYrQmdZYUFTK1F4RWpTY0RRaW5Nc0krdVJ2N2VUT1wvSUxVVWVKN3hnQU92QmlCbjQyMUpRdTZKVWJcL0RCSVFOcWl0azl4V2pBazBHWmVhdWptZGREVXh0VkRNWWxkQmFSYXhBRmZtMHA5dTlxMzIzQzBVaWRKMEFqSG0wbGkxME01RDBaaElTaU5QKzIxbSswaUltS0FYSzViZlFmZjZ1XC9Yclg0U2VKdHFCc0pTNndcL09FWklUdjlQM2dcL2RuN0szQ3plWmcyYWdpQzJDQ2NIcWROczVua3dIM1Q3OXpJY3Z0XC93MVk3SHUyODZHU3Z5aHFVbWEwRFU1ZFdyMGt0YWpsb3BkQitsZER5aWk4YWMrZWYzSFNHNERhOGdDeUJWeEtoSm9wQ0hQb2EzOHZ3eHFGVTQ2Mk1QSEZERzlXZWxRRTJldjJkdnZUM0ZwaW1KcEVVc3ZXWjRHaTZWRDJOK0YxR3d4bXhMR3BhWmZBNkJ6eUYxQjR4ODVxc0d0YkFpYU8yZ2tuWGdzelBpU3dFUjJVYUVtYUlpZllSUTVpaHZMbjhySFp4VEpQR3EyYnRLTmdcLzRvKzQwRmtGNUdWWnQ0VjFpcTNPc0JubEdWenFiajRLRFg5a2dRZFJOZ1wvaUEwVHR3ZnYzakpYVmVtT294aFk1TXBUZ3FmVnF2dnNSVWJ5VEE0WGZpV3o3Y0k2SjJhM2RDK2hoQ0FvV2YrSW9QWnhuZG5QN1hlOEFaTVZrcFZ3c0pXVHhtNkRTUkpmenpibG8zdTM0cGF6Q3oxTEJsdDdiOUgwWXFOUkNHWjlEbTBFYzdIRUcyalYrcW4wYklFbnlGYlZJUG00R1VDQTZLZEVJRklIbFVNZFdpS3RkeCt5bVpTNUkrOXE3dDlxWmZ2bitjSGlSeE9wZTg5Yk9wS0V6N1wvd1EzUTNVenNtbjlvQUJhdGsxMzNkZTdjTU1LNkd4THJMYTBGUEJ4elEycFNTNGZabEJnalhJc0pYZit1c1wvWDBzSm1JMzRad3F3PT0iLCJtYWMiOiI4MjNhNDJlYTMwOTlmY2VlYzgxNmU1N2JiM2QzODk5YjI5MDFhYThhMDBkYzNhODljOTRmMTMzMzk0YTM5OGIzIn0=&source=BANNER.2913&url=https://cityofrosedalems.com/
https://fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=https://cityofrosedalems.com/
https://pdcn.co/e/https://cityofrosedalems.com/
https://api.webconnex.com/v1/postmaster/track/click/4f8036d14ee545798599c8921fbfcd22/db005310dba511e89fb606f49a4ee876?url=https://cityofrosedalems.com/
https://www.lecake.com/stat/goto.php?url=https://cityofrosedalems.com/
https://www.im-harz.com/counter/counter.php?url=https://cityofrosedalems.com/
https://pzz.to/click?uid=8571&target_url=https://cityofrosedalems.com/
https://www.ricacorp.com/Ricapih09/redirect.aspx?link=https://cityofrosedalems.com/
https://www.tremblant.ca/Shared/LanguageSwitcher/ChangeCulture?culture=en&url=https://cityofrosedalems.com/
https://www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=https://cityofrosedalems.com/
https://moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://igert2011.videohall.com/to_client?target=https://cityofrosedalems.com/
https://sete.gr/app_plugins/newsletterstudio/pages/tracking/trackclick.aspx?nid=218221206039243162226109144018149003034132019130&e=000220142174231130224127060133189018075115154134&url=https://cityofrosedalems.com/
https://ovatu.com/e/c?url=https://cityofrosedalems.com/
https://primepartners.globalprime.com/afs/wcome.php?c=427|0|1&e=GP204519&url=https://cityofrosedalems.com/
https://ltp.org/home/setlocale?locale=en&returnUrl=https://cityofrosedalems.com/
https://www.morhipo.com/shared/partnercookie?k=gort&url=https://cityofrosedalems.com/
https://www.google.co.jp/url?q=https://cityofrosedalems.com/
https://images.google.de/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?q=https://cityofrosedalems.com/
https://za.zalo.me/v3/verifyv2/pc?token=OcNsmjfpL0XY2F3BtHzNRs4A-hhQ5q5sPXtbk3O&continue=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://cse.google.cz/url?q=https://cityofrosedalems.com/
https://scanmail.trustwave.com/?c=8510&d=4qa02KqxZJadHuhFUvy7ZCUfI_2L10yeH0EeBz7FGQ&u=https://cityofrosedalems.com/
https://images.google.co.kr/url?sa=t&url=https://cityofrosedalems.com/
https://animal.doctorsfile.jp/redirect/?ct=doctor&id=178583&url=https://cityofrosedalems.com/
https://hr.pecom.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://cse.google.ru/url?q=https://cityofrosedalems.com/
https://cse.google.com/url?q=https://cityofrosedalems.com/
https://jump2.bdimg.com/mo/q/checkurl?url=https://cityofrosedalems.com/
https://severeweather.wmo.int/cgi-bin/goto?where=https://cityofrosedalems.com/
https://beam.jpn.org/rank.cgi?mode=link&url=https://cityofrosedalems.com/
https://cse.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://blogranking.fc2.com/out.php?id=414788&url=https://cityofrosedalems.com/
https://wtk.db.com/777554543598768/optout?redirect=https://cityofrosedalems.com/
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=https://cityofrosedalems.com/
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=https://cityofrosedalems.com/
https://images.google.sk/url?q=https://cityofrosedalems.com/
https://images.google.gr/url?q=https://cityofrosedalems.com/
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=https://cityofrosedalems.com/
https://images.google.lv/url?sa=t&url=https://cityofrosedalems.com/
https://m.caijing.com.cn/member/logout?referer=https://cityofrosedalems.com/
https://jamesattorney.agilecrm.com/click?u=https://cityofrosedalems.com/
https://wikimapia.org/external_link?url=https://cityofrosedalems.com/
https://rsv.nta.co.jp/affiliate/set/af100101.aspx?site_id=66108024&redi_url=https://cityofrosedalems.com/
https://adengine.old.rt.ru/go.jsp?to=https://cityofrosedalems.com/
https://thediplomat.com/ads/books/ad.php?i=4&r=https://cityofrosedalems.com/
https://mitsui-shopping-park.com/lalaport/iwata/redirect.html?url=https://cityofrosedalems.com/
https://toolbarqueries.google.lt/url?q=https://cityofrosedalems.com/
https://clients1.google.de/url?q=https://cityofrosedalems.com/
https://clients1.google.es/url?q=https://cityofrosedalems.com/
https://clients1.google.co.uk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.jp/url?q=https://cityofrosedalems.com/
https://clients1.google.fr/url?q=https://cityofrosedalems.com/
https://clients1.google.it/url?q=https://cityofrosedalems.com/
https://clients1.google.com.br/url?q=https://cityofrosedalems.com/
https://clients1.google.co.in/url?q=https://cityofrosedalems.com/
https://clients1.google.ca/url?q=https://cityofrosedalems.com/
https://clients1.google.ru/url?q=https://cityofrosedalems.com/
https://clients1.google.com.hk/url?q=https://cityofrosedalems.com/
https://clients1.google.com.au/url?q=https://cityofrosedalems.com/
https://clients1.google.co.id/url?q=https://cityofrosedalems.com/
https://clients1.google.nl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tw/url?q=https://cityofrosedalems.com/
https://clients1.google.pl/url?q=https://cityofrosedalems.com/
https://clients1.google.be/url?q=https://cityofrosedalems.com/
https://clients1.google.co.th/url?q=https://cityofrosedalems.com/
https://clients1.google.at/url?q=https://cityofrosedalems.com/
https://clients1.google.cz/url?q=https://cityofrosedalems.com/
https://clients1.google.se/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mx/url?q=https://cityofrosedalems.com/
https://clients1.google.ch/url?q=https://cityofrosedalems.com/
https://clients1.google.com.vn/url?q=https://cityofrosedalems.com/
https://clients1.google.pt/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ua/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tr/url?q=https://cityofrosedalems.com/
https://clients1.google.ro/url?q=https://cityofrosedalems.com/
https://clients1.google.com.my/url?q=https://cityofrosedalems.com/
https://clients1.google.gr/url?q=https://cityofrosedalems.com/
https://clients1.google.dk/url?q=https://cityofrosedalems.com/
https://clients1.google.hu/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ar/url?q=https://cityofrosedalems.com/
https://clients1.google.fi/url?q=https://cityofrosedalems.com/
https://clients1.google.co.il/url?q=https://cityofrosedalems.com/
https://clients1.google.co.nz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.za/url?q=https://cityofrosedalems.com/
https://clients1.google.cl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.co/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sg/url?q=https://cityofrosedalems.com/
https://clients1.google.ie/url?q=https://cityofrosedalems.com/
https://clients1.google.sk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.kr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ph/url?q=https://cityofrosedalems.com/
https://clients1.google.no/url?q=https://cityofrosedalems.com/
https://clients1.google.lt/url?q=https://cityofrosedalems.com/
https://clients1.google.bg/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sa/url?q=https://cityofrosedalems.com/
https://clients1.google.hr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pe/url?q=https://cityofrosedalems.com/
https://clients1.google.ae/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ve/url?q=https://cityofrosedalems.com/
https://clients1.google.ee/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pk/url?q=https://cityofrosedalems.com/
https://clients1.google.rs/url?q=https://cityofrosedalems.com/
https://clients1.google.com.eg/url?q=https://cityofrosedalems.com/
https://clients1.google.si/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ec/url?q=https://cityofrosedalems.com/
https://clients1.google.com.qa/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pr/url?q=https://cityofrosedalems.com/
https://clients1.google.mu/url?q=https://cityofrosedalems.com/
https://clients1.google.li/url?q=https://cityofrosedalems.com/
https://clients1.google.lv/url?q=https://cityofrosedalems.com/
https://clients1.google.mn/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gt/url?q=https://cityofrosedalems.com/
https://clients1.google.co.cr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.uy/url?q=https://cityofrosedalems.com/
https://clients1.google.lu/url?q=https://cityofrosedalems.com/
https://clients1.google.ba/url?q=https://cityofrosedalems.com/
https://clients1.google.is/url?q=https://cityofrosedalems.com/
https://clients1.google.dz/url?q=https://cityofrosedalems.com/
https://clients1.google.kg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ke/url?q=https://cityofrosedalems.com/
https://clients1.google.az/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ng/url?q=https://cityofrosedalems.com/
https://clients1.google.com.np/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mt/url?q=https://cityofrosedalems.com/
https://clients1.google.bi/url?q=https://cityofrosedalems.com/
https://clients1.google.by/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bd/url?q=https://cityofrosedalems.com/
https://clients1.google.as/url?q=https://cityofrosedalems.com/
https://clients1.google.com.do/url?q=https://cityofrosedalems.com/
https://clients1.google.kz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ma/url?q=https://cityofrosedalems.com/
https://clients1.google.jo/url?q=https://cityofrosedalems.com/
https://clients1.google.lk/url?q=https://cityofrosedalems.com/
https://clients1.google.com.cu/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ai/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gi/url?q=https://cityofrosedalems.com/
https://clients1.google.cf/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ni/url?q=https://cityofrosedalems.com/
https://clients1.google.md/url?q=https://cityofrosedalems.com/
https://clients1.google.mg/url?q=https://cityofrosedalems.com/
https://clients1.google.la/url?q=https://cityofrosedalems.com/
https://clients1.google.com.jm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.vc/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tj/url?q=https://cityofrosedalems.com/
https://clients1.google.com.cy/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sv/url?q=https://cityofrosedalems.com/
https://clients1.google.rw/url?q=https://cityofrosedalems.com/
https://clients1.google.com.om/url?q=https://cityofrosedalems.com/
https://clients1.google.ps/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bo/url?q=https://cityofrosedalems.com/
https://clients1.google.tk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.mz/url?q=https://cityofrosedalems.com/
https://clients1.google.bs/url?q=https://cityofrosedalems.com/
https://clients1.google.mk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.bw/url?q=https://cityofrosedalems.com/
https://clients1.google.al/url?q=https://cityofrosedalems.com/
https://clients1.google.sm/url?q=https://cityofrosedalems.com/
https://clients1.google.co.zw/url?q=https://cityofrosedalems.com/
https://clients1.google.tm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bh/url?q=https://cityofrosedalems.com/
https://clients1.google.com.af/url?q=https://cityofrosedalems.com/
https://clients1.google.com.fj/url?q=https://cityofrosedalems.com/
https://clients1.google.com.kh/url?q=https://cityofrosedalems.com/
https://clients1.google.cg/url?q=https://cityofrosedalems.com/
https://clients1.google.ki/url?q=https://cityofrosedalems.com/
https://clients1.google.mw/url?q=https://cityofrosedalems.com/
https://clients1.google.com.kw/url?q=https://cityofrosedalems.com/
https://clients1.google.bf/url?q=https://cityofrosedalems.com/
https://clients1.google.com.lb/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ls/url?q=https://cityofrosedalems.com/
https://clients1.google.ms/url?q=https://cityofrosedalems.com/
https://clients1.google.ci/url?q=https://cityofrosedalems.com/
https://clients1.google.dm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sb/url?q=https://cityofrosedalems.com/
https://clients1.google.co.vi/url?q=https://cityofrosedalems.com/
https://clients1.google.so/url?q=https://cityofrosedalems.com/
https://clients1.google.nu/url?q=https://cityofrosedalems.com/
https://clients1.google.dj/url?q=https://cityofrosedalems.com/
https://clients1.google.hn/url?q=https://cityofrosedalems.com/
https://clients1.google.nr/url?q=https://cityofrosedalems.com/
https://clients1.google.co.tz/url?q=https://cityofrosedalems.com/
https://clients1.google.mv/url?q=https://cityofrosedalems.com/
https://clients1.google.tn/url?q=https://cityofrosedalems.com/
https://clients1.google.sc/url?q=https://cityofrosedalems.com/
https://clients1.google.com.py/url?q=https://cityofrosedalems.com/
https://clients1.google.sn/url?q=https://cityofrosedalems.com/
https://clients1.google.am/url?q=https://cityofrosedalems.com/
https://clients1.google.ad/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gh/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bz/url?q=https://cityofrosedalems.com/
https://clients1.google.iq/url?q=https://cityofrosedalems.com/
https://clients1.google.to/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bn/url?q=https://cityofrosedalems.com/
https://clients1.google.cat/url?q=https://cityofrosedalems.com/
https://clients1.google.sh/url?q=https://cityofrosedalems.com/
https://clients1.google.cm/url?q=https://cityofrosedalems.com/
https://clients1.google.gg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ug/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ly/url?q=https://cityofrosedalems.com/
https://clients1.google.co.uz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.zm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.na/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ag/url?q=https://cityofrosedalems.com/
https://clients1.google.me/url?q=https://cityofrosedalems.com/
https://clients1.google.cd/url?q=https://cityofrosedalems.com/
https://clients1.google.fm/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ck/url?q=https://cityofrosedalems.com/
https://clients1.google.com.et/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pa/url?q=https://cityofrosedalems.com/
https://clients1.google.je/url?q=https://cityofrosedalems.com/
https://clients1.google.gl/url?q=https://cityofrosedalems.com/
https://clients1.google.ge/url?q=https://cityofrosedalems.com/
https://clients1.google.ht/url?q=https://cityofrosedalems.com/
https://clients1.google.im/url?q=https://cityofrosedalems.com/
https://clients1.google.gy/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sl/url?q=https://cityofrosedalems.com/
https://clients1.google.bj/url?q=https://cityofrosedalems.com/
https://clients1.google.ml/url?q=https://cityofrosedalems.com/
https://clients1.google.cv/url?q=https://cityofrosedalems.com/
https://clients1.google.tl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mm/url?q=https://cityofrosedalems.com/
https://clients1.google.ga/url?q=https://cityofrosedalems.com/
https://clients1.google.bt/url?q=https://cityofrosedalems.com/
https://clients1.google.ac/url?q=https://cityofrosedalems.com/
https://clients1.google.st/url?q=https://cityofrosedalems.com/
https://clients1.google.td/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ao/url?q=https://cityofrosedalems.com/
https://clients1.google.gp/url?q=https://cityofrosedalems.com/
https://clients1.google.com.nf/url?q=https://cityofrosedalems.com/
https://clients1.google.ne/url?q=https://cityofrosedalems.com/
https://clients1.google.pn/url?q=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://ipv4.google.com/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://cse.google.com/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com/url?q=https://cityofrosedalems.com/
https://cse.google.com/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.com.om/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.com.om/url?q=https://cityofrosedalems.com/
https://images.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.mn/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.mn/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.mn/url?q=https://cityofrosedalems.com/
https://cse.google.mn/url?sa=i&url=https://cityofrosedalems.com/
https://www.google.mn/url?q=https://cityofrosedalems.com/
https://maps.google.dz/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.kg/url?q=https://cityofrosedalems.com/
https://www.google.kg/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.iq/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.iq/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.co.uz/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.dz/url?q=https://cityofrosedalems.com/
https://images.google.dz/url?q=https://cityofrosedalems.com/
https://images.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.ga/url?q=https://cityofrosedalems.com/
https://images.google.nu/url?q=https://cityofrosedalems.com/
https://images.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.nu/url?q=https://cityofrosedalems.com/
https://www.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.kg/url?q=https://cityofrosedalems.com/
https://maps.google.sc/url?q=https://cityofrosedalems.com/
https://maps.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.sc/url?q=https://cityofrosedalems.com/
https://maps.google.iq/url?q=https://cityofrosedalems.com/
https://images.google.ki/url?q=https://cityofrosedalems.com/
https://images.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.bt/url?q=https://cityofrosedalems.com/
https://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.bt/url?q=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://asia.google.com/url?q=https://cityofrosedalems.com/
http://www.macro.ua/out.php?link=https://cityofrosedalems.com/
http://www.aurki.com/jarioa/redirect?id_feed=510&url=https://cityofrosedalems.com/
http://www.cnainterpreta.it/redirect.asp?url=https://cityofrosedalems.com/
http://www.site-navi.net/sponavi/rank.cgi?mode=link&id=890&url=https://cityofrosedalems.com/
https://www.mattias.nu/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
http://teenstgp.us/cgi-bin/out.cgi?u=https://cityofrosedalems.com/
http://congovibes.com/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.humaniplex.com/jscs.html?hj=y&ru=https://cityofrosedalems.com/
http://www.qlt-online.de/cgi-bin/click/clicknlog.pl?link=https://cityofrosedalems.com/
http://ww.sdam-snimu.ru/redirect.php?url=https://cityofrosedalems.com/
http://www.uktrademarkregistration.co.uk/JumpTo.aspx?url=https://cityofrosedalems.com/
http://fosteringsuccessmichigan.com/?URL=https://cityofrosedalems.com/
http://fotos24.org/url?q=https://cityofrosedalems.com/
http://fouillez-tout.com/cgi-bin/redirurl.cgi?https://cityofrosedalems.com/
http://frag-den-doc.de/index.php?s=verlassen&url=https://cityofrosedalems.com/
http://freethailand.com/goto.php?url=https://cityofrosedalems.com/
http://freshcannedfrozen.ca/?URL=https://cityofrosedalems.com/
http://funkhouse.de/url?q=https://cityofrosedalems.com/
http://ga.naaar.nl/link/?url=https://cityofrosedalems.com/
http://gb.poetzelsberger.org/show.php?c453c4=https://cityofrosedalems.com/
http://getmethecd.com/?URL=https://cityofrosedalems.com/
http://goldankauf-oberberg.de/out.php?link=https://cityofrosedalems.com/
http://distributors.hrsprings.com/?URL=https://cityofrosedalems.com/
http://dorf-v8.de/url?q=https://cityofrosedalems.com/
http://doverwx.com/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://dr-guitar.de/quit.php?url=https://cityofrosedalems.com/
http://drdrum.biz/quit.php?url=https://cityofrosedalems.com/
http://ds-media.info/url?q=https://cityofrosedalems.com/
http://excitingperformances.com/?URL=https://cityofrosedalems.com/
http://familie-huettler.de/link.php?link=https://cityofrosedalems.com/
http://forum.vizslancs.hu/lnks.php?uid=net&url=https://cityofrosedalems.com/
http://forum.wonaruto.com/redirection.php?redirection=https://cityofrosedalems.com/
https://www.fairsandfestivals.net/?URL=https://cityofrosedalems.com/
http://chatx2.whocares.jp/redir.jsp?u=https://cityofrosedalems.com/
http://chuanroi.com/Ajax/dl.aspx?u=https://cityofrosedalems.com/
http://club.dcrjs.com/link.php?url=https://cityofrosedalems.com/
http://conny-grote.de/url?q=https://cityofrosedalems.com/
http://crazyfrag91.free.fr/?URL=https://cityofrosedalems.com/
http://crewe.de/url?q=https://cityofrosedalems.com/
http://cwa4100.org/uebimiau/redir.php?https://cityofrosedalems.com/
http://d-quintet.com/i/index.cgi?id=1&mode=redirect&no=494&ref_eid=33&url=https://cityofrosedalems.com/
http://data.allie.dbcls.jp/fct/rdfdesc/usage.vsp?g=https://cityofrosedalems.com/
http://data.linkedevents.org/describe/?url=https://cityofrosedalems.com/
http://dayviews.com/externalLinkRedirect.php?url=https://cityofrosedalems.com/
http://db.cbservices.org/cbs.nsf/forward?openform&https://cityofrosedalems.com/
http://simvol-veri.ru/xp/?goto=https://cityofrosedalems.com/
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=https://cityofrosedalems.com/
https://stroim100.ru/redirect?url=https://cityofrosedalems.com/
https://kakaku-navi.net/items/detail.php?url=https://cityofrosedalems.com/
http://www.paladiny.ru/go.php?url=https://cityofrosedalems.com/
http://tido.al/vazhdo.php?url=https://cityofrosedalems.com/
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=https://cityofrosedalems.com/
https://www.usjournal.com/go.php?campusID=190&url=https://cityofrosedalems.com/
https://temptationsaga.com/buy.php?url=https://cityofrosedalems.com/
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=https://cityofrosedalems.com/
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=https://cityofrosedalems.com/
https://spb-medcom.ru/redirect.php?https://cityofrosedalems.com/
https://www.info-realty.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.sermemole.com/public/serbook/redirect.php?url=https://cityofrosedalems.com/
https://www.hradycz.cz/redir.php?b=445&t=https://cityofrosedalems.com/
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=https://cityofrosedalems.com/
https://uk.kindofbook.com/redirect.php/?red=https://cityofrosedalems.com/
http://m.17ll.com/apply/tourl/?url=https://cityofrosedalems.com/
http://auyttrv.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bartos.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://cllfather.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://littlejohnny.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://semesmemos.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://faultypirations.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mmurugesamnfo.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://katihfmaxtron.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ikkemandar.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://googlejfgdlenewstoday.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bhrecadominicana.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://anupam-bestprice89.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bhrepublica.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://allinspirations.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://weallfreieds.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://shanmegurad.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://manualmfuctional.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://verybeayurifull.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://prasat.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://meri.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://prabu.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://easehipranaam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://nidatyi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://krodyit.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://naine.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://breajwasi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://beithe.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://allthingnbeyondblog.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://usafunworldt.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://financialallorner.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mjghouthernmatron.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://chandancomputers.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bingshopping.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ptritam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tech-universes.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://littleboy.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://correctinspiration.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://esujkamien.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ujkaeltnx.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ausnangck2809.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://supermu.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://buyfcyclingteam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tumari.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://hariomm.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://lejano.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://jotumko.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bestandroid.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://discoverable.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://puriagatratt.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://azizlemon.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ptrfsiitan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://boardgame-breking.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://flowwergulab.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bingcreater-uk.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://skinnyskin.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://amil.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mohan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://dilip.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://avinasg.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://virat.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://hsadyttk.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ashu-quality.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tinko.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://konkaruna.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://udavhav.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://matura.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://patana.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bopal.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://gwl.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://delhi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://gopi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://kripa.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://charanraj.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://pream.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://sang.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://pasas.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://kanchan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://assadaaka.nl/?URL=https://cityofrosedalems.com/
http://atchs.jp/jump?u=https://cityofrosedalems.com/
http://away3d.com/?URL=https://cityofrosedalems.com/
http://blackberryvietnam.net/proxy.php?link=https://cityofrosedalems.com/
http://boogiewoogie.com/?URL=https://cityofrosedalems.com/
http://bsumzug.de/url?q=https://cityofrosedalems.com/
http://business.eatonton.com/list?globaltemplate=https://cityofrosedalems.com/
http://archives.midweek.com/?URL=https://cityofrosedalems.com/
http://armdrag.com/mb/get.php?url=https://cityofrosedalems.com/
http://asai-kota.com/acc/acc.cgi?REDIRECT=https://cityofrosedalems.com/
http://ass-media.de/wbb2/redir.php?url=https://cityofrosedalems.com/
http://cdiabetes.com/redirects/offer.php?URL=https://cityofrosedalems.com/
https://padletcdn.com/cgi/fetch?disposition=attachment&url=https://cityofrosedalems.com/
http://cdn.iframe.ly/api/iframe?url=https://cityofrosedalems.com/
http://centuryofaction.org/?URL=https://cityofrosedalems.com/
http://Somewh.a.T.dfqw@www.newsdiffs.org/article-history/www.findabeautyschool.com/map.aspx?url=https://cityofrosedalems.com/
http://actontv.org/?URL=https://cityofrosedalems.com/
http://bigbarganz.com/g/?https://cityofrosedalems.com/
http://andreasgraef.de/url?q=https://cityofrosedalems.com/
http://app.espace.cool/ClientApi/SubscribeToCalendar/1039?url=https://cityofrosedalems.com/
http://archive.paulrucker.com/?URL=https://cityofrosedalems.com/
http://baseballpodcasts.net/Feed2JS/feed2js.php?src=https://cityofrosedalems.com/
http://bbs.mottoki.com/index?bbs=hpsenden&act=link&page=94&linkk=https://cityofrosedalems.com/
http://bernhardbabel.com/url?q=https://cityofrosedalems.com/
http://albertaaerialapplicators.com/?URL=https://cityofrosedalems.com/
http://alexanderroth.de/url?q=https://cityofrosedalems.com/
http://alt.baunetzwissen.de/rd/nl-track/?obj=bnw&cg1=redirect&cg2=wissen-newsletter:beton&cg3=partner&datum=2017-01&titel=partnerklick-beton&firma=informationszentrumbeton&link=https://cityofrosedalems.com/
https://duckduckgo.com/?q=https%3A%2F%2Fspo1top.com&t=h_&ia=web
https://www.google.com/search?q=https%3A%2F%2Fspo1top.com&sca_esv=567933587&sxsrf=AM9HkKkLNk4hFHvgRG-dTHcW476TZakh3Q%3A1695527512326&ei=WLIPZZPIE66t4-EPwYOS4AE&ved=0ahUKEwiT1NCYrMKBAxWu1jgGHcGBBBwQ4dUDCBA&uact=5&oq=https%3A%2F%2Fspo1top.com&gs_lp=Egxnd3Mtd2l6LXNlcnAiE2h0dHBzOi8vc3BvMXRvcC5jb20yBBAjGCdI2x9QkAtY2xpwAXgBkAEAmAGHAaABygeqAQMwLji4AQPIAQD4AQHCAgoQABhHGNYEGLAD4gMEGAAgQYgGAZAGBg&sclient=gws-wiz-serp
http://client.paltalk.com/client/webapp/client/External.wmt?url=https://cityofrosedalems.com/
http://community.nxp.com/t5/custom/page/page-id/ExternalRedirect?url=https://cityofrosedalems.com/
http://foro.infojardin.com/proxy.php?link=https://cityofrosedalems.com/
http://ceskapozice.lidovky.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mitsui-shopping-park.com/lalaport/ebina/redirect.html?url=https://cityofrosedalems.com/
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=https://cityofrosedalems.com/
http://fishbiz.seagrant.uaf.edu/click-thru.html?url=https://cityofrosedalems.com/
http://kupiauto.zr.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://ipx.bcove.me/?url=https://cityofrosedalems.com/
https://toolbarqueries.google.td/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
https://home.guanzhuang.org/link.php?url=https://cityofrosedalems.com/
http://dvd24online.de/url?q=https://cityofrosedalems.com/
http://twindish-electronics.de/url?q=https://cityofrosedalems.com/
http://www.beigebraunapartment.de/url?q=https://cityofrosedalems.com/
http://www.mix-choice.com/yomi/rank.cgi?mode=link&id=391&url=https://cityofrosedalems.com/
https://cse.google.fm/url?q=https://cityofrosedalems.com/
http://www.1soft-tennis.com/search/rank.cgi?mode=link&id=17&url=https://cityofrosedalems.com/
http://big-data-fr.com/linkedin.php?lien=https://cityofrosedalems.com/
http://reg.kost.ru/cgi-bin/go?https://cityofrosedalems.com/
http://www.kirstenulrich.de/url?q=https://cityofrosedalems.com/
http://www.inkwell.ru/redirect/?url=https://cityofrosedalems.com/
https://cse.google.co.je/url?q=https://cityofrosedalems.com/
http://click-navi.jp/cgi/service-search/rank.cgi?mode=link&id=121&url=https://cityofrosedalems.com/
https://n1653.funny.ge/redirect.php?url=https://cityofrosedalems.com/
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=https://cityofrosedalems.com/
http://maps.google.com/url?q=https://cityofrosedalems.com/
http://maps.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://plus.google.com/url?q=https://cityofrosedalems.com/
http://cse.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://clients1.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
http://forum.solidworks.com/external-link.jspa?url=https://cityofrosedalems.com/
http://www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
https://www.google.to/url?q=https://cityofrosedalems.com/
https://maps.google.bi/url?q=https://cityofrosedalems.com/
http://www.nickl-architects.com/url?q=https://cityofrosedalems.com/
https://www.ocbin.com/out.php?url=https://cityofrosedalems.com/
http://www.lobenhausen.de/url?q=https://cityofrosedalems.com/
https://image.google.bs/url?q=https://cityofrosedalems.com/
http://redirect.me/?https://cityofrosedalems.com/
http://kank.o.oo7.jp/cgi-bin/ys4/rank.cgi?mode=link&id=569&url=https://cityofrosedalems.com/
http://ruslog.com/forum/noreg.php?https://cityofrosedalems.com/
http://forum.ahigh.ru/away.htm?link=https://cityofrosedalems.com/
http://www.wildner-medien.de/url?q=https://cityofrosedalems.com/
http://www.tifosy.de/url?q=https://cityofrosedalems.com/
https://image.google.co.ck/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
http://ads.icorp.ro/others/STS/?t=CeNortjKxUjK0NDFVsgZcMBADAm4-&g=https://cityofrosedalems.com/
http://deai-ranking.org/search/rank.cgi?mode=link&id=28&url=https://cityofrosedalems.com/
https://izispicy.com/go.php?url=https://cityofrosedalems.com/
http://demo.vieclamcantho.vn/baohiemthatnghiep/Redirect.aspx?sms=90bb20bb20tbb20thc3%B4ng&link=https://cityofrosedalems.com/
http://www.city-fs.de/url?q=https://cityofrosedalems.com/
http://p.profmagic.com/urllink.php?url=https://cityofrosedalems.com/
https://www.google.md/url?q=https://cityofrosedalems.com/
https://maps.google.com.pa/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
http://www.arndt-am-abend.de/url?q=https://cityofrosedalems.com/
https://maps.google.com.vc/url?q=https://cityofrosedalems.com/
http://maxnetworks.org/searchlink/rank.cgi?mode=link&id=321&url=https://cityofrosedalems.com/
https://image.google.com.ng/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://www.kenzai-navi.com/location/location_topbanner.php?bs=238&m=899&href=https://cityofrosedalems.com/
https://www.anybeats.jp/jump/?https://cityofrosedalems.com/
http://url-collector.appspot.com/positiveVotes?topic=Hate%20speech&url=https://cityofrosedalems.com/
https://cse.google.com.cy/url?q=https://cityofrosedalems.com/
https://maps.google.be/url?sa=j&url=https://cityofrosedalems.com/
https://top.hange.jp/linkdispatch/dispatch?targetUrl=https://cityofrosedalems.com/
http://www.link.gokinjyo-eikaiwa.com/rank.cgi?mode=link&id=5&url=https://cityofrosedalems.com/
http://www.air-dive.com/au/mt4i.cgi?mode=redirect&ref_eid=697&url=https://cityofrosedalems.com/
http://okozukai.j-web.jp/j-web/okozukai/ys4/rank.cgi?mode=link&id=6817&url=https://cityofrosedalems.com/
https://sfmission.com/rss/feed2js.php?src=https://cityofrosedalems.com/
https://www.watersportstaff.co.uk/extern.aspx?src=https://cityofrosedalems.com/&cu=60096&page=1&t=1&s=42"
http://siamcafe.net/board/go/go.php?https://cityofrosedalems.com/
http://www.whitening-navi.info/cgi/search-smartphone/rank.cgi?mode=link&id=1431&url=https://cityofrosedalems.com/
http://www.youtube.com/redirect?q=https://cityofrosedalems.com/
http://www.youtube.com/redirect?event=channeldescription&q=https://cityofrosedalems.com/
http://www.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://go.e-frontier.co.jp/rd2.php?uri=https://cityofrosedalems.com/
http://adchem.net/Click.aspx?url=https://cityofrosedalems.com/
http://www.reddotmedia.de/url?q=https://cityofrosedalems.com/
https://10ways.com/fbredir.php?orig=https://cityofrosedalems.com/
https://emailtrackerapi.leadforensics.com/api/URLOpen?EmailSentRecordID=17006&URL=https://cityofrosedalems.com/
http://search.haga-f.net/rank.cgi?mode=link&url=https://cityofrosedalems.com/
http://7ba.ru/out.php?url=https://cityofrosedalems.com/
http://www.51queqiao.net/link.php?url=https://cityofrosedalems.com/
https://cse.google.dm/url?q=https://cityofrosedalems.com/
https://rsyosetsu.bookmarks.jp/ys4/rank.cgi?mode=link&id=3519&url=https://cityofrosedalems.com/
https://ulfishing.ru/forum/go.php?https://cityofrosedalems.com/
https://underwood.ru/away.html?url=https://cityofrosedalems.com/
https://unicom.ru/links.php?go=https://cityofrosedalems.com/
http://www.unifin.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://uogorod.ru/feed/520?redirect=https://cityofrosedalems.com/
http://uvbnb.ru/go?https://cityofrosedalems.com/
https://skibaza.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://smartservices.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.topkam.ru/gtu/?url=https://cityofrosedalems.com/
https://torggrad.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://torgi-rybinsk.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://tpprt.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://clients1.google.al/url?q=https://cityofrosedalems.com/
https://cse.google.al/url?q=https://cityofrosedalems.com/
https://images.google.al/url?q=https://cityofrosedalems.com/
http://toolbarqueries.google.al/url?q=https://cityofrosedalems.com/
https://www.google.al/url?q=https://cityofrosedalems.com/
https://www.snek.ai/redirect?url=https://cityofrosedalems.com/
http://gals.graphis.ne.jp/mkr/out.cgi?id=01019&go=https://cityofrosedalems.com/
http://www.capitalbikepark.se/bok/go.php?url=https://cityofrosedalems.com/
http://cooltgp.org/tgp/click.php?id=370646&u=https://cityofrosedalems.com/
https://joomlinks.org/?url=https://cityofrosedalems.com/
https://managementportal.de/modules/mod_jw_srfr/redir.php?url=https://cityofrosedalems.com/
http://www.green-cross.pro/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://m.adlf.jp/jump.php?l=https://cityofrosedalems.com/
https://www.moonbbs.com/dm/dmlink.php?dmurl=https://cityofrosedalems.com/
http://www.mac52ipod.cn/urlredirect.php?go=https://cityofrosedalems.com/
http://chrison.net/ct.ashx?id=dad2849a-8862-4a6d-b842-ef628cbfd3c3&url=https://cityofrosedalems.com/
http://datasheetcatalog.biz/url.php?url=https://cityofrosedalems.com/
http://minlove.biz/out.html?id=nhmode&go=https://cityofrosedalems.com/
http://www.diwaxx.ru/win/redir.php?redir=https://cityofrosedalems.com/
http://request-response.com/blog/ct.ashx?url=https://cityofrosedalems.com/
https://turbazar.ru/url/index?url=https://cityofrosedalems.com/
http://members.asoa.org/sso/logout.aspx?returnurl=https://cityofrosedalems.com/
http://login.mediafort.ru/autologin/mail/?code=14844×02ef859015x290299&url=https://cityofrosedalems.com/
https://www.sports-central.org/cgi-bin/axs/ax.pl?https://cityofrosedalems.com/
http://dot.boss.sk/o/rs.php?ssid=1&szid=4&dsid=12&dzid=32&url=https://cityofrosedalems.com/
http://www.americanstylefridgefreezer.co.uk/go.php?url=https://cityofrosedalems.com/
https://www.newzealandgirls.co.nz/auckland/escorts/clicks.php?click_id=NavBar_AF_AdultDirectory&target=https://cityofrosedalems.com/
https://wdesk.ru/go?https://cityofrosedalems.com/
http://edcommunity.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://page.yicha.cn/tp/j?url=https://cityofrosedalems.com/
http://www.internettrafficreport.com/cgi-bin/cgirdir.exe?https://cityofrosedalems.com/
http://www.interfacelift.com/goto.php?url=https://cityofrosedalems.com/
https://good-surf.ru/r.php?g=https://cityofrosedalems.com/
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=https://cityofrosedalems.com/
https://gektor-nsk.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://lekoufa.ru/banner/go?banner_id=4&link=https://cityofrosedalems.com/
https://forsto.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://cityofrosedalems.com/
http://www.gigatran.ru/go?url=https://cityofrosedalems.com/
http://www.gigaalert.com/view.php?h=&s=https://cityofrosedalems.com/
http://www.jkes.tyc.edu.tw/dyna/webs/gotourl.php?id=357&url=https://cityofrosedalems.com/
http://www.laopinpai.com/gourl.asp?url=/gourl.asp?url=https://cityofrosedalems.com/
http://www.humanbrainmapping.org/i4a/etrack/track.cfm?rType=2&campaignID=3572&contactID=4524&origURL=https://cityofrosedalems.com/
https://gcup.ru/go?https://cityofrosedalems.com/
http://www.gearguide.ru/phpbb/go.php?https://cityofrosedalems.com/
https://es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
https://passport.sfacg.com/LoginOut.aspx?Returnurl=https://cityofrosedalems.com/
https://offers.sidex.ru/stat_ym_new.php?redir=https://cityofrosedalems.com/&hash=1577762
https://globalmedia51.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://ramset.com.au/document/url/?url=https://cityofrosedalems.com/
https://www.vicsport.com.au/analytics/outbound?url=https://cityofrosedalems.com/
http://www.cyprus-net.com/banner_click.php?banid=4&link=https://cityofrosedalems.com/
http://a.gongkong.com/db/adredir.asp?id=16757&url=https://cityofrosedalems.com/
http://gfaq.ru/go?https://cityofrosedalems.com/
http://gbi-12.ru/links.php?go=https://cityofrosedalems.com/
http://www.global-flat.com/mobile/mobile_switch.php?dir=tofull&url=https://cityofrosedalems.com/
https://golden-resort.ru/out.php?out=https://cityofrosedalems.com/
http://avalon.gondor.ru/away.php?link=https://cityofrosedalems.com/
http://www.laosubenben.com/home/link.php?url=https://cityofrosedalems.com/
http://www.lifeshow.com.tw/show.php?ty5_id=1599&url=https://cityofrosedalems.com/
http://games.cheapdealuk.co.uk/go.php?url=https://cityofrosedalems.com/
https://www.netwalkerstore.com/redirect.asp?accid=18244&adcampaignid=2991&adcampaigntype=2&affduration=30&url=https://cityofrosedalems.com/
http://tsugarubrand.jp/blog/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://blackhistorydaily.com/black_historylinks/link.asp?linkid=5&URL=https://cityofrosedalems.com/
http://www.kitchencabinetsdirectory.com/redirect.asp?url=https://cityofrosedalems.com/
http://cityprague.ru/go.php?go=https://cityofrosedalems.com/
https://www.hachimantaishi.com/click3/click3.cgi?cnt=c5&url=https://cityofrosedalems.com/
http://earnupdates.com/goto.php?url=https://cityofrosedalems.com/
http://www.careerbuilder.com.cn/cn/tallyredirector.aspx?task=Ushi&objName=SnsShare&redirectUrl=https://cityofrosedalems.com/&methodName=SetSnsShareLink
http://banners.knollenstein.com/adnetwork/servlet/advertBeans.TrackingServlet?seid=27709655&t=100&redirectTo=https://cityofrosedalems.com/&cid=DECONL&vid=986809&externalsource=jobbsquareppc
https://meyeucon.org/ext-click.php?url=https://cityofrosedalems.com/
http://www.cnmhe.fr/spip.php?action=cookie&url=https://cityofrosedalems.com/
https://perezvoni.com/blog/away?url=https://cityofrosedalems.com/
https://www.ciymca.org/af/register-redirect/71104?url=https://cityofrosedalems.com/
https://whizpr.nl/tracker.php?u=https://cityofrosedalems.com/
http://bw.irr.by/knock.php?bid=252583&link=https://cityofrosedalems.com/
http://www.beeicons.com/redirect.php?site=https://cityofrosedalems.com/
http://www.diwaxx.ru/hak/redir.php?redir=https://cityofrosedalems.com/
http://www.actuaries.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMVFIELD]EMAIL[EMV/FIELD]&cat=Techniquesculturales&url=https://cityofrosedalems.com/
https://www.jukujo.gs/bin/out.cgi?id=kimiomof&url=https://cityofrosedalems.com/
http://hansolav.net/blog/ct.ashx?id=0af6301b-e71f-44be-838f-905709eee792&url=https://cityofrosedalems.com/
http://bitrix.adlr.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://m.snek.ai/redirect?url=https://cityofrosedalems.com/
http://www.officeservice.com.br/trackMKT/trackerFolha.aspx?DESCRIPTION=HotSite_EcrmContabil&FROM=Workshop&DESTINATION=https://cityofrosedalems.com/
https://www.arbsport.ru/gotourl.php?url=https://cityofrosedalems.com/
http://www.strana.co.il/finance/redir.aspx?site=https://cityofrosedalems.com/
https://www.tourplanisrael.com/redir/?url=https://cityofrosedalems.com/
http://www.mutualgravity.com/archangel/webcontact/d_signinsimple.php?action=signup&CID=240&EID=&S=default.css&return=https://cityofrosedalems.com/
http://buildingreputation.com/lib/exe/fetch.php?media=https://cityofrosedalems.com/
http://gals.graphis.ne.jp/mkr/out.cgi?id=04489&go=https://cityofrosedalems.com/
http://dir.tetsumania.net/search/rank.cgi?mode=link&id=3267&url=https://cityofrosedalems.com/
http://www.project24.info/mmview.php?dest=https://cityofrosedalems.com/
http://tfads.testfunda.com/TFServeAds.aspx?strTFAdVars=4a086196-2c64-4dd1-bff7-aa0c7823a393,TFvar,00319d4f-d81c-4818-81b1-a8413dc614e6,TFvar,GYDH-Y363-YCFJ-DFGH-5R6H,TFvar,https://cityofrosedalems.com/
http://www.request-response.com/blog/ct.ashx?id=d827b163-39dd-48f3-b767-002147c94e05&url=https://cityofrosedalems.com/
http://www.cooltgp.org/tgp/click.php?id=370646&u=https://cityofrosedalems.com/
http://sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=785&redirect=https://cityofrosedalems.com/
http://www.kyoto-osaka.com/search/rank.cgi?mode=link&url=https://cityofrosedalems.com/
http://metalist.co.il/redirect.asp?url=https://cityofrosedalems.com/
http://i-marine.eu/pages/goto.aspx?link=https://cityofrosedalems.com/
http://www.teenlove.biz/cgibin/atc/out.cgi?id=24&u=https://cityofrosedalems.com/
http://www.altoona.com/newsletter/click.php?volume=52&url=https://cityofrosedalems.com/
http://www.katjushik.ru/link.php?to=https://cityofrosedalems.com/
http://gondor.ru/go.php?url=https://cityofrosedalems.com/
http://proekt-gaz.ru/go?https://cityofrosedalems.com/
https://www.tricitiesapartmentguide.com/MobileDefault.aspx?reff=https://cityofrosedalems.com/
http://www.vietnamsingle.com/vn/banners/redirect.asp?url=https://cityofrosedalems.com/
https://www.voxlocalis.net/enlazar/?url=https://cityofrosedalems.com/
http://no-smok.net/nsmk/InterWiki?action=goto&oe=EUC-KR&url=https://cityofrosedalems.com/
http://www.stalker-modi.ru/go?https://cityofrosedalems.com/
http://www.p1-uranai.com/rank.cgi?mode=link&id=538&url=https://cityofrosedalems.com/
http://earthsciencescanada.com/modules/babel/redirect.php?newlang=en_us&newurl=https://cityofrosedalems.com/
http://psykodynamiskt.nu/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
http://www.pcbheaven.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.castellodivezio.it/lingua.php?lingua=EN&url=https://cityofrosedalems.com/
http://moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.fairpoint.net/~jensen1242/gbook/go.php?url=https://cityofrosedalems.com/
https://lirasport.com.ar/bloques/bannerclick.php?id=7&url=https://cityofrosedalems.com/
http://www.knabstrupper.se/guestbook/go.php?url=https://cityofrosedalems.com/
https://www.pba.ph/redirect?url=https://cityofrosedalems.com/&id=3&type=tab
https://bondage-guru.net/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.westbloomfieldlibrary.org/includes/statistics.php?StatType=Link&StatID=Facebook&weblink=https://cityofrosedalems.com/
https://tenisnews.com.br/clickTracker/clickTracker.php?u=https://cityofrosedalems.com/
http://www.stevestechspot.com/ct.ashx?id=9fdcf4a4-6e09-4807-bc31-ac1adf836f6c&url=https://cityofrosedalems.com/
http://rcoi71.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://www.laselection.net/redir.php3?cat=int&url=https://cityofrosedalems.com/
http://www.masai-mara.com/cgi-bin/link2.pl?grp=mm&link=https://cityofrosedalems.com/
http://evenemangskalender.se/redirect/?id=15723&lank=https://cityofrosedalems.com/
http://anifre.com/out.html?go=https://cityofrosedalems.com/
http://www.restavracije-gostilne.si/banner.php?id=44&url=https://cityofrosedalems.com/
http://hobbyplastic.co.uk/trigger.php?r_link=https://cityofrosedalems.com/
http://ads.pukpik.com/myads/click.php?banner_id=316&banner_url=https://cityofrosedalems.com/
https://www.adminer.org/redirect/?sa=t&url=https://cityofrosedalems.com/
http://www.bigblackmamas.com/cgi-bin/sites/out.cgi?url=https://cityofrosedalems.com/
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=https://cityofrosedalems.com/
https://primorye.ru/go.php?id=19&url=https://cityofrosedalems.com/
https://www.123gomme.it/it/ViewSwitcher/SwitchView?mobile=True&returnUrl=https://cityofrosedalems.com/
http://youmydaddy.com/cgi-bin/at3/out.cgi?id=88&trade=https://cityofrosedalems.com/
https://naviking.localking.com.tw/about/redirect.aspx?mid=7&url=https://cityofrosedalems.com/
http://sms-muzeji.si/Home/ChangeCulture?lang=sl&returnUrl=https://cityofrosedalems.com/
http://r.emeraldexpoinfo.com/s.ashx?ms=EXI2:125462_115115&e=krubin723@aol.com&eId=440186886&c=h&url=https://cityofrosedalems.com/
https://apps.firstrain.com/service/openurl.jsp?action=titleclick&src=rss&u=https://cityofrosedalems.com/
http://cdp.thegoldwater.com/click.php?id=101&url=https://cityofrosedalems.com/
http://www.appenninobianco.it/ads/adclick.php?bannerid=159&zoneid=8&source=&dest=https://cityofrosedalems.com/
https://www.eduplus.hk/special/emailalert/goURL.jsp?clickURL=https://cityofrosedalems.com/
http://www.japan.road.jp/navi/navi.cgi?jump=226&url=https://cityofrosedalems.com/
http://www.arch.iped.pl/artykuly.php?id=1&cookie=1&url=https://cityofrosedalems.com/
http://hirlevelcenter.eu/click.php?hirlevel_id=13145441085508&url=https://cityofrosedalems.com/
https://birds.cz/avif/redirect.php?from=avif.obs.php&url=https://cityofrosedalems.com/
http://www.bigblackbootywatchers.com/cgi-bin/sites/out.cgi?url=https://cityofrosedalems.com/
http://barykin.com/go.php?https://cityofrosedalems.com/
https://forum.everleap.com/proxy.php?link=https://cityofrosedalems.com/
http://www.mosig-online.de/url?q=https://cityofrosedalems.com/
http://www.hccincorporated.com/?URL=https://cityofrosedalems.com/
http://fatnews.com/?URL=https://cityofrosedalems.com/
https://csirealty.com/?URL=https://cityofrosedalems.com/
http://asadi.de/url?q=https://cityofrosedalems.com/
http://treblin.de/url?q=https://cityofrosedalems.com/
https://kentbroom.com/?URL=https://cityofrosedalems.com/
http://0845.boo.jp/cgi/mt3/mt4i.cgi?id=24&mode=redirect&no=15&ref_eid=3387&url=https://cityofrosedalems.com/
http://110.164.66.211/ULIB6//dublin.linkout.php?url=https://cityofrosedalems.com/
http://110.164.92.12/ULIB//dublin.linkout.php?url=https://cityofrosedalems.com/
http://198.54.125.86.myopenlink.net/describe/?url=https://cityofrosedalems.com/
http://202.144.225.38/jmp?url=https://cityofrosedalems.com/
http://2cool2.be/url?q=https://cityofrosedalems.com/
http://4coma.net/cgi/mt4/mt4i.cgi?cat=12&mode=redirect&ref_eid=3231&url=https://cityofrosedalems.com/
http://4travel.jp/dynamic/redirect.php?mode=dm_tour&url=https://cityofrosedalems.com/
http://imaginingourselves.globalfundforwomen.org/pb/External.aspx?url=https://cityofrosedalems.com/
https://clients1.google.cd/url?q=https://cityofrosedalems.com/
https://clients1.google.co.id/url?q=https://cityofrosedalems.com/
https://clients1.google.co.in/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ag/url?q=https://cityofrosedalems.com/
https://clients1.google.com.et/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ua/url?q=https://cityofrosedalems.com/
https://clients1.google.fm/url?q=https://cityofrosedalems.com/
https://clients1.google.hu/url?q=https://cityofrosedalems.com/
https://clients1.google.md/url?q=https://cityofrosedalems.com/
https://clients1.google.mw/url?q=https://cityofrosedalems.com/
https://clients1.google.nu/url?sa=j&url=https://cityofrosedalems.com/
https://clients1.google.rw/url?q=https://cityofrosedalems.com/
http://search.pointcom.com/k.php?ai=&url=https://cityofrosedalems.com/
http://www.cercasostituto.it/index.php?name=GestBanner&file=counter&idbanner=40&dir_link=https://cityofrosedalems.com/
http://vsvejr.dk/mt/plugins/stationExtremes/redirect.php?url=https://cityofrosedalems.com/
http://www.meteo-leran.fr/meteotemplate/template/plugins/deviations/redirect.php?url=https://cityofrosedalems.com/
https://www.eurobichons.com/fda%20alerts.php?url=https://cityofrosedalems.com/
https://mydojo.at/de_AT/karate/weiterleitung?redirect=https://cityofrosedalems.com/
http://click.phosphodiesterase4.com/k.php?ai=&url=https://cityofrosedalems.com/
http://www.mariahownersclub.com/forum/redirect-to/?redirect=https://cityofrosedalems.com/
http://www.wildromance.com/buy.php?url=https://cityofrosedalems.com/&store=iBooks&book=omk-ibooks-us
http://mapleriverweather.com/mobile/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://www.archijob.co.il/index/comp_website.asp?companyId=1469&website=https://cityofrosedalems.com/
https://student-helpr.rminds.dev/redirect?redirectTo=https://cityofrosedalems.com/
http://www.kalinna.de/url?q=https://cityofrosedalems.com/
http://www.hartmanngmbh.de/url?q=https://cityofrosedalems.com/
https://www.the-mainboard.com/proxy.php?link=https://cityofrosedalems.com/
https://www.betamachinery.com/?URL=https://cityofrosedalems.com/
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=https://cityofrosedalems.com/
http://www.sprang.net/url?q=https://cityofrosedalems.com/
https://img.2chan.net/bin/jump.php?https://cityofrosedalems.com/
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=https://cityofrosedalems.com/
http://local.rongbachkim.com/rdr.php?url=https://cityofrosedalems.com/
http://bachecauniversitaria.it/link/frm_top.php?url=https://cityofrosedalems.com/
https://www.stcwdirect.com/redirect.php?url=https://cityofrosedalems.com/
http://forum.vcoderz.com/externalredirect.php?url=https://cityofrosedalems.com/
https://www.momentumstudio.com/?URL=https://cityofrosedalems.com/
https://s-p.me/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://ww.thesteelbrothers.com/buy.php?store=iBooks&url=https://cityofrosedalems.com/
http://thdt.vn/convert/convert.php?link=https://cityofrosedalems.com/
http://www.doitweb365.de/scripts/doitweb.exe/rasklickzaehler2?https://cityofrosedalems.com/
https://www.guadamur.eu/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
https://weather.cube.com.gr/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://www.fimmgviterbo.org/mobfimmgviterbo/index.php?nametm=counter&idbanner=4&dir_link=https://cityofrosedalems.com/
http://www.mckinneyfarm.com/template/plugins/stationExtremes/redirect.php?url=https://cityofrosedalems.com/
https://utmagazine.ru/r?url=https://cityofrosedalems.com/
http://www.evrika41.ru/redirect?url=https://cityofrosedalems.com/
http://expomodel.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://facto.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://fallout3.ru/utils/ref.php?url=https://cityofrosedalems.com/
https://fc-zenit.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://forum-region.ru/forum/away.php?s=https://cityofrosedalems.com/
http://forumdate.ru/redirect-to/?redirect=https://cityofrosedalems.com/
http://shckp.ru/ext_link?url=https://cityofrosedalems.com/
https://shinglas.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://sibran.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://sintez-oka.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://app.espace.cool/clientapi/subscribetocalendar/974?url=https://cityofrosedalems.com/
http://111056.net/yomisearch/rank.cgi?mode=link&id=6205&url=https://cityofrosedalems.com/
http://stanko.tw1.ru/redirect.php?url=https://cityofrosedalems.com/
http://www.sozialemoderne.de/url?q=https://cityofrosedalems.com/
http://www.showb.com/search/ranking.cgi?mode=link&id=7083&url=https://cityofrosedalems.com/
http://imagelibrary.asprey.com/?URL=www.https://cityofrosedalems.com/
http://ime.nu/https://cityofrosedalems.com/
http://interflex.biz/url?q=https://cityofrosedalems.com/
http://ivvb.de/url?q=https://cityofrosedalems.com/
http://j.lix7.net/?https://cityofrosedalems.com/
http://jacobberger.com/?URL=www.https://cityofrosedalems.com/
http://jahn.eu/url?q=https://cityofrosedalems.com/
http://jamesvelvet.com/?URL=www.https://cityofrosedalems.com/
http://jla.drmuller.net/r.php?url=https://cityofrosedalems.com/
http://jump.pagecs.net/https://cityofrosedalems.com/
http://kagarin.net/cgi/mt/mt4i.cgi?id=2&mode=redirect&no=330&ref_eid=103&url=https://cityofrosedalems.com/
http://karkom.de/url?q=https://cityofrosedalems.com/
http://kens.de/url?q=https://cityofrosedalems.com/
http://kinderundjugendpsychotherapie.de/url?q=https://cityofrosedalems.com/
http://kinhtexaydung.net/redirect/?url=https://cityofrosedalems.com/
http://neoromance.info/link/rank.cgi?mode=link&id=26&url=https://cityofrosedalems.com/
http://www.hainberg-gymnasium.com/url?q=https://cityofrosedalems.com/
https://befonts.com/checkout/redirect?url=https://cityofrosedalems.com/
https://www.usap.gov/externalsite.cfm?https://cityofrosedalems.com/
https://maps.google.com.ua/url?rct=j&sa=t&url=https://cityofrosedalems.com/
http://images.google.hu/url?q=https://cityofrosedalems.com/
http://images.google.com.mx/url?q=https://cityofrosedalems.com/
http://www.google.com.mx/url?q=https://cityofrosedalems.com/
http://images.google.com.hk/url?q=https://cityofrosedalems.com/
http://images.google.fi/url?q=https://cityofrosedalems.com/
http://maps.google.fi/url?q=https://cityofrosedalems.com/
http://www.shinobi.jp/etc/goto.html?https://cityofrosedalems.com/
http://images.google.co.id/url?q=https://cityofrosedalems.com/
http://maps.google.co.id/url?q=https://cityofrosedalems.com/
http://images.google.no/url?q=https://cityofrosedalems.com/
http://maps.google.no/url?q=https://cityofrosedalems.com/
http://images.google.co.th/url?q=https://cityofrosedalems.com/
http://maps.google.co.th/url?q=https://cityofrosedalems.com/
http://www.google.co.th/url?q=https://cityofrosedalems.com/
http://maps.google.co.za/url?q=https://cityofrosedalems.com/
http://images.google.ro/url?q=https://cityofrosedalems.com/
http://maps.google.ro/url?q=https://cityofrosedalems.com/
http://cse.google.dk/url?q=https://cityofrosedalems.com/
http://cse.google.com.tr/url?q=https://cityofrosedalems.com/
http://cse.google.hu/url?q=https://cityofrosedalems.com/
http://cse.google.com.hk/url?q=https://cityofrosedalems.com/
http://cse.google.fi/url?q=https://cityofrosedalems.com/
http://images.google.com.sg/url?q=https://cityofrosedalems.com/
http://cse.google.pt/url?q=https://cityofrosedalems.com/
http://cse.google.co.nz/url?q=https://cityofrosedalems.com/
http://images.google.com.ar/url?q=https://cityofrosedalems.com/
http://cse.google.co.id/url?q=https://cityofrosedalems.com/
http://images.google.com.ua/url?q=https://cityofrosedalems.com/
http://cse.google.no/url?q=https://cityofrosedalems.com/
http://cse.google.co.th/url?q=https://cityofrosedalems.com/
http://cse.google.ro/url?q=https://cityofrosedalems.com/
http://images.google.com.tr/url?q=https://cityofrosedalems.com/
http://maps.google.dk/url?q=https://cityofrosedalems.com/
http://www.google.fi/url?q=https://cityofrosedalems.com/
https://supplier-portal-uat.daimler.com/external-link.jspa?url=https://cityofrosedalems.com/
https://strelmag.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://spb90.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://spartak.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://sovaisova.ru/bitrix/redirect.php?event1=2009_mainpage&event2=go_www&event3=&goto=https://cityofrosedalems.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
https://seoandme.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://s-online.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://rssfeeds.wtsp.com/~/t/0/0/wtsp/home/~https://cityofrosedalems.com/
https://rostovmama.ru/redirect?url=https://cityofrosedalems.com/
https://rev1.reversion.jp/redirect?url=https://cityofrosedalems.com/
https://www.star174.ru/redir.php?url=https://cityofrosedalems.com/
https://staten.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://stopcran.ru/go?https://cityofrosedalems.com/
http://studioad.ru/go?https://cityofrosedalems.com/
http://swepub.kb.se/setattribute?language=en&redirect=https://cityofrosedalems.com/
https://www.licnioglasi.org/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=https://cityofrosedalems.com/
http://www2.smartmail.com.ar/tl.php?p=hqf/f94/rs/1fp/4c0/rs//https://cityofrosedalems.com/
http://old.roofnet.org/external.php?link=https://cityofrosedalems.com/
http://www.bucatareasa.ro/link.php?url=https://cityofrosedalems.com/
http://mosprogulka.ru/go?https://cityofrosedalems.com/
https://uniline.co.nz/Document/Url/?url=https://cityofrosedalems.com/
https://tracker.onrecruit.net/api/v1/redirect/?redirect_to=https://cityofrosedalems.com/
http://slipknot1.info/go.php?url=https://cityofrosedalems.com/
https://www.samovar-forum.ru/go?https://cityofrosedalems.com/
http://staldver.ru/go.php?go=https://cityofrosedalems.com/
https://posts.google.com/url?q=https://cityofrosedalems.com/
https://plus.google.com/url?q=https://cityofrosedalems.com/
https://naruto.su/link.ext.php?url=https://cityofrosedalems.com/
https://justpaste.it/redirect/172fy/https://cityofrosedalems.com/
https://jt-pr-dot-yamm-track.appspot.com/Redirect?ukey=1iXi3dF8AuzUIsgifbcVnqqx-anF4B8R-9PC3UGhHO3E-1131306248&key=YAMMID-79725512&link=https://cityofrosedalems.com/
https://ipv4.google.com/url?q=https://cityofrosedalems.com/
https://forum.solidworks.com/external-link.jspa?url=https://cityofrosedalems.com/
https://foro.infojardin.com/proxy.php?link=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://de.flavii.de/index.php?flavii=linker&link=https://cityofrosedalems.com/
https://dakke.co/redirect/?url=https://cityofrosedalems.com/
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_attribute_to_url=https://cityofrosedalems.com/
https://contacts.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://community.rsa.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.nxp.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.esri.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.cypress.com/external-link.jspa?url=https://cityofrosedalems.com/
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=https://cityofrosedalems.com/
https://cdn.iframe.ly/api/iframe?url=https://cityofrosedalems.com/
https://bukkit.org/proxy.php?link=https://cityofrosedalems.com/
https://branch.app.link/?$deeplink_path=article%2Fjan%2F123&$fallback_url=https://cityofrosedalems.com/&channel=facebook&feature=affiliate
https://boowiki.info/go.php?go=https://cityofrosedalems.com/
https://blaze.su/bitrix/redirect.php?event1=&event2=&event3=&goto=https://cityofrosedalems.com/
https://bbs.pku.edu.cn/v2/jump-to.php?url=https://cityofrosedalems.com/
https://bares.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
https://www.flyingsamaritans.net/Startup/SetupSite.asp?RestartPage=https://cityofrosedalems.com/
https://www.ewind.cz/index.php?page=home/redirect&url=https://cityofrosedalems.com/
https://www.eas-racing.se/gbook/go.php?url=https://cityofrosedalems.com/
https://www.direkt-einkauf.de/includes/refer.php?id=170&url=https://cityofrosedalems.com/
https://www.dialogportal.com/Services/Forward.aspx?link=https://cityofrosedalems.com/
https://www.curseforge.com/linkout?remoteUrl=https://cityofrosedalems.com/
https://www.counterwelt.com/charts/click.php?user=14137&link=https://cityofrosedalems.com/
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=https://cityofrosedalems.com/
https://www.autopartskart.com/buyfromamzon.php?url=https://cityofrosedalems.com/
https://www.autoandrv.com/linkout.aspx?websiteurl=https://cityofrosedalems.com/
https://www.art-prizes.com/AdRedirector.aspx?ad=MelbPrizeSculpture_2017&target=https://cityofrosedalems.com/
https://wirelessestimator.com/advertise/newsletter_redirect.php?url=https://cityofrosedalems.com/
https://wasitviewed.com/index.php?href=https://cityofrosedalems.com/
https://tvtropes.org/pmwiki/no_outbounds.php?o=https://cityofrosedalems.com/
https://trello.com/add-card?source=mode=popup&name=click+here&desc=https://cityofrosedalems.com/
https://sutd.ru/links.php?go=https://cityofrosedalems.com/
https://abiznes.com.ua/bitrix/redirect.php?event1=&event2=&event3=&goto=https://cityofrosedalems.com/
http://www.sv-mama.ru/shared/go.php?url=https://cityofrosedalems.com/
http://www.sofion.ru/banner.php?r1=41&r2=2234&goto=https://cityofrosedalems.com/
http://www.rss.geodles.com/fwd.php?url=https://cityofrosedalems.com/
http://www.nuttenzone.at/jump.php?url=https://cityofrosedalems.com/
http://www.myhottiewife.com/cgi-bin/arpro/out.cgi?id=Jojo&url=https://cityofrosedalems.com/
http://www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=https://cityofrosedalems.com/
http://www.johnvorhees.com/gbook/go.php?url=https://cityofrosedalems.com/
http://www.imsnet.at/LangChange.aspx?uri=https://cityofrosedalems.com/
http://www.hon-cafe.net/cgi-bin/re.cgi?lid=hmw&url=https://cityofrosedalems.com/
http://www.glorioustronics.com/redirect.php?link=https://cityofrosedalems.com/
http://www.etis.ford.com/externalURL.do?url=https://cityofrosedalems.com/
http://www.chungshingelectronic.com/redirect.asp?url=https://cityofrosedalems.com/
http://www.bdsmandfetish.com/cgi-bin/sites/out.cgi?id=mandymon&url=https://cityofrosedalems.com/
http://visits.seogaa.ru/redirect/?g=https://cityofrosedalems.com/
http://tharp.me/?url_to_shorten=https://cityofrosedalems.com/
http://speakrus.ru/links.php?go=https://cityofrosedalems.com/
http://spbstroy.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://solo-center.ru/links.php?go=https://cityofrosedalems.com/
http://sennheiserstore.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://school364.spb.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://sc.sie.gov.hk/TuniS/https://cityofrosedalems.com/
http://rzngmu.ru/go?https://cityofrosedalems.com/
http://rostovklad.ru/go.php?https://cityofrosedalems.com/
http://portalnp.snauka.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://markiza.me/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://jump.5ch.net/?https://cityofrosedalems.com/
http://imperialoptical.com/news-redirect.aspx?url=https://cityofrosedalems.com/
http://hellothai.com/wwwlink/wwwredirect.asp?hp_id=1242&url=https://cityofrosedalems.com/
http://guru.sanook.com/?URL=https://cityofrosedalems.com/
http://fr.knubic.com/redirect_to?url=https://cityofrosedalems.com/
http://branch.app.link/?$deeplink_path=article/jan/123&$fallback_url=https://cityofrosedalems.com/
http://biz-tech.org/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://2010.russianinternetweek.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://xat.com/web_gear/chat/linkvalidator.php?link=https://cityofrosedalems.com/
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=https://cityofrosedalems.com/
https://www.woodlist.us/delete-company?nid=13964&element=https://cityofrosedalems.com/
https://www.viecngay.vn/go?to=https://cityofrosedalems.com/
https://www.uts.edu.co/portal/externo.php?id=https://cityofrosedalems.com/
https://www.talgov.com/Main/exit.aspx?url=https://cityofrosedalems.com/
https://www.serie-a.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://www.russianrobotics.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.ruchnoi.ru/ext_link?url=https://cityofrosedalems.com/
https://www.rprofi.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.roccotube.com/cgi-bin/at3/out.cgi?id=27&tag=toplist&trade=https://cityofrosedalems.com/
https://www.nyl0ns.com/cgi-bin/a2/out.cgi?id=43&l=btop&u=https://cityofrosedalems.com/
https://www.meetme.com/apps/redirect/?url=https://cityofrosedalems.com/
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=https://cityofrosedalems.com/
https://www.interpals.net/url_redirect.php?href=https://cityofrosedalems.com/
https://www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=https://cityofrosedalems.com/
https://www.hottystop.com/cgi-bin/at3/out.cgi?id=12&trade=https://cityofrosedalems.com/
https://www.hobowars.com/game/linker.php?url=https://cityofrosedalems.com/
https://www.hentainiches.com/index.php?id=derris&tour=https://cityofrosedalems.com/
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=https://cityofrosedalems.com/
https://www.greencom.ru/catalog/irrigation_systems.html?jumpsite=2008&url=https://cityofrosedalems.com/
https://www.girlznation.com/cgi-bin/atc/out.cgi?id=50&l=side&u=https://cityofrosedalems.com/
https://www.funeralunion.org/delete-company?nid=39&element=https://cityofrosedalems.com/
https://www.fudbal91.com/tz.php?zone=America/Iqaluit&r=https://cityofrosedalems.com/
https://www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://cityofrosedalems.com/
https://kryvbas.at.ua/go?https://cityofrosedalems.com/
https://google.cat/url?q=https://cityofrosedalems.com/
https://joomluck.com/go/?https://cityofrosedalems.com/
https://www.anonymz.com/?https://cityofrosedalems.com/
https://weburg.net/redirect?url=https://cityofrosedalems.com/
https://tw6.jp/jump/?url=https://cityofrosedalems.com/
https://www.spainexpat.com/?URL=https://cityofrosedalems.com/
https://www.fotka.pl/link.php?u=https://cityofrosedalems.com/
https://www.lolinez.com/?https://cityofrosedalems.com/
https://nanos.jp/jmp?url=https://cityofrosedalems.com/
https://www.fca.gov/?URL=https://cityofrosedalems.com/
https://savvylion.com/?bmDomain=https://cityofrosedalems.com/
https://www.soyyooestacaido.com/https://cityofrosedalems.com/
https://www.gta.ru/redirect/www.https://cityofrosedalems.com/
https://directx10.org/go?https://cityofrosedalems.com/
https://mejeriet.dk/link.php?id=https://cityofrosedalems.com/
https://ezdihan.do.am/go?https://cityofrosedalems.com/
https://fishki.net/click?https://cityofrosedalems.com/
https://hiddenrefer.com/?https://cityofrosedalems.com/
https://romhacking.ru/go?https://cityofrosedalems.com/
https://turion.my1.ru/go?https://cityofrosedalems.com/
https://kassirs.ru/sweb.asp?url=https://cityofrosedalems.com/
https://www.allods.net/redirect/https://cityofrosedalems.com/
https://icook.ucoz.ru/go?https://cityofrosedalems.com/
https://www.pasco.k12.fl.us/?URL=https://cityofrosedalems.com/
https://anolink.com/?link=https://cityofrosedalems.com/
https://www.questsociety.ca/?URL=https://cityofrosedalems.com/
https://www.disl.edu/?URL=https://cityofrosedalems.com/
https://holidaykitchens.com/?URL=https://cityofrosedalems.com/
https://www.mbcarolinas.org/?URL=https://cityofrosedalems.com/
https://www.anibox.org/go?https://cityofrosedalems.com/
https://google.info/url?q=https://cityofrosedalems.com/
https://atlantis-tv.ru/go?https://cityofrosedalems.com/
https://otziv.ucoz.com/go?https://cityofrosedalems.com/
https://www.sgvavia.ru/go?https://cityofrosedalems.com/
https://element.lv/go?url=https://cityofrosedalems.com/
https://karanova.ru/?goto=https://cityofrosedalems.com/
https://789.ru/go.php?url=https://cityofrosedalems.com/
https://krasnoeselo.su/go?https://cityofrosedalems.com/
https://game-era.do.am/go?https://cityofrosedalems.com/
https://kudago.com/go/?to=https://cityofrosedalems.com/
https://after.ucoz.net/go?https://cityofrosedalems.com/
https://kinteatr.at.ua/go?https://cityofrosedalems.com/
https://nervetumours.org.uk/?URL=https://cityofrosedalems.com/
https://kopyten.clan.su/go?https://cityofrosedalems.com/
https://www.taker.im/go/?u=https://cityofrosedalems.com/
https://usehelp.clan.su/go?https://cityofrosedalems.com/
https://sepoa.fr/wp/go.php?https://cityofrosedalems.com/
https://world-source.ru/go?https://cityofrosedalems.com/
https://mail2.mclink.it/SRedirect/https://cityofrosedalems.com/
https://www.swleague.ru/go?https://cityofrosedalems.com/
https://nazgull.ucoz.ru/go?https://cityofrosedalems.com/
https://www.rosbooks.ru/go?https://cityofrosedalems.com/
https://beskuda.ucoz.ru/go?https://cityofrosedalems.com/
https://richmonkey.biz/go/?https://cityofrosedalems.com/
https://vlpacific.ru/?goto=https://cityofrosedalems.com/
https://google.co.ck/url?q=https://cityofrosedalems.com/
https://google.co.uz/url?q=https://cityofrosedalems.com/
https://google.co.ls/url?q=https://cityofrosedalems.com/
https://google.co.zm/url?q=https://cityofrosedalems.com/
https://google.co.ve/url?q=https://cityofrosedalems.com/
https://google.co.zw/url?q=https://cityofrosedalems.com/
https://google.co.uk/url?q=https://cityofrosedalems.com/
https://google.co.ao/url?q=https://cityofrosedalems.com/
https://google.co.cr/url?q=https://cityofrosedalems.com/
https://google.co.nz/url?q=https://cityofrosedalems.com/
https://google.co.th/url?q=https://cityofrosedalems.com/
https://google.co.ug/url?q=https://cityofrosedalems.com/
https://google.co.ma/url?q=https://cityofrosedalems.com/
https://google.co.za/url?q=https://cityofrosedalems.com/
https://google.co.kr/url?q=https://cityofrosedalems.com/
https://google.co.mz/url?q=https://cityofrosedalems.com/
https://google.co.vi/url?q=https://cityofrosedalems.com/
https://google.co.ke/url?q=https://cityofrosedalems.com/
https://google.co.hu/url?q=https://cityofrosedalems.com/
https://google.co.tz/url?q=https://cityofrosedalems.com/
https://google.co.jp/url?q=https://cityofrosedalems.com/
https://eric.ed.gov/?redir=https://cityofrosedalems.com/
https://www.usich.gov/?URL=https://cityofrosedalems.com/
https://sec.pn.to/jump.php?https://cityofrosedalems.com/
https://www.earth-policy.org/?URL=https://cityofrosedalems.com/
https://www.silverdart.co.uk/?URL=https://cityofrosedalems.com/
https://pr-cy.ru/jump/?url=https://cityofrosedalems.com/
https://google.co.bw/url?q=https://cityofrosedalems.com/
https://google.co.id/url?q=https://cityofrosedalems.com/
https://google.co.in/url?q=https://cityofrosedalems.com/
https://google.co.il/url?q=https://cityofrosedalems.com/
https://pikmlm.ru/out.php?p=https://cityofrosedalems.com/
https://regnopol.clan.su/go?https://cityofrosedalems.com/
https://mss.in.ua/go.php?to=https://cityofrosedalems.com/
https://owohho.com/away?url=https://cityofrosedalems.com/
https://www.youa.eu/r.php?u=https://cityofrosedalems.com/
https://cool4you.ucoz.ru/go?https://cityofrosedalems.com/
https://rg4u.clan.su/go?https://cityofrosedalems.com/
https://dawnofwar.org.ru/go?https://cityofrosedalems.com/
https://www.de-online.ru/go?https://cityofrosedalems.com/
https://www.allpn.ru/redirect/?url=https://cityofrosedalems.com/
https://click.start.me/?url=https://cityofrosedalems.com/
https://prizraks.clan.su/go?https://cityofrosedalems.com/
https://flyd.ru/away.php?to=https://cityofrosedalems.com/
https://risunok.ucoz.com/go?https://cityofrosedalems.com/
https://www.google.ca/url?q=https://cityofrosedalems.com/
https://www.google.fr/url?q=https://cityofrosedalems.com/
https://cse.google.mk/url?q=https://cityofrosedalems.com/
https://cse.google.ki/url?q=https://cityofrosedalems.com/
https://www.google.sn/url?q=https://cityofrosedalems.com/
https://cse.google.sr/url?q=https://cityofrosedalems.com/
https://www.google.so/url?q=https://cityofrosedalems.com/
https://www.google.cl/url?q=https://cityofrosedalems.com/
https://www.google.sc/url?q=https://cityofrosedalems.com/
https://www.google.iq/url?q=https://cityofrosedalems.com/
https://www.semanticjuice.com/site/https://cityofrosedalems.com/
https://cse.google.kz/url?q=https://cityofrosedalems.com/
https://www.google.gy/url?q=https://cityofrosedalems.com/
https://s79457.gridserver.com/?URL=https://cityofrosedalems.com/
https://cdl.su/redirect?url=https://cityofrosedalems.com/
https://www.fondbtvrtkovic.hr/?URL=https://cityofrosedalems.com/
https://lostnationarchery.com/?URL=https://cityofrosedalems.com/
https://www.booktrix.com/live/?URL=https://cityofrosedalems.com/
https://www.google.ro/url?q=https://cityofrosedalems.com/
https://www.google.tm/url?q=https://cityofrosedalems.com/
https://www.marcellusmatters.psu.edu/?URL=https://cityofrosedalems.com/
https://cse.google.vu/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.vg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.td/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.so/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.sn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.se/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ne/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.mu/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ml/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.kz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.hn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gy/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gp/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ge/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.dj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cv/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.vc/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.tj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.sl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.sb/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.py/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ph/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.pg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.np/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.nf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.mt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ly/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.lb/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.kw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.kh/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.jm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.gi/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.gh/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.fj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.et/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.do/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.cy/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bo/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ai/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ag/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.af/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.zw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.zm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.vi/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.uz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.tz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.mz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ma/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ls/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ke/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ck/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.bw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ci/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cd/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cat/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.am/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.al/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ad/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ac/url?sa=i&url=https://cityofrosedalems.com/
https://maps.google.ws/url?q=https://cityofrosedalems.com/
https://maps.google.tn/url?q=https://cityofrosedalems.com/
https://maps.google.tl/url?q=https://cityofrosedalems.com/
https://maps.google.tk/url?q=https://cityofrosedalems.com/
https://maps.google.td/url?q=https://cityofrosedalems.com/
https://maps.google.st/url?q=https://cityofrosedalems.com/
https://maps.google.sn/url?q=https://cityofrosedalems.com/
https://maps.google.sm/url?q=https://cityofrosedalems.com/
https://maps.google.si/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.sh/url?q=https://cityofrosedalems.com/
https://maps.google.se/url?q=https://cityofrosedalems.com/
https://maps.google.rw/url?q=https://cityofrosedalems.com/
https://maps.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ru/url?q=https://cityofrosedalems.com/
https://maps.google.rs/url?q=https://cityofrosedalems.com/
https://maps.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.pt/url?q=https://cityofrosedalems.com/
https://maps.google.pn/url?q=https://cityofrosedalems.com/
https://maps.google.pl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.pl/url?q=https://cityofrosedalems.com/
https://maps.google.nr/url?q=https://cityofrosedalems.com/
https://maps.google.no/url?q=https://cityofrosedalems.com/
https://maps.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ne/url?q=https://cityofrosedalems.com/
https://maps.google.mw/url?q=https://cityofrosedalems.com/
https://maps.google.mu/url?q=https://cityofrosedalems.com/
https://maps.google.ms/url?q=https://cityofrosedalems.com/
https://maps.google.mn/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ml/url?q=https://cityofrosedalems.com/
https://maps.google.mk/url?q=https://cityofrosedalems.com/
https://maps.google.mg/url?q=https://cityofrosedalems.com/
https://maps.google.lv/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.lt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.lt/url?q=https://cityofrosedalems.com/
https://maps.google.lk/url?q=https://cityofrosedalems.com/
https://maps.google.li/url?q=https://cityofrosedalems.com/
https://maps.google.la/url?q=https://cityofrosedalems.com/
https://maps.google.kz/url?q=https://cityofrosedalems.com/
https://maps.google.ki/url?q=https://cityofrosedalems.com/
https://maps.google.jo/url?q=https://cityofrosedalems.com/
https://maps.google.je/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?q=https://cityofrosedalems.com/
https://maps.google.gg/url?q=https://cityofrosedalems.com/
https://maps.google.ge/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ge/url?q=https://cityofrosedalems.com/
https://maps.google.fr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fr/url?q=https://cityofrosedalems.com/
https://maps.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ee/url?q=https://cityofrosedalems.com/
https://maps.google.dm/url?q=https://cityofrosedalems.com/
https://maps.google.dk/url?q=https://cityofrosedalems.com/
https://maps.google.de/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cz/url?q=https://cityofrosedalems.com/
https://maps.google.cv/url?q=https://cityofrosedalems.com/
https://maps.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com/url?q=https://cityofrosedalems.com/
https://maps.google.com.uy/url?q=https://cityofrosedalems.com/
https://maps.google.com.ua/url?q=https://cityofrosedalems.com/
https://maps.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tw/url?q=https://cityofrosedalems.com/
https://maps.google.com.sg/url?q=https://cityofrosedalems.com/
https://maps.google.com.sb/url?q=https://cityofrosedalems.com/
https://maps.google.com.qa/url?q=https://cityofrosedalems.com/
https://maps.google.com.py/url?q=https://cityofrosedalems.com/
https://maps.google.com.ph/url?q=https://cityofrosedalems.com/
https://maps.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.com.ni/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ni/url?q=https://cityofrosedalems.com/
https://maps.google.com.na/url?q=https://cityofrosedalems.com/
https://maps.google.com.mx/url?q=https://cityofrosedalems.com/
https://maps.google.com.mt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ly/url?q=https://cityofrosedalems.com/
https://maps.google.com.lb/url?q=https://cityofrosedalems.com/
https://maps.google.com.kw/url?q=https://cityofrosedalems.com/
https://maps.google.com.kh/url?q=https://cityofrosedalems.com/
https://maps.google.com.jm/url?q=https://cityofrosedalems.com/
https://maps.google.com.gt/url?q=https://cityofrosedalems.com/
https://maps.google.com.gh/url?q=https://cityofrosedalems.com/
https://maps.google.com.fj/url?q=https://cityofrosedalems.com/
https://maps.google.com.et/url?q=https://cityofrosedalems.com/
https://maps.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bz/url?q=https://cityofrosedalems.com/
https://maps.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?q=https://cityofrosedalems.com/
https://maps.google.com.bn/url?q=https://cityofrosedalems.com/
https://maps.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.au/url?q=https://cityofrosedalems.com/
https://maps.google.com.ar/url?q=https://cityofrosedalems.com/
https://maps.google.com.ai/url?q=https://cityofrosedalems.com/
https://maps.google.com.ag/url?q=https://cityofrosedalems.com/
https://maps.google.co.zm/url?q=https://cityofrosedalems.com/
https://maps.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.vi/url?q=https://cityofrosedalems.com/
https://maps.google.co.ug/url?q=https://cityofrosedalems.com/
https://maps.google.co.tz/url?q=https://cityofrosedalems.com/
https://maps.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.nz/url?q=https://cityofrosedalems.com/
https://maps.google.co.ls/url?q=https://cityofrosedalems.com/
https://maps.google.co.kr/url?q=https://cityofrosedalems.com/
https://maps.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.il/url?q=https://cityofrosedalems.com/
https://maps.google.co.id/url?q=https://cityofrosedalems.com/
https://maps.google.co.cr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.ck/url?q=https://cityofrosedalems.com/
https://maps.google.co.bw/url?q=https://cityofrosedalems.com/
https://maps.google.co.ao/url?q=https://cityofrosedalems.com/
https://maps.google.cm/url?q=https://cityofrosedalems.com/
https://maps.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ci/url?q=https://cityofrosedalems.com/
https://maps.google.ch/url?q=https://cityofrosedalems.com/
https://maps.google.cg/url?q=https://cityofrosedalems.com/
https://maps.google.cf/url?q=https://cityofrosedalems.com/
https://maps.google.cd/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cd/url?q=https://cityofrosedalems.com/
https://maps.google.bs/url?q=https://cityofrosedalems.com/
https://maps.google.bj/url?q=https://cityofrosedalems.com/
https://maps.google.bi/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?q=https://cityofrosedalems.com/
https://maps.google.bf/url?q=https://cityofrosedalems.com/
https://maps.google.be/url?q=https://cityofrosedalems.com/
https://maps.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.at/url?q=https://cityofrosedalems.com/
https://maps.google.ad/url?q=https://cityofrosedalems.com/
https://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.vg/url?q=https://cityofrosedalems.com/
https://images.google.tt/url?q=https://cityofrosedalems.com/
https://images.google.tm/url?q=https://cityofrosedalems.com/
https://images.google.tk/url?q=https://cityofrosedalems.com/
https://images.google.tg/url?q=https://cityofrosedalems.com/
https://images.google.sk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.si/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.sh/url?q=https://cityofrosedalems.com/
https://images.google.se/url?q=https://cityofrosedalems.com/
https://images.google.pt/url?q=https://cityofrosedalems.com/
https://images.google.ps/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pn/url?q=https://cityofrosedalems.com/
https://images.google.pl/url?q=https://cityofrosedalems.com/
https://images.google.nr/url?q=https://cityofrosedalems.com/
https://images.google.no/url?q=https://cityofrosedalems.com/
https://images.google.mw/url?q=https://cityofrosedalems.com/
https://images.google.mv/url?q=https://cityofrosedalems.com/
https://images.google.ml/url?q=https://cityofrosedalems.com/
https://images.google.mg/url?q=https://cityofrosedalems.com/
https://images.google.me/url?q=https://cityofrosedalems.com/
https://images.google.lk/url?q=https://cityofrosedalems.com/
https://images.google.li/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.la/url?q=https://cityofrosedalems.com/
https://images.google.kz/url?q=https://cityofrosedalems.com/
https://images.google.kg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.kg/url?q=https://cityofrosedalems.com/
https://images.google.je/url?q=https://cityofrosedalems.com/
https://images.google.it/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.it/url?q=https://cityofrosedalems.com/
https://images.google.im/url?q=https://cityofrosedalems.com/
https://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hu/url?q=https://cityofrosedalems.com/
https://images.google.ht/url?q=https://cityofrosedalems.com/
https://images.google.hn/url?q=https://cityofrosedalems.com/
https://images.google.gy/url?q=https://cityofrosedalems.com/
https://images.google.gp/url?q=https://cityofrosedalems.com/
https://images.google.gm/url?q=https://cityofrosedalems.com/
https://images.google.gg/url?q=https://cityofrosedalems.com/
https://images.google.ge/url?q=https://cityofrosedalems.com/
https://images.google.fr/url?q=https://cityofrosedalems.com/
https://images.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.fi/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dm/url?q=https://cityofrosedalems.com/
https://images.google.de/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?q=https://cityofrosedalems.com/
https://images.google.com/url?q=https://cityofrosedalems.com/
https://images.google.com.vn/url?q=https://cityofrosedalems.com/
https://images.google.com.vc/url?q=https://cityofrosedalems.com/
https://images.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tj/url?q=https://cityofrosedalems.com/
https://images.google.com.sl/url?q=https://cityofrosedalems.com/
https://images.google.com.sb/url?q=https://cityofrosedalems.com/
https://images.google.com.qa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.py/url?q=https://cityofrosedalems.com/
https://images.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ph/url?q=https://cityofrosedalems.com/
https://images.google.com.pa/url?q=https://cityofrosedalems.com/
https://images.google.com.ni/url?q=https://cityofrosedalems.com/
https://images.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.na/url?q=https://cityofrosedalems.com/
https://images.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.mm/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ly/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ly/url?q=https://cityofrosedalems.com/
https://images.google.com.lb/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kw/url?q=https://cityofrosedalems.com/
https://images.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kh/url?q=https://cityofrosedalems.com/
https://images.google.com.jm/url?q=https://cityofrosedalems.com/
https://images.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.gt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.gi/url?q=https://cityofrosedalems.com/
https://images.google.com.gh/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.fj/url?q=https://cityofrosedalems.com/
https://images.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.eg/url?q=https://cityofrosedalems.com/
https://images.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cy/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cy/url?q=https://cityofrosedalems.com/
https://images.google.com.bz/url?q=https://cityofrosedalems.com/
https://images.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bd/url?q=https://cityofrosedalems.com/
https://images.google.com.au/url?q=https://cityofrosedalems.com/
https://images.google.com.ag/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ag/url?q=https://cityofrosedalems.com/
https://images.google.co.zw/url?q=https://cityofrosedalems.com/
https://images.google.co.zm/url?q=https://cityofrosedalems.com/
https://images.google.co.za/url?q=https://cityofrosedalems.com/
https://images.google.co.vi/url?q=https://cityofrosedalems.com/
https://images.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.ve/url?q=https://cityofrosedalems.com/
https://images.google.co.uz/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?q=https://cityofrosedalems.com/
https://images.google.co.ug/url?q=https://cityofrosedalems.com/
https://images.google.co.tz/url?q=https://cityofrosedalems.com/
https://images.google.co.nz/url?q=https://cityofrosedalems.com/
https://images.google.co.mz/url?q=https://cityofrosedalems.com/
https://images.google.co.ma/url?q=https://cityofrosedalems.com/
https://images.google.co.jp/url?q=https://cityofrosedalems.com/
https://images.google.co.id/url?q=https://cityofrosedalems.com/
https://images.google.co.cr/url?q=https://cityofrosedalems.com/
https://images.google.co.ck/url?q=https://cityofrosedalems.com/
https://images.google.co.bw/url?q=https://cityofrosedalems.com/
https://images.google.cm/url?q=https://cityofrosedalems.com/
https://images.google.ci/url?q=https://cityofrosedalems.com/
https://images.google.ch/url?q=https://cityofrosedalems.com/
https://images.google.cg/url?q=https://cityofrosedalems.com/
https://images.google.cf/url?q=https://cityofrosedalems.com/
https://images.google.cat/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ca/url?q=https://cityofrosedalems.com/
https://images.google.by/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bs/url?q=https://cityofrosedalems.com/
https://images.google.bj/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.bf/url?q=https://cityofrosedalems.com/
https://images.google.be/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ba/url?q=https://cityofrosedalems.com/
https://images.google.at/url?q=https://cityofrosedalems.com/
https://images.google.am/url?q=https://cityofrosedalems.com/
https://images.google.ad/url?q=https://cityofrosedalems.com/
https://images.google.ac/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.iq/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hu/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ht/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hn/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gy/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gp/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gl/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ge/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ga/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.es/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ee/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dk/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.de/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cv/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.kh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.hk/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gt/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.fj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.et/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.eg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ec/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.do/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.cy/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.cu/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.co/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.br/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bo/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bn/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bd/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.au/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ar/url?sa=i&url=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ar/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ai/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ag/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.af/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.il/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.id/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.ck/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.ao/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cl/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ci/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ch/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cf/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cd/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cc/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cat/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ca/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.by/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bt/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bs/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bf/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.be/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ba/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.az/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.at/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.as/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.am/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.al/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ae/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ad/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ac/url?q=https://cityofrosedalems.com/
http://maps.google.vu/url?q=https://cityofrosedalems.com/
http://maps.google.vg/url?q=https://cityofrosedalems.com/
http://maps.google.tt/url?q=https://cityofrosedalems.com/
http://maps.google.sk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.si/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.sc/url?q=https://cityofrosedalems.com/
http://maps.google.ru/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ro/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.pt/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.pl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.nl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.mv/url?q=https://cityofrosedalems.com/
http://maps.google.mn/url?q=https://cityofrosedalems.com/
http://maps.google.ml/url?q=https://cityofrosedalems.com/
http://maps.google.lv/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.lt/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.je/url?q=https://cityofrosedalems.com/
http://maps.google.it/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.im/url?q=https://cityofrosedalems.com/
http://maps.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ie/url?q=https://cityofrosedalems.com/
http://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ht/url?q=https://cityofrosedalems.com/
http://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.gm/url?q=https://cityofrosedalems.com/
http://maps.google.fr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.fm/url?q=https://cityofrosedalems.com/
http://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.es/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ee/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.dk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.dj/url?q=https://cityofrosedalems.com/
http://maps.google.cz/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.om/url?q=https://cityofrosedalems.com/
http://maps.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.mm/url?q=https://cityofrosedalems.com/
http://maps.google.com.ly/url?q=https://cityofrosedalems.com/
http://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.gi/url?q=https://cityofrosedalems.com/
http://maps.google.com.fj/url?q=https://cityofrosedalems.com/
http://maps.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.cu/url?q=https://cityofrosedalems.com/
http://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.bh/url?q=https://cityofrosedalems.com/
http://maps.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.zw/url?q=https://cityofrosedalems.com/
http://maps.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.mz/url?q=https://cityofrosedalems.com/
http://maps.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.cl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ch/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.cg/url?q=https://cityofrosedalems.com/
http://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.bt/url?q=https://cityofrosedalems.com/
http://maps.google.bi/url?q=https://cityofrosedalems.com/
http://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.be/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ba/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.at/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.as/url?q=https://cityofrosedalems.com/
http://maps.google.ae/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.vu/url?q=https://cityofrosedalems.com/
http://images.google.vg/url?q=https://cityofrosedalems.com/
http://images.google.sr/url?q=https://cityofrosedalems.com/
http://images.google.sn/url?q=https://cityofrosedalems.com/
http://images.google.sm/url?q=https://cityofrosedalems.com/
http://images.google.sk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.si/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.sh/url?q=https://cityofrosedalems.com/
http://images.google.sc/url?q=https://cityofrosedalems.com/
http://images.google.rw/url?q=https://cityofrosedalems.com/
http://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ro/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ps/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.nr/url?q=https://cityofrosedalems.com/
http://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.mw/url?q=https://cityofrosedalems.com/
http://images.google.mv/url?q=https://cityofrosedalems.com/
http://images.google.ms/url?q=https://cityofrosedalems.com/
http://images.google.mn/url?q=https://cityofrosedalems.com/
http://images.google.ml/url?q=https://cityofrosedalems.com/
http://images.google.mg/url?q=https://cityofrosedalems.com/
http://images.google.md/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.lk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.li/url?q=https://cityofrosedalems.com/
http://images.google.la/url?q=https://cityofrosedalems.com/
http://images.google.kg/url?q=https://cityofrosedalems.com/
http://images.google.je/url?q=https://cityofrosedalems.com/
http://images.google.it/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.iq/url?q=https://cityofrosedalems.com/
http://images.google.im/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?q=https://cityofrosedalems.com/
http://images.google.hu/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ht/url?q=https://cityofrosedalems.com/
http://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.gm/url?q=https://cityofrosedalems.com/
http://images.google.gg/url?q=https://cityofrosedalems.com/
http://images.google.fr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.fm/url?q=https://cityofrosedalems.com/
http://images.google.fi/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ee/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.dm/url?q=https://cityofrosedalems.com/
http://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.dj/url?q=https://cityofrosedalems.com/
http://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.uy/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tj/url?q=https://cityofrosedalems.com/
http://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.na/url?q=https://cityofrosedalems.com/
http://images.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.mm/url?q=https://cityofrosedalems.com/
http://images.google.com.jm/url?q=https://cityofrosedalems.com/
http://images.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.gi/url?q=https://cityofrosedalems.com/
http://images.google.com.fj/url?q=https://cityofrosedalems.com/
http://images.google.com.et/url?q=https://cityofrosedalems.com/
http://images.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ec/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.cy/url?q=https://cityofrosedalems.com/
http://images.google.com.cu/url?q=https://cityofrosedalems.com/
http://images.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.bz/url?q=https://cityofrosedalems.com/
http://images.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.bn/url?q=https://cityofrosedalems.com/
http://images.google.com.bh/url?q=https://cityofrosedalems.com/
http://images.google.com.bd/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ag/url?q=https://cityofrosedalems.com/
http://images.google.com.af/url?q=https://cityofrosedalems.com/
http://images.google.co.zw/url?q=https://cityofrosedalems.com/
http://images.google.co.zm/url?q=https://cityofrosedalems.com/
http://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.vi/url?q=https://cityofrosedalems.com/
http://images.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.tz/url?q=https://cityofrosedalems.com/
http://images.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.mz/url?q=https://cityofrosedalems.com/
http://images.google.co.ma/url?q=https://cityofrosedalems.com/
http://images.google.co.ls/url?q=https://cityofrosedalems.com/
http://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.ck/url?q=https://cityofrosedalems.com/
http://images.google.co.bw/url?q=https://cityofrosedalems.com/
http://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ci/url?q=https://cityofrosedalems.com/
http://images.google.ch/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.cg/url?q=https://cityofrosedalems.com/
http://images.google.cd/url?q=https://cityofrosedalems.com/
http://images.google.ca/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.bt/url?q=https://cityofrosedalems.com/
http://images.google.bs/url?q=https://cityofrosedalems.com/
http://images.google.bj/url?q=https://cityofrosedalems.com/
http://images.google.bi/url?q=https://cityofrosedalems.com/
http://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.be/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.az/url?q=https://cityofrosedalems.com/
http://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.as/url?q=https://cityofrosedalems.com/
http://images.google.al/url?q=https://cityofrosedalems.com/
http://images.google.ae/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ad/url?q=https://cityofrosedalems.com/
http://cse.google.com.ly/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.lb/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.kw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.kh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.jm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.hk/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gt/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.fj/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.et/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.eg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ec/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.do/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.cy/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.co/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.br/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bo/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bn/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bd/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.au/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ai/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ag/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.af/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.zw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.zm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.za/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.vi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ve/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.uz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.uk/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ug/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.tz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.th/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.nz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.mz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ma/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ls/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.kr/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ke/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.jp/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.in/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.il/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.id/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.cr/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ck/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.bw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ao/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cl/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ci/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ch/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cf/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cd/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cat/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ca/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.by/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bs/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bj/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bf/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.be/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ba/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.az/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.at/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.as/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.am/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.al/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ae/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ad/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ac/url?sa=i&url=https://cityofrosedalems.com/
https://google.ws/url?q=https://cityofrosedalems.com/
https://google.vu/url?q=https://cityofrosedalems.com/
https://google.vg/url?q=https://cityofrosedalems.com/
https://google.tt/url?q=https://cityofrosedalems.com/
https://google.to/url?q=https://cityofrosedalems.com/
https://google.tm/url?q=https://cityofrosedalems.com/
https://google.tl/url?q=https://cityofrosedalems.com/
https://google.tk/url?q=https://cityofrosedalems.com/
https://google.tg/url?q=https://cityofrosedalems.com/
https://google.st/url?q=https://cityofrosedalems.com/
https://google.sr/url?q=https://cityofrosedalems.com/
https://google.so/url?q=https://cityofrosedalems.com/
https://google.sm/url?q=https://cityofrosedalems.com/
https://google.sh/url?q=https://cityofrosedalems.com/
https://google.sc/url?q=https://cityofrosedalems.com/
https://google.rw/url?q=https://cityofrosedalems.com/
https://google.ps/url?q=https://cityofrosedalems.com/
https://google.pn/url?q=https://cityofrosedalems.com/
https://google.nu/url?q=https://cityofrosedalems.com/
https://google.nr/url?q=https://cityofrosedalems.com/
https://google.ne/url?q=https://cityofrosedalems.com/
https://google.mw/url?q=https://cityofrosedalems.com/
https://google.mv/url?q=https://cityofrosedalems.com/
https://google.ms/url?q=https://cityofrosedalems.com/
https://google.ml/url?q=https://cityofrosedalems.com/
https://google.mg/url?q=https://cityofrosedalems.com/
https://google.md/url?q=https://cityofrosedalems.com/
https://google.lk/url?q=https://cityofrosedalems.com/
https://google.la/url?q=https://cityofrosedalems.com/
https://google.kz/url?q=https://cityofrosedalems.com/
https://google.ki/url?q=https://cityofrosedalems.com/
https://google.kg/url?q=https://cityofrosedalems.com/
https://google.iq/url?q=https://cityofrosedalems.com/
https://google.im/url?q=https://cityofrosedalems.com/
https://google.ht/url?q=https://cityofrosedalems.com/
https://google.hn/url?sa=t&url=https://cityofrosedalems.com/
https://google.gm/url?q=https://cityofrosedalems.com/
https://google.gl/url?q=https://cityofrosedalems.com/
https://google.gg/url?q=https://cityofrosedalems.com/
https://google.ge/url?q=https://cityofrosedalems.com/
https://google.ga/url?q=https://cityofrosedalems.com/
https://google.dz/url?q=https://cityofrosedalems.com/
https://google.dm/url?q=https://cityofrosedalems.com/
https://google.dj/url?q=https://cityofrosedalems.com/
https://google.cv/url?q=https://cityofrosedalems.com/
https://google.com.vc/url?q=https://cityofrosedalems.com/
https://google.com.tj/url?q=https://cityofrosedalems.com/
https://google.com.sv/url?sa=t&url=https://cityofrosedalems.com/
https://google.com.sb/url?q=https://cityofrosedalems.com/
https://google.com.pa/url?q=https://cityofrosedalems.com/
https://google.com.om/url?q=https://cityofrosedalems.com/
https://google.com.ni/url?q=https://cityofrosedalems.com/
https://google.com.na/url?q=https://cityofrosedalems.com/
https://google.com.kw/url?q=https://cityofrosedalems.com/
https://google.com.kh/url?q=https://cityofrosedalems.com/
https://google.com.jm/url?q=https://cityofrosedalems.com/
https://google.com.gi/url?q=https://cityofrosedalems.com/
https://google.com.gh/url?q=https://cityofrosedalems.com/
https://google.com.fj/url?q=https://cityofrosedalems.com/
https://google.com.et/url?q=https://cityofrosedalems.com/
https://google.com.do/url?q=https://cityofrosedalems.com/
https://google.com.bz/url?q=https://cityofrosedalems.com/
https://google.com.ai/url?q=https://cityofrosedalems.com/
https://google.com.ag/url?q=https://cityofrosedalems.com/
https://google.com.af/url?q=https://cityofrosedalems.com/
https://google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://google.cm/url?q=https://cityofrosedalems.com/
https://google.ci/url?sa=t&url=https://cityofrosedalems.com/
https://google.cg/url?q=https://cityofrosedalems.com/
https://google.cf/url?q=https://cityofrosedalems.com/
https://google.cd/url?q=https://cityofrosedalems.com/
https://google.bt/url?q=https://cityofrosedalems.com/
https://google.bj/url?q=https://cityofrosedalems.com/
https://google.bf/url?q=https://cityofrosedalems.com/
https://google.am/url?q=https://cityofrosedalems.com/
https://google.al/url?q=https://cityofrosedalems.com/
https://google.ad/url?q=https://cityofrosedalems.com/
https://google.ac/url?q=https://cityofrosedalems.com/
https://www.google.vg/url?q=https://cityofrosedalems.com/
https://www.google.tt/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.tl/url?q=https://cityofrosedalems.com/
https://www.google.st/url?q=https://cityofrosedalems.com/
https://www.google.nu/url?q=https://cityofrosedalems.com/
https://www.google.ms/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.it/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.it/url?q=https://cityofrosedalems.com/
https://www.google.is/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.gl/url?q=https://cityofrosedalems.com/
https://www.google.fm/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.es/url?q=https://cityofrosedalems.com/
https://www.google.dm/url?q=https://cityofrosedalems.com/
https://www.google.cz/url?q=https://cityofrosedalems.com/
https://www.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.uy/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.ua/url?q=https://cityofrosedalems.com/
https://www.google.com.sl/url?q=https://cityofrosedalems.com/
https://www.google.com.sg/url?q=https://cityofrosedalems.com/
https://www.google.com.pr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.om/url?q=https://cityofrosedalems.com/
https://www.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.ec/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.au/url?q=https://cityofrosedalems.com/
https://www.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ma/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ck/url?q=https://cityofrosedalems.com/
https://www.google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.ch/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.cd/url?q=https://cityofrosedalems.com/
https://www.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.by/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.bt/url?q=https://cityofrosedalems.com/
https://www.google.be/url?q=https://cityofrosedalems.com/
https://www.google.as/url?sa=t&url=https://cityofrosedalems.com/
http://www.google.sk/url?q=https://cityofrosedalems.com/
http://www.google.ro/url?q=https://cityofrosedalems.com/
http://www.google.pt/url?q=https://cityofrosedalems.com/
http://www.google.no/url?q=https://cityofrosedalems.com/
http://www.google.lt/url?q=https://cityofrosedalems.com/
http://www.google.ie/url?q=https://cityofrosedalems.com/
http://www.google.com.vn/url?q=https://cityofrosedalems.com/
http://www.google.com.ua/url?q=https://cityofrosedalems.com/
http://www.google.com.tr/url?q=https://cityofrosedalems.com/
http://www.google.com.ph/url?q=https://cityofrosedalems.com/
http://www.google.com.ar/url?q=https://cityofrosedalems.com/
http://www.google.co.za/url?q=https://cityofrosedalems.com/
http://www.google.co.nz/url?q=https://cityofrosedalems.com/
http://www.google.co.kr/url?q=https://cityofrosedalems.com/
http://www.google.co.il/url?q=https://cityofrosedalems.com/
http://www.google.co.id/url?q=https://cityofrosedalems.com/
https://www.google.ac/url?q=https://cityofrosedalems.com/
https://www.google.ad/url?q=https://cityofrosedalems.com/
https://www.google.ae/url?q=https://cityofrosedalems.com/
https://www.google.am/url?q=https://cityofrosedalems.com/
https://www.google.as/url?q=https://cityofrosedalems.com/
https://www.google.at/url?q=https://cityofrosedalems.com/
https://www.google.az/url?q=https://cityofrosedalems.com/
https://www.google.bg/url?q=https://cityofrosedalems.com/
https://www.google.bi/url?q=https://cityofrosedalems.com/
https://www.google.bj/url?q=https://cityofrosedalems.com/
https://www.google.bs/url?q=https://cityofrosedalems.com/
https://www.google.by/url?q=https://cityofrosedalems.com/
https://www.google.cf/url?q=https://cityofrosedalems.com/
https://www.google.cg/url?q=https://cityofrosedalems.com/
https://www.google.ch/url?q=https://cityofrosedalems.com/
https://www.google.ci/url?q=https://cityofrosedalems.com/
https://www.google.cm/url?q=https://cityofrosedalems.com/
https://www.google.co.ao/url?q=https://cityofrosedalems.com/
https://www.google.co.bw/url?q=https://cityofrosedalems.com/
https://www.google.co.cr/url?q=https://cityofrosedalems.com/
https://www.google.co.id/url?q=https://cityofrosedalems.com/
https://www.google.co.in/url?q=https://cityofrosedalems.com/
https://www.google.co.kr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ma/url?q=https://cityofrosedalems.com/
https://www.google.co.mz/url?q=https://cityofrosedalems.com/
https://www.google.co.nz/url?q=https://cityofrosedalems.com/
https://www.google.co.th/url?q=https://cityofrosedalems.com/
https://www.google.co.tz/url?q=https://cityofrosedalems.com/
https://www.google.co.ug/url?q=https://cityofrosedalems.com/
https://www.google.co.uk/url?q=https://cityofrosedalems.com/
https://www.google.co.uz/url?q=https://cityofrosedalems.com/
https://www.google.co.uz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ve/url?q=https://cityofrosedalems.com/
https://www.google.co.vi/url?q=https://cityofrosedalems.com/
https://www.google.co.za/url?q=https://cityofrosedalems.com/
https://www.google.co.zm/url?q=https://cityofrosedalems.com/
https://www.google.co.zw/url?q=https://cityofrosedalems.com/
https://www.google.com.ai/url?q=https://cityofrosedalems.com/
https://www.google.com.ar/url?q=https://cityofrosedalems.com/
https://www.google.com.bd/url?q=https://cityofrosedalems.com/
https://www.google.com.bh/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.bo/url?q=https://cityofrosedalems.com/
https://www.google.com.br/url?q=https://cityofrosedalems.com/
https://www.google.com.bz/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://www.google.com.cu/url?q=https://cityofrosedalems.com/
https://www.google.com.cy/url?q=https://cityofrosedalems.com/
https://www.google.com.ec/url?q=https://cityofrosedalems.com/
https://www.google.com.fj/url?q=https://cityofrosedalems.com/
https://www.google.com.gh/url?q=https://cityofrosedalems.com/
https://www.google.com.hk/url?q=https://cityofrosedalems.com/
https://www.google.com.jm/url?q=https://cityofrosedalems.com/
https://www.google.com.kh/url?q=https://cityofrosedalems.com/
https://www.google.com.kw/url?q=https://cityofrosedalems.com/
https://www.google.com.lb/url?q=https://cityofrosedalems.com/
https://www.google.com.ly/url?q=https://cityofrosedalems.com/
https://www.google.com.mm/url?q=https://cityofrosedalems.com/
https://www.google.com.mt/url?q=https://cityofrosedalems.com/
https://www.google.com.mx/url?q=https://cityofrosedalems.com/
https://www.google.com.my/url?q=https://cityofrosedalems.com/
https://www.google.com.nf/url?q=https://cityofrosedalems.com/
https://www.google.com.ng/url?q=https://cityofrosedalems.com/
https://www.google.com.ni/url?q=https://cityofrosedalems.com/
https://www.google.com.pa/url?q=https://cityofrosedalems.com/
https://www.google.com.pe/url?q=https://cityofrosedalems.com/
https://www.google.com.pg/url?q=https://cityofrosedalems.com/
https://www.google.com.ph/url?q=https://cityofrosedalems.com/
https://www.google.com.pk/url?q=https://cityofrosedalems.com/
https://www.google.com.pr/url?q=https://cityofrosedalems.com/
https://www.google.com.py/url?q=https://cityofrosedalems.com/
https://www.google.com.qa/url?q=https://cityofrosedalems.com/
https://www.google.com.sa/url?q=https://cityofrosedalems.com/
https://www.google.com.sb/url?q=https://cityofrosedalems.com/
https://www.google.com.sv/url?q=https://cityofrosedalems.com/
https://www.google.com.tj/url?sa=i&url=https://cityofrosedalems.com/
https://www.google.com.tr/url?q=https://cityofrosedalems.com/
https://www.google.com.tw/url?q=https://cityofrosedalems.com/
https://www.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.uy/url?q=https://cityofrosedalems.com/
https://www.google.com.vn/url?q=https://cityofrosedalems.com/
https://www.google.com/url?q=https://cityofrosedalems.com/
https://www.google.com/url?sa=i&rct=j&url=https://cityofrosedalems.com/
https://www.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.de/url?q=https://cityofrosedalems.com/
https://www.google.dj/url?q=https://cityofrosedalems.com/
https://www.google.dk/url?q=https://cityofrosedalems.com/
https://www.google.dz/url?q=https://cityofrosedalems.com/
https://www.google.ee/url?q=https://cityofrosedalems.com/
https://www.google.fi/url?q=https://cityofrosedalems.com/
https://www.google.fm/url?q=https://cityofrosedalems.com/
https://www.google.ga/url?q=https://cityofrosedalems.com/
https://www.google.ge/url?q=https://cityofrosedalems.com/
https://www.google.gg/url?q=https://cityofrosedalems.com/
https://www.google.gm/url?q=https://cityofrosedalems.com/
https://www.google.gp/url?q=https://cityofrosedalems.com/
https://www.google.gr/url?q=https://cityofrosedalems.com/
https://www.google.hn/url?q=https://cityofrosedalems.com/
https://www.google.hr/url?q=https://cityofrosedalems.com/
https://www.google.ht/url?q=https://cityofrosedalems.com/
https://www.google.hu/url?q=https://cityofrosedalems.com/
https://www.google.ie/url?q=https://cityofrosedalems.com/
https://www.google.jo/url?q=https://cityofrosedalems.com/
https://www.google.ki/url?q=https://cityofrosedalems.com/
https://www.google.la/url?q=https://cityofrosedalems.com/
https://www.google.lk/url?q=https://cityofrosedalems.com/
https://www.google.lt/url?q=https://cityofrosedalems.com/
https://www.google.lu/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.lv/url?q=https://cityofrosedalems.com/
https://www.google.mg/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.mk/url?q=https://cityofrosedalems.com/
https://www.google.ml/url?q=https://cityofrosedalems.com/
https://www.google.mn/url?q=https://cityofrosedalems.com/
https://www.google.ms/url?q=https://cityofrosedalems.com/
https://www.google.mu/url?q=https://cityofrosedalems.com/
https://www.google.mv/url?q=https://cityofrosedalems.com/
https://www.google.mw/url?q=https://cityofrosedalems.com/
https://www.google.ne/url?q=https://cityofrosedalems.com/
https://www.google.nl/url?q=https://cityofrosedalems.com/
https://www.google.no/url?q=https://cityofrosedalems.com/
https://www.google.nr/url?q=https://cityofrosedalems.com/
https://www.google.pl/url?q=https://cityofrosedalems.com/
https://www.google.pt/url?q=https://cityofrosedalems.com/
https://www.google.rs/url?q=https://cityofrosedalems.com/
https://www.google.ru/url?q=https://cityofrosedalems.com/
https://www.google.se/url?q=https://cityofrosedalems.com/
https://www.google.sh/url?q=https://cityofrosedalems.com/
https://www.google.si/url?q=https://cityofrosedalems.com/
https://www.google.sk/url?q=https://cityofrosedalems.com/
https://www.google.sm/url?q=https://cityofrosedalems.com/
https://www.google.sr/url?q=https://cityofrosedalems.com/
https://www.google.tg/url?q=https://cityofrosedalems.com/
https://www.google.tk/url?q=https://cityofrosedalems.com/
https://www.google.tn/url?q=https://cityofrosedalems.com/
https://www.google.tt/url?q=https://cityofrosedalems.com/
https://www.google.vu/url?q=https://cityofrosedalems.com/
https://www.google.ws/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.uk/url?sa=i&url=https://cityofrosedalems.com/
https://toolbarqueries.google.je/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ms/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.vg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.vu/url?q=https://cityofrosedalems.com/
http://toolbarqueries.google.com.tj/url?sa=t&url=https://cityofrosedalems.com/
https://cse.google.ba/url?q=https://cityofrosedalems.com/
https://cse.google.bj/url?q=https://cityofrosedalems.com/
https://cse.google.cat/url?q=https://cityofrosedalems.com/
https://cse.google.co.bw/url?q=https://cityofrosedalems.com/
https://cse.google.co.kr/url?q=https://cityofrosedalems.com/
https://cse.google.co.nz/url?q=https://cityofrosedalems.com/
https://cse.google.co.zw/url?q=https://cityofrosedalems.com/
https://cse.google.com.ai/url?q=https://cityofrosedalems.com/
https://cse.google.com.ly/url?q=https://cityofrosedalems.com/
https://cse.google.com.sb/url?q=https://cityofrosedalems.com/
https://cse.google.com.sv/url?q=https://cityofrosedalems.com/
https://cse.google.com.vc/url?q=https://cityofrosedalems.com/
https://cse.google.ge/url?q=https://cityofrosedalems.com/
https://cse.google.gy/url?q=https://cityofrosedalems.com/
https://cse.google.hn/url?q=https://cityofrosedalems.com/
https://cse.google.ht/url?q=https://cityofrosedalems.com/
https://cse.google.iq/url?q=https://cityofrosedalems.com/
https://cse.google.lk/url?q=https://cityofrosedalems.com/
https://cse.google.no/url?q=https://cityofrosedalems.com/
https://cse.google.se/url?q=https://cityofrosedalems.com/
https://cse.google.sn/url?q=https://cityofrosedalems.com/
https://cse.google.st/url?q=https://cityofrosedalems.com/
https://cse.google.td/url?q=https://cityofrosedalems.com/
https://cse.google.ws/url?q=https://cityofrosedalems.com/
http://maps.google.ad/url?q=https://cityofrosedalems.com/
http://maps.google.at/url?q=https://cityofrosedalems.com/
http://maps.google.ba/url?q=https://cityofrosedalems.com/
http://maps.google.be/url?q=https://cityofrosedalems.com/
http://maps.google.bf/url?q=https://cityofrosedalems.com/
http://maps.google.bg/url?q=https://cityofrosedalems.com/
http://maps.google.bj/url?q=https://cityofrosedalems.com/
http://maps.google.bs/url?q=https://cityofrosedalems.com/
http://maps.google.by/url?q=https://cityofrosedalems.com/
http://maps.google.cd/url?q=https://cityofrosedalems.com/
http://maps.google.cf/url?q=https://cityofrosedalems.com/
http://maps.google.ch/url?q=https://cityofrosedalems.com/
http://maps.google.ci/url?q=https://cityofrosedalems.com/
http://maps.google.cl/url?q=https://cityofrosedalems.com/
http://maps.google.cm/url?q=https://cityofrosedalems.com/
http://maps.google.co.ao/url?q=https://cityofrosedalems.com/
http://maps.google.co.bw/url?q=https://cityofrosedalems.com/
http://maps.google.co.ck/url?q=https://cityofrosedalems.com/
http://maps.google.co.cr/url?q=https://cityofrosedalems.com/
http://maps.google.co.il/url?q=https://cityofrosedalems.com/
http://maps.google.co.kr/url?q=https://cityofrosedalems.com/
http://maps.google.co.ls/url?q=https://cityofrosedalems.com/
http://maps.google.co.nz/url?q=https://cityofrosedalems.com/
http://maps.google.co.tz/url?q=https://cityofrosedalems.com/
http://maps.google.co.ug/url?q=https://cityofrosedalems.com/
http://maps.google.co.ve/url?q=https://cityofrosedalems.com/
http://maps.google.co.vi/url?q=https://cityofrosedalems.com/
http://maps.google.co.zm/url?q=https://cityofrosedalems.com/
http://maps.google.com.ag/url?q=https://cityofrosedalems.com/
http://maps.google.com.ai/url?q=https://cityofrosedalems.com/
http://maps.google.com.ar/url?q=https://cityofrosedalems.com/
http://maps.google.com.au/url?q=https://cityofrosedalems.com/
http://maps.google.com.bn/url?q=https://cityofrosedalems.com/
http://maps.google.com.bz/url?q=https://cityofrosedalems.com/
http://maps.google.com.ec/url?q=https://cityofrosedalems.com/
http://maps.google.com.eg/url?q=https://cityofrosedalems.com/
http://maps.google.com.et/url?q=https://cityofrosedalems.com/
http://maps.google.com.gh/url?q=https://cityofrosedalems.com/
http://maps.google.com.gt/url?q=https://cityofrosedalems.com/
http://maps.google.com.jm/url?q=https://cityofrosedalems.com/
http://maps.google.com.mt/url?q=https://cityofrosedalems.com/
http://maps.google.com.mx/url?q=https://cityofrosedalems.com/
http://maps.google.com.na/url?q=https://cityofrosedalems.com/
http://maps.google.com.ng/url?q=https://cityofrosedalems.com/
http://maps.google.com.pe/url?q=https://cityofrosedalems.com/
http://maps.google.com.ph/url?q=https://cityofrosedalems.com/
http://maps.google.com.pr/url?q=https://cityofrosedalems.com/
http://maps.google.com.py/url?q=https://cityofrosedalems.com/
http://maps.google.com.qa/url?q=https://cityofrosedalems.com/
http://maps.google.com.sb/url?q=https://cityofrosedalems.com/
http://maps.google.com.sg/url?q=https://cityofrosedalems.com/
http://maps.google.com.tw/url?q=https://cityofrosedalems.com/
http://maps.google.com.uy/url?q=https://cityofrosedalems.com/
http://maps.google.com.vc/url?q=https://cityofrosedalems.com/
http://maps.google.cv/url?q=https://cityofrosedalems.com/
http://maps.google.cz/url?q=https://cityofrosedalems.com/
http://maps.google.dm/url?q=https://cityofrosedalems.com/
http://maps.google.dz/url?q=https://cityofrosedalems.com/
http://maps.google.ee/url?q=https://cityofrosedalems.com/
http://maps.google.fr/url?q=https://cityofrosedalems.com/
http://maps.google.ga/url?q=https://cityofrosedalems.com/
http://maps.google.ge/url?q=https://cityofrosedalems.com/
http://maps.google.gg/url?q=https://cityofrosedalems.com/
http://maps.google.gp/url?q=https://cityofrosedalems.com/
http://maps.google.hr/url?q=https://cityofrosedalems.com/
http://maps.google.hu/url?q=https://cityofrosedalems.com/
http://maps.google.iq/url?q=https://cityofrosedalems.com/
http://maps.google.kg/url?q=https://cityofrosedalems.com/
http://maps.google.ki/url?q=https://cityofrosedalems.com/
http://maps.google.kz/url?q=https://cityofrosedalems.com/
http://maps.google.la/url?q=https://cityofrosedalems.com/
http://maps.google.li/url?q=https://cityofrosedalems.com/
http://maps.google.lt/url?q=https://cityofrosedalems.com/
http://maps.google.mg/url?q=https://cityofrosedalems.com/
http://maps.google.mk/url?q=https://cityofrosedalems.com/
http://maps.google.ms/url?q=https://cityofrosedalems.com/
http://maps.google.mu/url?q=https://cityofrosedalems.com/
http://maps.google.mw/url?q=https://cityofrosedalems.com/
http://maps.google.ne/url?q=https://cityofrosedalems.com/
http://maps.google.nr/url?q=https://cityofrosedalems.com/
http://maps.google.pl/url?q=https://cityofrosedalems.com/
http://maps.google.pn/url?q=https://cityofrosedalems.com/
http://maps.google.pt/url?q=https://cityofrosedalems.com/
http://maps.google.rs/url?q=https://cityofrosedalems.com/
http://maps.google.ru/url?q=https://cityofrosedalems.com/
http://maps.google.rw/url?q=https://cityofrosedalems.com/
http://maps.google.se/url?q=https://cityofrosedalems.com/
http://maps.google.sh/url?q=https://cityofrosedalems.com/
http://maps.google.si/url?q=https://cityofrosedalems.com/
http://maps.google.sm/url?q=https://cityofrosedalems.com/
http://maps.google.sn/url?q=https://cityofrosedalems.com/
http://maps.google.st/url?q=https://cityofrosedalems.com/
http://maps.google.td/url?q=https://cityofrosedalems.com/
http://maps.google.tl/url?q=https://cityofrosedalems.com/
http://maps.google.tn/url?q=https://cityofrosedalems.com/
http://maps.google.ws/url?q=https://cityofrosedalems.com/
https://maps.google.ae/url?q=https://cityofrosedalems.com/
https://maps.google.as/url?q=https://cityofrosedalems.com/
https://maps.google.cat/url?q=https://cityofrosedalems.com/
https://maps.google.co.ck/url?sa=t&source=web&rct=j&url=https://cityofrosedalems.com/
https://maps.google.co.cr/url?q=https://cityofrosedalems.com/
https://maps.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.in/url?q=https://cityofrosedalems.com/
https://maps.google.co.jp/url?q=https://cityofrosedalems.com/
https://maps.google.co.ke/url?q=https://cityofrosedalems.com/
https://maps.google.co.mz/url?q=https://cityofrosedalems.com/
https://maps.google.co.th/url?q=https://cityofrosedalems.com/
https://maps.google.co.ug/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?q=https://cityofrosedalems.com/
https://maps.google.co.za/url?q=https://cityofrosedalems.com/
https://maps.google.co.zw/url?q=https://cityofrosedalems.com/
https://maps.google.com.bd/url?q=https://cityofrosedalems.com/
https://maps.google.com.bh/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.br/url?q=https://cityofrosedalems.com/
https://maps.google.com.co/url?q=https://cityofrosedalems.com/
https://maps.google.com.cu/url?q=https://cityofrosedalems.com/
https://maps.google.com.do/url?q=https://cityofrosedalems.com/
https://maps.google.com.fj/url?sa=t&rct=j&url=https://cityofrosedalems.com/
https://maps.google.com.gi/url?q=https://cityofrosedalems.com/
https://maps.google.com.hk/url?q=https://cityofrosedalems.com/
https://maps.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ly/url?sa=i&rct=j&url=https://cityofrosedalems.com/
https://maps.google.com.mm/url?q=https://cityofrosedalems.com/
https://maps.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.np/url?q=https://cityofrosedalems.com/
https://maps.google.com.om/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.pa/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.pg/url?q=https://cityofrosedalems.com/
https://maps.google.com.sa/url?q=https://cityofrosedalems.com/
https://maps.google.com.sl/url?q=https://cityofrosedalems.com/
https://maps.google.com.sv/url?q=https://cityofrosedalems.com/
https://maps.google.com.tr/url?q=https://cityofrosedalems.com/
https://maps.google.de/url?q=https://cityofrosedalems.com/
https://maps.google.dj/url?q=https://cityofrosedalems.com/
https://maps.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.es/url?q=https://cityofrosedalems.com/
https://maps.google.fi/url?q=https://cityofrosedalems.com/
https://maps.google.fm/url?q=https://cityofrosedalems.com/
https://maps.google.gl/url?q=https://cityofrosedalems.com/
https://maps.google.gm/url?q=https://cityofrosedalems.com/
https://maps.google.gr/url?q=https://cityofrosedalems.com/
https://maps.google.gy/url?q=https://cityofrosedalems.com/
https://maps.google.hn/url?q=https://cityofrosedalems.com/
https://maps.google.ht/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?sa=j&rct=j&url=https://cityofrosedalems.com/
https://maps.google.im/url?q=https://cityofrosedalems.com/
https://maps.google.is/url?q=https://cityofrosedalems.com/
https://maps.google.it/url?q=https://cityofrosedalems.com/
https://maps.google.lk/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.lv/url?q=https://cityofrosedalems.com/
https://maps.google.ml/url?sa=i&url=https://cityofrosedalems.com/
https://maps.google.mn/url?q=https://cityofrosedalems.com/
https://maps.google.mv/url?q=https://cityofrosedalems.com/
https://maps.google.nl/url?q=https://cityofrosedalems.com/
https://maps.google.no/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ro/url?q=https://cityofrosedalems.com/
https://maps.google.sk/url?q=https://cityofrosedalems.com/
https://maps.google.so/url?q=https://cityofrosedalems.com/
https://maps.google.tg/url?q=https://cityofrosedalems.com/
https://maps.google.to/url?q=https://cityofrosedalems.com/
https://maps.google.tt/url?q=https://cityofrosedalems.com/
https://maps.google.vg/url?q=https://cityofrosedalems.com/
https://maps.google.vu/url?q=https://cityofrosedalems.com/
http://www.google.be/url?q=https://cityofrosedalems.com/
http://www.google.bf/url?q=https://cityofrosedalems.com/
http://www.google.bt/url?q=https://cityofrosedalems.com/
http://www.google.ca/url?q=https://cityofrosedalems.com/
http://www.google.cd/url?q=https://cityofrosedalems.com/
http://www.google.cl/url?q=https://cityofrosedalems.com/
http://www.google.co.ck/url?q=https://cityofrosedalems.com/
http://www.google.co.ls/url?q=https://cityofrosedalems.com/
http://www.google.com.af/url?q=https://cityofrosedalems.com/
http://www.google.com.au/url?q=https://cityofrosedalems.com/
http://www.google.com.bn/url?q=https://cityofrosedalems.com/
http://www.google.com.do/url?q=https://cityofrosedalems.com/
http://www.google.com.eg/url?q=https://cityofrosedalems.com/
http://www.google.com.et/url?q=https://cityofrosedalems.com/
http://www.google.com.gi/url?q=https://cityofrosedalems.com/
http://www.google.com.na/url?q=https://cityofrosedalems.com/
http://www.google.com.np/url?q=https://cityofrosedalems.com/
http://www.google.com.sg/url?q=https://cityofrosedalems.com/
http://www.google.com/url?q=https://cityofrosedalems.com/
http://www.google.cv/url?q=https://cityofrosedalems.com/
http://www.google.dm/url?q=https://cityofrosedalems.com/
http://www.google.es/url?q=https://cityofrosedalems.com/
http://www.google.iq/url?q=https://cityofrosedalems.com/
http://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0cdiqfjaa&url=https://cityofrosedalems.com/
http://www.google.kg/url?q=https://cityofrosedalems.com/
http://www.google.kz/url?q=https://cityofrosedalems.com/
http://www.google.li/url?q=https://cityofrosedalems.com/
http://www.google.me/url?q=https://cityofrosedalems.com/
http://www.google.pn/url?q=https://cityofrosedalems.com/
http://www.google.ps/url?q=https://cityofrosedalems.com/
http://www.google.sn/url?q=https://cityofrosedalems.com/
http://www.google.so/url?q=https://cityofrosedalems.com/
http://www.google.st/url?q=https://cityofrosedalems.com/
http://www.google.td/url?q=https://cityofrosedalems.com/
http://www.google.tm/url?q=https://cityofrosedalems.com/
https://image.google.com.kw/url?sa=t&rct=j&url=https://cityofrosedalems.com/
https://image.google.com.nf/url?sa=j&url=https://cityofrosedalems.com/
https://image.google.tn/url?q=j&sa=t&url=https://cityofrosedalems.com/
https://images.google.ad/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.as/url?q=https://cityofrosedalems.com/
https://images.google.az/url?q=https://cityofrosedalems.com/
https://images.google.be/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?q=https://cityofrosedalems.com/
https://images.google.bi/url?q=https://cityofrosedalems.com/
https://images.google.bs/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cat/url?q=https://cityofrosedalems.com/
https://images.google.cd/url?q=https://cityofrosedalems.com/
https://images.google.cl/url?q=https://cityofrosedalems.com/
https://images.google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?q=https://cityofrosedalems.com/
https://images.google.co.ke/url?q=https://cityofrosedalems.com/
https://images.google.co.kr/url?q=https://cityofrosedalems.com/
https://images.google.co.ls/url?q=https://cityofrosedalems.com/
https://images.google.co.th/url?q=https://cityofrosedalems.com/
https://images.google.co.zm/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.af/url?q=https://cityofrosedalems.com/
https://images.google.com.ai/url?q=https://cityofrosedalems.com/
https://images.google.com.ar/url?q=https://cityofrosedalems.com/
https://images.google.com.bd/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bn/url?q=https://cityofrosedalems.com/
https://images.google.com.bo/url?q=https://cityofrosedalems.com/
https://images.google.com.br/url?q=https://cityofrosedalems.com/
https://images.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cu/url?q=https://cityofrosedalems.com/
https://images.google.com.do/url?q=https://cityofrosedalems.com/
https://images.google.com.et/url?q=https://cityofrosedalems.com/
https://images.google.com.gh/url?q=https://cityofrosedalems.com/
https://images.google.com.hk/url?q=https://cityofrosedalems.com/
https://images.google.com.lb/url?q=https://cityofrosedalems.com/
https://images.google.com.mm/url?q=https://cityofrosedalems.com/
https://images.google.com.mx/url?q=https://cityofrosedalems.com/
https://images.google.com.my/url?q=https://cityofrosedalems.com/
https://images.google.com.np/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pg/url?q=https://cityofrosedalems.com/
https://images.google.com.pk/url?q=https://cityofrosedalems.com/
https://images.google.com.pr/url?q=https://cityofrosedalems.com/
https://images.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?q=https://cityofrosedalems.com/
https://images.google.com.sv/url?q=https://cityofrosedalems.com/
https://images.google.com.tr/url?q=https://cityofrosedalems.com/
https://images.google.com.tw/url?q=https://cityofrosedalems.com/
https://images.google.com.ua/url?q=https://cityofrosedalems.com/
https://images.google.cv/url?q=https://cityofrosedalems.com/
https://images.google.cz/url?sa=i&url=https://cityofrosedalems.com/
https://images.google.dj/url?q=https://cityofrosedalems.com/
https://images.google.dk/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?q=https://cityofrosedalems.com/
https://images.google.es/url?q=https://cityofrosedalems.com/
https://images.google.fm/url?q=https://cityofrosedalems.com/
https://images.google.fr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gl/url?q=https://cityofrosedalems.com/
https://images.google.hr/url?q=https://cityofrosedalems.com/
https://images.google.iq/url?q=https://cityofrosedalems.com/
https://images.google.jo/url?q=https://cityofrosedalems.com/
https://images.google.lk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.lt/url?q=https://cityofrosedalems.com/
https://images.google.lu/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.md/url?q=https://cityofrosedalems.com/
https://images.google.mk/url?q=https://cityofrosedalems.com/
https://images.google.mn/url?q=https://cityofrosedalems.com/
https://images.google.ms/url?q=https://cityofrosedalems.com/
https://images.google.ne/url?q=https://cityofrosedalems.com/
https://images.google.ng/url?q=https://cityofrosedalems.com/
https://images.google.nl/url?q=https://cityofrosedalems.com/
https://images.google.ps/url?q=https://cityofrosedalems.com/
https://images.google.ro/url?q=https://cityofrosedalems.com/
https://images.google.ru/url?q=https://cityofrosedalems.com/
https://images.google.rw/url?q=https://cityofrosedalems.com/
https://images.google.si/url?q=https://cityofrosedalems.com/
https://images.google.sm/url?q=https://cityofrosedalems.com/
https://images.google.sn/url?q=https://cityofrosedalems.com/
https://images.google.so/url?q=https://cityofrosedalems.com/
https://images.google.sr/url?q=https://cityofrosedalems.com/
https://images.google.st/url?q=https://cityofrosedalems.com/
https://images.google.tl/url?q=https://cityofrosedalems.com/
https://images.google.tn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.to/url?q=https://cityofrosedalems.com/
https://images.google.vu/url?q=https://cityofrosedalems.com/
http://images.google.am/url?q=https://cityofrosedalems.com/
http://images.google.ba/url?q=https://cityofrosedalems.com/
http://images.google.bf/url?q=https://cityofrosedalems.com/
http://images.google.co.ao/url?q=https://cityofrosedalems.com/
http://images.google.co.jp/url?q=https://cityofrosedalems.com/
http://images.google.co.nz/url?q=https://cityofrosedalems.com/
http://images.google.co.ug/url?q=https://cityofrosedalems.com/
http://images.google.co.uk/url?q=https://cityofrosedalems.com/
http://images.google.co.uz/url?q=https://cityofrosedalems.com/
http://images.google.co.ve/url?q=https://cityofrosedalems.com/
http://images.google.com.co/url?q=https://cityofrosedalems.com/
http://images.google.com.ly/url?q=https://cityofrosedalems.com/
http://images.google.com.ng/url?q=https://cityofrosedalems.com/
http://images.google.com.om/url?q=https://cityofrosedalems.com/
http://images.google.com.qa/url?q=https://cityofrosedalems.com/
http://images.google.com.sb/url?q=https://cityofrosedalems.com/
http://images.google.com.sl/url?q=https://cityofrosedalems.com/
http://images.google.com.uy/url?q=https://cityofrosedalems.com/
http://images.google.com.vc/url?q=https://cityofrosedalems.com/
http://images.google.de/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.is/url?q=https://cityofrosedalems.com/
http://images.google.it/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?q=https://cityofrosedalems.com/
http://images.google.me/url?q=https://cityofrosedalems.com/
http://images.google.mu/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?q=https://cityofrosedalems.com/
http://images.google.rs/url?q=https://cityofrosedalems.com/
http://images.google.td/url?q=https://cityofrosedalems.com/
http://images.google.tm/url?q=https://cityofrosedalems.com/
http://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.om/url?q=https://cityofrosedalems.com/
http://images.google.com.qa/url?q=https://cityofrosedalems.com/
http://images.google.com.sb/url?q=https://cityofrosedalems.com/
http://images.google.com.sl/url?q=https://cityofrosedalems.com/
http://images.google.com.uy/url?q=https://cityofrosedalems.com/
http://images.google.com.vc/url?q=https://cityofrosedalems.com/
http://images.google.de/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.is/url?q=https://cityofrosedalems.com/
http://images.google.it/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?q=https://cityofrosedalems.com/
http://images.google.me/url?q=https://cityofrosedalems.com/
http://images.google.mu/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?q=https://cityofrosedalems.com/
http://images.google.rs/url?q=https://cityofrosedalems.com/
http://images.google.td/url?q=https://cityofrosedalems.com/
http://images.google.tm/url?q=https://cityofrosedalems.com/
http://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://medium.com/@adam.scotty.best
https://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://www.cityofrosedalems.com/
http://cityofrosedalems.com/
https://cityofrosedalems.com/
http://www.cityofrosedalems.com/
안전해외배팅사이트 https://cityofrosedalems.com/
해외 스포츠 배팅 사이트 https://cityofrosedalems.com/
해외배팅사이트 먹튀검증 https://cityofrosedalems.com/
안전배팅사이트 https://cityofrosedalems.com/
안전 온라인 카지노 https://cityofrosedalems.com/
해외 온라인 카지노 https://cityofrosedalems.com/
유럽 온라인 카지노 https://cityofrosedalems.com/
안전 온라인 바카라 https://cityofrosedalems.com/
해외 스포츠 배팅 안전주소 https://cityofrosedalems.com/
해외 스포츠 배팅 먹튀검증 https://cityofrosedalems.com/
안전 해외 카지노사이트 https://cityofrosedalems.com/
안전 해외 바카라사이트 https://cityofrosedalems.com/
헤라카지노 https://cityofrosedalems.com/헤라카지노/
아시안커넥트 https://ac-tm66.net/
에볼루션 카지노 https://cityofrosedalems.com/에볼루션카지노/
247벳코리아 https://cityofrosedalems.com/247벳코리아/
쿨카지노 https://cityofrosedalems.com/쿨카지노/
뉴헤븐카지노 https://cityofrosedalems.com/뉴헤븐카지노/
파라오카지노 https://cityofrosedalems.com/파라오카지노/
맥스벳 https://ac-tm66.net/
비티아이 스포츠 https://ac-tm66.net/
스보벳 https://ac-tm66.net/
1xbet https://cityofrosedalems.com/247벳코리아/
피나클 https://ac-tm66.net/
https://gamma.app/public/247-652nia2b0wmcbkc
https://gamma.app/public/247-6jcr5hfz3zo7hto
https://gamma.app/public/-n3szrlsehdr6wt3
https://sebsauvage.net/paste/?8c54344c01775406#XeaJJXo/6gTGsWMOAgoezJjCtAo7R/wLiaMhWSvxmu0=
https://sites.google.com/view/newhc/%ED%99%88
https://sites.google.com/view/newhc/
https://sites.google.com/view/newhc/%20
http://sites.google.com/view/newhc
https://sites.google.com/view/newhc/
https://sites.google.com/view/newhc/%2F
http://sites.google.com/view/newhc/%20
http://sites.google.com/view/newhc/
http://www.sites.google.com/view/newhc/
https://www.sites.google.com/view/newhc/
http://www.sites.google.com/view/newhc/%20
https://www.sites.google.com/view/newhc/%20
http://www.sites.google.com/view/newhc/%2F
https://www.sites.google.com/view/newhc/%2F
https://sites.google.com/view/247betkor/%ED%99%88
https://sites.google.com/view/247betkor/
https://sites.google.com/view/247betkor/%20
http://sites.google.com/view/247betkor
https://sites.google.com/view/247betkor/
https://sites.google.com/view/247betkor/%2F
http://sites.google.com/view/247betkor/%20
http://sites.google.com/view/247betkor/
http://www.sites.google.com/view/247betkor/
https://www.sites.google.com/view/247betkor/
http://www.sites.google.com/view/247betkor/%20
https://www.sites.google.com/view/247betkor/%20
http://www.sites.google.com/view/247betkor/%2F
https://www.sites.google.com/view/247betkor/%2F
https://sites.google.com/view/coolcasin0/%ED%99%88
https://sites.google.com/view/coolcasin0/
https://sites.google.com/view/coolcasin0/%20
http://sites.google.com/view/coolcasin0
https://sites.google.com/view/coolcasin0/
https://sites.google.com/view/coolcasin0/%2F
http://sites.google.com/view/coolcasin0/%20
http://sites.google.com/view/coolcasin0/
http://www.sites.google.com/view/coolcasin0/
https://www.sites.google.com/view/coolcasin0/
http://www.sites.google.com/view/coolcasin0/%20
https://www.sites.google.com/view/coolcasin0/%20
http://www.sites.google.com/view/coolcasin0/%2F
https://www.sites.google.com/view/coolcasin0/%2F
https://sites.google.com/view/herac/%ED%99%88
https://sites.google.com/view/herac/
https://sites.google.com/view/herac/%20
http://sites.google.com/view/herac
https://sites.google.com/view/herac/
https://sites.google.com/view/herac/%2F
http://sites.google.com/view/herac/%20
http://sites.google.com/view/herac/
http://www.sites.google.com/view/herac/
https://www.sites.google.com/view/herac/
http://www.sites.google.com/view/herac/%20
https://www.sites.google.com/view/herac/%20
http://www.sites.google.com/view/herac/%2F
https://www.sites.google.com/view/herac/%2F
https://codrose8.blogspot.com/
https://codtulip1.blogspot.com/
https://codlily3.blogspot.com/
https://codorchid00.blogspot.com/
https://codlotus5.blogspot.com/
https://codrose8.mystrikingly.com/
https://codtulip1.mystrikingly.com/
https://codlily3.mystrikingly.com/
https://codorchid00.mystrikingly.com/
https://codlotus5.mystrikingly.com/
codrose8.wordpress.com/
codtulip1.wordpress.com/
codorchid00.wordpress.com/
codlotus5.wordpress.com/
https://adamscottybest.wixsite.com/asianconnect
https://sites.google.com/view/maxbet7/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/btisports/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/sbobet10/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/asianconect/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://sites.google.com/view/pinbet88/%EC%95%84%EC%8B%9C%EC%95%88%EC%BB%A4%EB%84%A5%ED%8A%B8
https://docs.google.com/drawings/d/1Rw9uy_zVHH-HFMfC6zzDogmKWEX4QfRJKouLbBVDX9Q/
https://docs.google.com/drawings/d/1jfDjmepvqsFmjddMk9ExSpiucLuLCooG25nHYJJv32E/
https://docs.google.com/drawings/d/1aOosLvI9NmB14qzndI73GAlLEbqLMx9ch9DKc66TIRE/
https://docs.google.com/drawings/d/1iw_h50dwb711A3vZJdapzQeqNjP8qSSAzOLTq1txjX4/
https://docs.google.com/drawings/d/1UlCrDTUNaz-oNqO7oehLZVMbQML_uu3mTVwxeOAUAno/
https://duckduckgo.com/?va=p&t=hc&q=https%3A%2F%2Fcityofrosedalems.com%2F&ia=web
https://www.google.com/search?q=https%3A%2F%2Fcityofrosedalems.com%2F&sca_esv=569194044&hl=ko&sxsrf=AM9HkKmEN6znojH7Kxw4Q8vGgB5hN2__yg%3A1702917062922&ei=xnOAZcruN5r2seMPx6u7cA&ved=0ahUKEwjK6fe0tJmDAxUae2wGHcfVDg4Q4dUDCBA&uact=5&oq=https%3A%2F%2Fcityofrosedalems.com%2F&gs_lp=Egxnd3Mtd2l6LXNlcnAiHWh0dHBzOi8vY2l0eW9mcm9zZWRhbGVtcy5jb20vMgQQIxgnSKhaUMlDWMlDcAJ4AJABAJgBUqABUqoBATG4AQPIAQD4AQL4AQHiAwQYASBBiAYB&sclient=gws-wiz-serp
https://www.google.com/search?q=site%3Acityofrosedalems.com%2F&sca_esv=569194044&hl=ko&sxsrf=AM9HkKkQ5LwIAxKBzwN5nI5szVREfrLPxw%3A1702966192183&ei=sDOBZajZCq_i4-EPh8mf8Aw&ved=0ahUKEwio8cu365qDAxUv8TgGHYfkB84Q4dUDCBA&uact=5&oq=site%3Acityofrosedalems.com%2F&gs_lp=Egxnd3Mtd2l6LXNlcnAiGnNpdGU6Y2l0eW9mcm9zZWRhbGVtcy5jb20vSI5vUIlNWPZYcAV4AJABAJgBY6ABpAOqAQE1uAEDyAEA-AEB4gMEGAEgQYgGAQ&sclient=gws-wiz-serp#ip=1
https://www.google.com/search?q=https%3A%2F%2Fcityofrosedalems.com%2F&tbm=isch&ved=2ahUKEwja_6Tb9JqDAxX1e2wGHStbAbEQ2-cCegQIABAA&oq=https%3A%2F%2Fcityofrosedalems.com%2F&gs_lcp=CgNpbWcQAzIECCMQJ1DVNljVNmCTP2gAcAB4AIABkQGIAaIDkgEDMC4zmAEAoAEBqgELZ3dzLXdpei1pbWfAAQE&sclient=img&ei=aj2BZdqiOvX3seMPq7aFiAs&bih=991&biw=2048
https://www.bing.com/search?q=cityofrosedalems.com%2F&form=QBLH&sp=-1&lq=0&pq=cityofrosedalems.com%2F&sc=3-21&qs=n&sk=&cvid=D93E7BBA539348C9B1981797A2C693C0&ghsh=0&ghacc=0&ghpl=
https://ph.search.yahoo.com/yhs/search?hspart=bc&hsimp=yhs-fresh&type=jjdeaf&p=cityofrosedalems.com%2F
mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=https://cityofrosedalems.com/
materinstvo.ru/forward?link=https://cityofrosedalems.com/
http://computer-chess.org/lib/exe/fetch.php?media=https://cityofrosedalems.com/
https://enchantedcottageshop.com/shop/trigger.php?r_link=https://cityofrosedalems.com/
https://buist-keatch.org/sphider/include/click_counter.php?url=https://cityofrosedalems.com/
www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=https://cityofrosedalems.com/
rel.chubu-gu.ac.jp/soumokuji/cgi-bin/go.cgi?https://cityofrosedalems.com/
fallout3.ru/utils/ref.php?url=https://cityofrosedalems.com/
www.veloxbox.us/link/?h=https://cityofrosedalems.com/
www.adult-plus.com/ys/rank.php?mode=link&id=592&url=https://cityofrosedalems.com/
mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=https://cityofrosedalems.com/
sparktime.justclick.ru/lms/api-login/?hash=MO18szcRUQdzpT%2FrstSCW5K8Gz6ts1NvTJLVa34vf1A%3D&authBhvr=1&email=videotrend24%40mail.ru&expire=1585462818&lms%5BrememberMe%5D=1&targetPath=https://cityofrosedalems.com/
http://vcteens.com/cgi-bin/at3/out.cgi?trade=https://cityofrosedalems.com/
www.bquest.org/Links/Redirect.aspx?ID=164&url=https://cityofrosedalems.com/
www.stipendije.info/phpAdsNew/adclick.php?bannerid=129&zoneid=1&source=&dest=https://cityofrosedalems.com/
today.od.ua/redirect.php?url=https://cityofrosedalems.com/
search.kcm.co.kr/jump.php?url=https://cityofrosedalems.com/
laskma.megastart-slot.ru/redirect/?g=https://cityofrosedalems.com/
www.uktrademarkregistration.co.uk/JumpTo.aspx?url=https://cityofrosedalems.com/
www.mir-stalkera.ru/go?https://cityofrosedalems.com/
duhocphap.edu.vn/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
www.millerovo161.ru/go?https://cityofrosedalems.com/
www.naturaltranssexuals.com/cgi-bin/a2/out.cgi?id=97&l=toplist&u=https://cityofrosedalems.com/
https://amanaimages.com/lsgate/?lstid=pM6b0jdQgVM-Y9ibFgTe6Zv1N0oD2nYuMA&lsurl=https://cityofrosedalems.com/
planszowkiap.pl/trigger.php?r_link=https://cityofrosedalems.com/
http://covenantpeoplesministry.org/cpm/wp/sermons/?show&url=https://cityofrosedalems.com/
www.sporteasy.net/redirect/?url=https://cityofrosedalems.com/
mightypeople.asia/link.php?id=M0ZGNHFISkd2bFh0RmlwSFU4bDN4QT09&destination=https://cityofrosedalems.com/
www.chinaleatheroid.com/redirect.php?url=https://cityofrosedalems.com/
i.s0580.cn/module/adsview/content/?action=click&bid=5&aid=163&url=https://cityofrosedalems.com/&variable=&source=https%3A%2F%2Fcutepix.info%2Fsex%2Friley-reyes.php
jsv3.recruitics.com/redirect?rx_cid=506&rx_jobId=39569207&rx_url=https://cityofrosedalems.com/
https://jobinplanet.com/away?link=https://cityofrosedalems.com/
www.supermoto8.com/sidebanner/62?href=https://cityofrosedalems.com/
presse.toyota.dk/login.aspx?returnurl=https://cityofrosedalems.com/
http://www.youtube.de/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.com/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.es/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ca/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.nl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.pl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ch/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.be/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.se/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.dk/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.pt/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.no/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.gr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.cl/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.at/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.bg/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.sk/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.rs/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.lt/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.si/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.hr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ee/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.lu/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.tn/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co.ke/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.co.cr/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.kz/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.cat/redirect?event=channel_description&q=https://cityofrosedalems.com/
http://www.youtube.ge/redirect?event=channel_description&q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?event=channel_description&q=https://cityofrosedalems.com/&gl=ml
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=DE
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=IT
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=AR
https://www.youtube.at/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ch/redirect?q=https://cityofrosedalems.com/
https://www.youtube.fr/redirect?q=https://cityofrosedalems.com/
https://www.youtube.es/redirect?q=https://cityofrosedalems.com/
https://www.youtube.jp/redirect?q=https://cityofrosedalems.com/
https://www.youtube.co.uk/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ru/redirect?q=https://cityofrosedalems.com/
https://www.youtube.pl/redirect?q=https://cityofrosedalems.com/
https://www.youtube.gr/redirect?q=https://cityofrosedalems.com/
https://www.youtube.nl/redirect?q=https://cityofrosedalems.com/
https://www.youtube.ca/redirect?q=https://cityofrosedalems.com/
https://www.youtube.cz/redirect?q=https://cityofrosedalems.com/
https://www.youtube.com/redirect?q=https://cityofrosedalems.com/&gl=AU
https://www.youtube.com.tw/redirect?q=https://cityofrosedalems.com/
golfpark.jp/banner/counter.aspx?url=https://cityofrosedalems.com/
www.mexicolore.co.uk/click.php?url=https://cityofrosedalems.com/
www.tiersertal.com/clicks/uk_banner_click.php?url=https://cityofrosedalems.com/
www.invisalign-doctor.in/api/redirect?url=https://cityofrosedalems.com/
www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMV%20FIELD]EMAIL[EMV%20/FIELD]&cat=Techniques+culturales&url=https://cityofrosedalems.com/
eatart.dk/Home/ChangeCulture?lang=da&returnUrl=https://cityofrosedalems.com/
www.hschina.net/ADClick.aspx?SiteID=206&ADID=1&URL=https://cityofrosedalems.com/
cipresso.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.mydosti.com/Advertisement/updateadvhits.aspx?adid=48&gourl=https://cityofrosedalems.com/
www.beeicons.com/redirect.php?site=https://cityofrosedalems.com/
hirlevel.pte.hu/site/redirect?newsletter_id=UFV1UG5yZ3hOaWFyQVhvSUFoRmRQUT09&recipient=Y25zcm1ZaGxvR0xJMFNtNmhwdmpPNFlVSzlpS2c4ZnA1NzRPWjJKY3QrND0=&address=https://cityofrosedalems.com/
www.joeshouse.org/booking?link=https://cityofrosedalems.com/&ID=1112
hanhphucgiadinh.vn/ext-click.php?url=https://cityofrosedalems.com/
http://okane-antena.com/redirect/index/fid100269/?u=https://cityofrosedalems.com/
www.gmwebsite.com/web/redirect.asp?url=https://cityofrosedalems.com/
swra.backagent.net/ext/rdr/?https://cityofrosedalems.com/
namiotle.pl/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
www.wqketang.com/logout?goto=https://cityofrosedalems.com/
access.bridges.com/externalRedirector.do?url=https://cityofrosedalems.com/
www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://cityofrosedalems.com/
bot.buymeapie.com/recipe?url=https://cityofrosedalems.com/
www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://cityofrosedalems.com/
extremaduraempresarial.juntaex.es/cs/c/document_library/find_file_entry?p_l_id=47702&noSuchEntryRedirect=https://cityofrosedalems.com/
rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://cityofrosedalems.com/
www.knet-web.net/m/pRedirect.php?uID=2&iID=259&iURL=https://cityofrosedalems.com/
holmss.lv/bancp/www/delivery/ck.php?ct=1&oaparams=2bannerid=44__zoneid=1__cb=7743e8d201__oadest=https://cityofrosedalems.com/ ?
www.mintmail.biz/track/clicks/v2/?messageid=1427&cid=54657&url=https://cityofrosedalems.com/
marciatravessoni.com.br/revive/www/delivery/ck.php?ct=1&oaparams=2__bannerid=40__zoneid=16__cb=fc1d72225c__oadest=https://cityofrosedalems.com/
psylive.ru/success.aspx?id=0&goto=https://cityofrosedalems.com/
https://sohodiffusion.com/mod/mod_langue.asp?action=francais&url=https://cityofrosedalems.com/
www.mybunnies.net/te3/out.php?u=https://cityofrosedalems.com/
lubaczowskie.pl/rdir/?l=https://cityofrosedalems.com/&lid=1315
www.ieslaasuncion.org/enlacesbuscar/clicsenlaces.asp?Idenlace=411&url=https://cityofrosedalems.com/
www.wave24.net/cgi-bin/linkrank/out.cgi?id=106248&cg=1&url=https://cityofrosedalems.com/
www.tssweb.co.jp/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
e.ourger.com/?c=scene&a=link&id=47154371&url=https://cityofrosedalems.com/
zh-hk.guitarians.com/home/redirect/ubid/1015?r=https://cityofrosedalems.com/
www.mastercleaningsupply.com/trigger.php?r_link=https://cityofrosedalems.com/
www.cumshoter.com/cgi-bin/at3/out.cgi?id=98&tag=top&trade=https://cityofrosedalems.com/
shp.hu/hpc_uj/click.php?ml=5&url=https://cityofrosedalems.com/
https://indonesianmma.com/modules/mod_jw_srfr/redir.php?url=https://cityofrosedalems.com/
www.dive-international.net/places/redirect.php?b=797&web=www.https://cityofrosedalems.com/
www.themza.com/redirect.php?r=https://cityofrosedalems.com/
lambda.ecommzone.com/lz/srr/00as0z/06e397d17325825ee6006c3c5ee495f922/actions/redirect.aspx?url=http://https://cityofrosedalems.com/
v.wcj.dns4.cn/?c=scene&a=link&id=8833621&url=https://cityofrosedalems.com/
spb-medcom.ru/redirect.php?https://cityofrosedalems.com/
forest.ru/links.php?go=https://cityofrosedalems.com/
reefcentral.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
bsau.ru/bitrix/redirect.php?event1=news_out&event2=2Fiblock9CB0%D1D0D0D0%B0BB87B0%D1D1D0D1%82B5%D1D0%B8B0%D0D1D0D1%81828C+2.pdf&goto=https://cityofrosedalems.com/
https://trackdaytoday.com/redirect-out?url=https://cityofrosedalems.com/
https://bigjobslittlejobs.com/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=bigjobslittlejobs.com&rgp_m=title23&et=4495
ekovjesnik.hr/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=4__zoneid=4__cb=68dbdae1d1__oadest=https://cityofrosedalems.com/
www.obertaeva.com/include/get.php?go=https://cityofrosedalems.com/
https://studiohire.com/admin-web-tel-process.php?memberid=4638&indentifier=weburl&websitelinkhitnumber=7&telnumberhitnumber=0&websiteurl=https://cityofrosedalems.com/
www.quanmama.com/t/goto.aspx?url=https://cityofrosedalems.com/
quartiernetz-friesenberg.ch/links-go.php?to=https://cityofrosedalems.com/
http://anorexicpornmovies.com/cgi-bin/atc/out.cgi?id=20&u=https://cityofrosedalems.com/
www.maultalk.com/url.php?to=https://cityofrosedalems.com/
www.infohakodate.com/ps/ps_search.cgi?act=jump&url=https://cityofrosedalems.com/
www.e-expo.net/category/click_url.html?url=https://cityofrosedalems.com/
www.chitaitext.ru/bitrix/redirect.php?event1=utw&event2=utw1&event3=&goto=https://cityofrosedalems.com/
www.realsubliminal.com/newsletter/t/c/11098198/c?dest=https://cityofrosedalems.com/
http://getdatasheet.com/url.php?url=https://cityofrosedalems.com/
www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://cityofrosedalems.com/
https://kirei-style.info/st-manager/click/track?id=7643&type=raw&url=https://cityofrosedalems.com/
www.aldolarcher.com/tools/esstat/esdown.asp?File=https://cityofrosedalems.com/
embed.gabrielny.com/embedlink?key=f12cc3d5-e680-47b0-8914-a6ce19556f96&width=100%25&height=1200&division=bridal&no_chat=1&domain=https://cityofrosedalems.com/
cs.payeasy.com.tw/click?url=https://cityofrosedalems.com/
www.168web.com.tw/in/front/bin/adsclick.phtml?Nbr=114_02&URL=https://cityofrosedalems.com/
http://tswzjs.com/go.asp?url=https://cityofrosedalems.com/
asstomouth.guru/out.php?url=https://cityofrosedalems.com/
advtest.exibart.com/adv/adv.php?id_banner=7201&link=https://cityofrosedalems.com/
https://thairesidents.com/l.php?b=85&p=2,5&l=https://cityofrosedalems.com/
www.latestnigeriannews.com/link_channel.php?channel=https://cityofrosedalems.com/
www.haogaoyao.com/proad/default.aspx?url=https://cityofrosedalems.com/
globalmedia51.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
citysafari.nl/Home/setCulture?language=en&returnUrl=https://cityofrosedalems.com/
es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
lorena-kuhni.kz/redirect?link=https://cityofrosedalems.com/
www.webshoptrustmark.fr/Change/en?returnUrl=https://cityofrosedalems.com/
https://processon.com/setting/locale?language=zh&back=https://cityofrosedalems.com/
c.yam.com/srh/wsh/r.c?https://cityofrosedalems.com/
www.jolletorget.no/J/l.php?l=https://cityofrosedalems.com/
www.bobclubsau.com/cmshome/WebsiteAuditor/6744?url=https://cityofrosedalems.com/
www.moonbbs.com/dm/dmlink.php?dmurl=https://cityofrosedalems.com/
www.sparetimeteaching.dk/forward.php?link=https://cityofrosedalems.com/
https://paspn.net/default.asp?p=90&gmaction=40&linkid=52&linkurl=https://cityofrosedalems.com/
www.dialogportal.com/Services/Forward.aspx?link=https://cityofrosedalems.com/
www.poddebiczak.pl/?action=set-desktop&url=https://cityofrosedalems.com/
ant53.ru/file/link.php?url=https://cityofrosedalems.com/
www.docin.com/jsp_cn/mobile/tip/android_v1.jsp?forward=https://cityofrosedalems.com/
old.magictower.ru/cgi-bin/redir/redir.pl?https://cityofrosedalems.com/
go.flx1.com/click?id=1&m=11&pl=113&dmcm=16782&euid=16603484876&out=https://cityofrosedalems.com/
moba-hgh.de/link2http.php?href=https://cityofrosedalems.com/
https://gazetablic.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=34__zoneid=26__cb=0e0dfef92b__oadest=https://cityofrosedalems.com/
watchvideo.co/go.php?url=https://cityofrosedalems.com/
www.todoku.info/gpt/rank.cgi?mode=link&id=29649&url=https://cityofrosedalems.com/
www.fotochki.com/redirect.php?go=https://cityofrosedalems.com/
bayerwald.tips/plugins/bannerverwaltung/bannerredirect.php?bannerid=1&url=https://cityofrosedalems.com/
ms1.caps.ntct.edu.tw/school/netlink/hits.php?id=107&url=https://cityofrosedalems.com/
www.widgetinfo.net/read.php?sym=FRA_LM&url=https://cityofrosedalems.com/
arctic.nyheter24.se/rdb/nyheter24_eed6ad4b451f2fb8193922f832bc91ed/5?url=https://cityofrosedalems.com/
www.sdam-snimu.ru/redirect.php?url=https://cityofrosedalems.com/
school.mosreg.ru/soc/moderation/abuse.aspx?link=https://cityofrosedalems.com/
affiliates.kanojotoys.com/affiliate/scripts/click.php?a_aid=widdi77&desturl=https://cityofrosedalems.com/
www.gotomypctech.com/affiliates/scripts/click.php?a_aid=ed983915&a_bid=&desturl=https://cityofrosedalems.com/
www.my-sms.ru/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/&rel=external
www.genderpsychology.com/https://cityofrosedalems.com/
http://chtbl.com/track/118167/https://cityofrosedalems.com/
www.360wichita.com/amp-banner-tracking?adid=192059&url=https://cityofrosedalems.com/
www.tsv-bad-blankenburg.de/cms/page/mod/url/url.php?eid=16&urlpf=https://cityofrosedalems.com/
https://fishki.net/click?https://cityofrosedalems.com/
https://zerlong.com/bitrix/redirect.php?goto=https://cityofrosedalems.com/
catalog.flexcom.ru/go?z=36047&i=55&u=https://cityofrosedalems.com/
www.wellvit.nl/response/forward/c1e41491e30c5af3c20f80a2af44e440.php?link=0&target=https://cityofrosedalems.com/
www.ident.de/adserver/www/delivery/ck.php?ct=1&oaparams=2__bannerid=76__zoneid=2__cb=8a18c95a9e__oadest=https://cityofrosedalems.com/
www.fertilab.net/background_manager.aspx?ajxName=link_banner&id_banner=50&url=https://cityofrosedalems.com/
shop-uk.fmworld.com/Queue/Index?url=https://cityofrosedalems.com/
www.weddinginlove.com/redirect/?url=https://cityofrosedalems.com/
nieuws.rvent.nl/bitmailer/statistics/mailstatclick/42261?link=https://cityofrosedalems.com/
https://jobanticipation.com/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=jobanticipation.com
www.xsbaseball.com/tracker/index.html?t=ad&pool_id=3&ad_id=5&url=https://cityofrosedalems.com/
eos.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
b.sm.su/click.php?bannerid=56&zoneid=10&source=&dest=https://cityofrosedalems.com/
https://bethlehem-alive.com/abnrs/countguideclicks.cfm?targeturl=https://cityofrosedalems.com/&businessid=29579
www.bookmark-favoriten.com/?goto=https://cityofrosedalems.com/
shop.yuliyababich.eu/RU/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/
www.depmode.com/go.php?https://cityofrosedalems.com/
https://urgankardesler.com/anasayfa/yonlen?link=https://cityofrosedalems.com/
www.metalindex.ru/netcat/modules/redir/?&site=https://cityofrosedalems.com/
www.rprofi.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://darudar.org/external/?link=https://cityofrosedalems.com/
www.postsabuy.com/autopost4/page/generate/?link=https://cityofrosedalems.com/&list=PL9d7lAncfCDSkF4UPyhzO59Uh8cOoD-8q&fb_node=942812362464093&picture&name=%E0%B9%82%E0%B8%9B%E0%B8%A3%E0%B9%81%E0%B8%81%E0%B8%A3%E0%B8%A1%E0%B9%82%E0%B8%9E%E0%B8%AA%E0%B8%82%E0%B8%B2%E0%B8%A2%E0%B8%AA%E0%B8%B4%E0%B8%99%E0%B8%84%E0%B9%89%E0%B8%B2%E0%B8%AD%E0%B8%AD%E0%B8%99%E0%B9%84%E0%B8%A5%E0%B8%99%E0%B9%8C+&caption=%E0%B9%80%E0%B8%A5%E0%B8%82%E0%B8%B2%E0%B8%AA%E0%B9%88%E0%B8%A7%E0%B8%99%E0%B8%95%E0%B8%B1%E0%B8%A7+%E0%B8%97%E0%B8%B5%E0%B8%84%E0%B8%B8%E0%B8%93%E0%B8%A5%E0%B8%B7%E0%B8%A1%E0%B9%84%E0%B8%A1%E0%B9%88%E0%B8%A5%E0%B8%87+Line+%40postsabuy&description=%E0%B8%A3%E0%B8%B2%E0%B8%84%E0%B8%B2%E0%B8%96%E0%B8%B9%E0%B8%81%E0%B8%97%E0%B8%B5%E0%B9%88%E0%B8%AA%E0%B8%B8%E0%B8%94%E0%B9%83%E0%B8%99+3+%E0%B9%82%E0%B8%A5%E0%B8%81+%E0%B8%AD%E0%B8%B4%E0%B8%AD%E0%B8%B4
www.perimeter.org/track.pdf?url=https://cityofrosedalems.com/
forum.darievna.ru/go.php?https://cityofrosedalems.com/
techlab.rarus.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.spiritualforums.com/vb/redir.php?link=https://cityofrosedalems.com/
www.review-mag.com/cdn/www/delivery/view.php?ct=1&oaparams=2__bannerid=268__zoneid=1__cb=8c1317f219__oadest=https://cityofrosedalems.com/
belantara.or.id/lang/s/ID?url=https://cityofrosedalems.com/
ele-market.ru/consumer.php?url=https://cityofrosedalems.com/
https://www.хорошие-сайты.рф/r.php?r=https://cityofrosedalems.com/
https://jobsflagger.com/jobclick/?RedirectURL=https://cityofrosedalems.com/
https://gpoltava.com/away/?go=https://cityofrosedalems.com/
www.cardexchange.com/index.php/tools/packages/tony_mailing_list/services/?mode=link&mlm=62&mlu=0&u=2&url=https://cityofrosedalems.com/
services.nfpa.org/Authentication/GetSSOSession.aspx?return=https://cityofrosedalems.com/
http://spaceup.org/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
yarko-zhivi.ru/redirect?url=https://cityofrosedalems.com/
365sekretov.ru/redirect.php?action=url&goto=https://cityofrosedalems.com/%20
www.sgdrivingtest.com/redirect.php?page=https://cityofrosedalems.com/
www.jxren.com/news/link/link.asp?id=7&url=https://cityofrosedalems.com/
particularcareers.co.uk/jobclick/?RedirectURL=https://cityofrosedalems.com/
https://bsaonline.com/MunicipalDirectory/SelectUnit?unitId=411&returnUrl=https://cityofrosedalems.com/&sitetransition=true
www.elit-apartament.ru/go?https://cityofrosedalems.com/
sendai.japansf.net/rank.cgi?mode=link&id=1216&url=https://cityofrosedalems.com/
www.bmwfanatics.ru/goto.php?l=https://cityofrosedalems.com/
www.saabsportugal.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=https://cityofrosedalems.com/
cms.sive.it/Jump.aspx?gotourl=https://cityofrosedalems.com/
largusladaclub.ru/go/url=https:/https://cityofrosedalems.com/
https://kekeeimpex.com/Home/ChangeCurrency?urls=https://cityofrosedalems.com/&cCode=GBP&cRate=77.86247
mycounter.com.ua/go.php?https://cityofrosedalems.com/
l2base.su/go?https://cityofrosedalems.com/
https://freeseotool.org/url/?q=https://cityofrosedalems.com/
www.duomodicagliari.it/reg_link.php?link_ext=https://cityofrosedalems.com/&prov=1
assine.hostnet.com.br/cadastro/?rep=17&url=https://cityofrosedalems.com/
www.dvnlp.de/profile/gruppe/redirect/5?url=https://cityofrosedalems.com/
http://hotmaturegirlfriends.com/cl.php?porno=MzB4MzB4NjEw&url=https://cityofrosedalems.com/
www.smkn5pontianak.sch.id/redirect/?alamat=https://cityofrosedalems.com/
www.cheapdealuk.co.uk/go.php?url=https://cityofrosedalems.com/
bilometro.brksedu.com.br/tracking?url=https://cityofrosedalems.com/&zorigem=hotsite-blackfriday
www.omschweiz.ch/select-your-country?publicUrl=https://cityofrosedalems.com/
https://anacolle.net/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
www.cubamusic.com/Home/ChangeLanguage?lang=es-ES&returnUrl=https://cityofrosedalems.com/
expoclub.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.deypenburgschecourant.nl/reklame/www/delivery/ck.php?oaparams=2__bannerid=44__zoneid=11__cb=078c2a52ea__oadest=https://cityofrosedalems.com/
tramplintk.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
cnc.extranet.gencat.cat/treball_cnc/AppJava/FileDownload.do?pdf=https://cityofrosedalems.com/&codi_cnv=9998045
www.gamecollections.co.uk/search/redirect.php?retailer=127&deeplink=https://cityofrosedalems.com/
bearcong.no1.sexy/hobby-delicious/rank.cgi?mode=link&id=19&url=https://cityofrosedalems.com/
dawnofwar.org.ru/go?https://cityofrosedalems.com/
www.surinenglish.com/backend/conectar.php?url=https://cityofrosedalems.com/
www.reference-cannabis.com/interface/sortie.php?adresse=https://cityofrosedalems.com/
login.0×69416d.co.uk/sso/logout?tenantId=tnl&gotoUrl=https://cityofrosedalems.com/&domain=0×69416d.co.uk
blog.link-usa.jp/emi?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://damki.net/go/?https://cityofrosedalems.com/
opensesame.wellymulia.zaxaa.com/b/66851136?s=1&redir=https://cityofrosedalems.com/
ism3.infinityprosports.com/ismdata/2009100601/std-sitebuilder/sites/200901/www/en/tracker/index.html?t=ad&pool_id=1&ad_id=112&url=https://cityofrosedalems.com/
apps.cancaonova.com/ads/www/delivery/ck.php?ct=1&oaparams=2__bannerid=149__zoneid=20__cb=87d2c6208d__oadest=https://cityofrosedalems.com/
www.lespritjardin.be/?advp_click_bimage_id=19&url=https://cityofrosedalems.com/&shortcode_id=10
www.dresscircle-net.com/psr/rank.cgi?mode=link&id=14&url=https://cityofrosedalems.com/
www.counterwelt.com/charts/click.php?user=14137&link=https://cityofrosedalems.com/
fms.csonlineschool.com.au/changecurrency/1?returnurl=https://cityofrosedalems.com/
www.v-archive.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.jagat.co.jp/analysis/analysis.php?url=https://cityofrosedalems.com/
https://vse-doski.com/redirect/?go=https://cityofrosedalems.com/
www.karatetournaments.net/link7.asp?LRURL=https://cityofrosedalems.com/&LRTYP=O
simracing.su/go/?https://cityofrosedalems.com/
click.cheshi.com/go.php?proid=218&clickid=1393306648&url=https://cityofrosedalems.com/
fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=https://cityofrosedalems.com/
flypoet.toptenticketing.com/index.php?url=https://cityofrosedalems.com/
www.horsesmouth.com/LinkTrack.aspx?u=https://cityofrosedalems.com/
d-click.fiemg.com.br/u/18081/131/75411/137_0/82cb7/?url=https://cityofrosedalems.com/
www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=https://cityofrosedalems.com/
www.packmage.net/uc/goto/?url=https://cityofrosedalems.com/
my.9991.com/login_for_index_0327.php?action=logout&forward=https://cityofrosedalems.com/
www.sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=986&redirect=https://cityofrosedalems.com/
www.naturum.co.jp/ad/linkshare/?siteID=p_L785d6UQY-V4Fh4Rxs7wNzOPgtzv95Tg&lsurl=https://cityofrosedalems.com/
www.d-e-a.eu/newsletter/redirect.php?link=https://cityofrosedalems.com/
www.bom.ai/goweburl?go=https://cityofrosedalems.com/
enews2.sfera.net/newsletter/redirect.php?id=sabricattani@gmail.com_0000006566_144&link=https://cityofrosedalems.com/
underwater.com.au/redirect_url/id/7509/?redirect=https://cityofrosedalems.com/
https://eqsoftwares.com/languages/setlanguage?languagesign=en&redirect=https://cityofrosedalems.com/
www.jagdambasarees.com/Home/ChangeCurrency?urls=https://cityofrosedalems.com/&cCode=MYR&cRate=14.554
www.priegeltje.nl/gastenboek/go.php?url=https://cityofrosedalems.com/
www.serie-a.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.rz114.cn/url.html?url=https://cityofrosedalems.com/
www.greatdealsindia.com/redirects/infibeam.aspx?url=https://cityofrosedalems.com/
rs.345kei.net/rank.php?id=37&mode=link&url=https://cityofrosedalems.com/
webapp.jgz.la/?c=scene&a=link&id=8665466&url=https://cityofrosedalems.com/
www.erotiikkalelut.com/url.php?link=https://cityofrosedalems.com/
vicsport.com.au/analytics/outbound?url=https://cityofrosedalems.com/
https://fachowiec.com/zliczanie-bannera?id=24&url=https://cityofrosedalems.com/
ieea.ir/includes/change_lang.php?lang=en&goto=https://cityofrosedalems.com/
scribe.mmonline.io/click?evt_nm=Clicked+Registration+Completion&evt_typ=clickEmail&app_id=m4marry&eml_sub=Registration+Successful&usr_did=4348702&cpg_sc=NA&cpg_md=email&cpg_nm=&cpg_cnt=&cpg_tm=NA&link_txt=Live+Chat&em_type=Notification&url=https://cityofrosedalems.com/
polo-v1.feathr.co/v1/analytics/crumb?flvr=email_link_click&rdr=https://cityofrosedalems.com/
sintesi.provincia.mantova.it/portale/LinkClick.aspx?link=https://cityofrosedalems.com/
https://careerchivy.com/jobclick/?RedirectURL=https://cityofrosedalems.com/
shinsekai.type.org/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
https://maned.com/scripts/lm/lm.php?tk=CQkJZWNuZXdzQGluZm90b2RheS5jb20JW05ld3NdIE1FSSBBbm5vdW5jZXMgUGFydG5lcnNoaXAgV2l0aCBUd2l4bCBNZWRpYQkxNjcyCVBSIE1lZGlhIENvbnRhY3RzCTI1OQljbGljawl5ZXMJbm8=&url=https://cityofrosedalems.com/
www.etaigou.com/turn2.php?ad_id=276&link=https://cityofrosedalems.com/
crewroom.alpa.org/SAFETY/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=12872
www.tagirov.org/out.php?url=https://cityofrosedalems.com/
startlist.club/MSF/Language/Set?languageIsoCode=en&returnUrl=https://cityofrosedalems.com/
www.tetsumania.net/search/rank.cgi?mode=link&id=947&url=https://cityofrosedalems.com/
https://imperial-info.net/link?idp=125&url=https://cityofrosedalems.com/
www.brainlanguage-sa.com/setcookie.php?lang=en&file=https://cityofrosedalems.com/
www.asensetranslations.com/modules/babel/redirect.php?newlang=en_US&newurl=https://cityofrosedalems.com/
gameshock.jeez.jp/rank.cgi?mode=link&id=307&url=https://cityofrosedalems.com/
https://tripyar.com/go.php?https://cityofrosedalems.com/
www.floridafilmofficeinc.com/?goto=https://cityofrosedalems.com/
tsvc1.teachiworld.com/bin/checker?mode=4&module=11&mailidx=19130&dmidx=0&emidx=0&service=0&cidx=&etime=20120328060000&seqidx=3&objidx=22&encoding=0&url=https://cityofrosedalems.com/
rechner.atikon.at/lbg.at/newsletter/linktracking?subscriber=&delivery=38116&url=https://cityofrosedalems.com/
www.pcreducator.com/Common/SSO.aspx?returnUrl=https://cityofrosedalems.com/
go.eniro.dk/lg/ni/cat-2611/http:/https://cityofrosedalems.com/
www.fisherly.com/redirect?type=website&ref=listing_detail&url=https://cityofrosedalems.com/
bio-pack.ru/bitrix/redirect.php?goto=http://https://cityofrosedalems.com/
fid.com.ua/redirect/?go=https://cityofrosedalems.com/
www.modernipanelak.cz/?b=618282165&redirect=https://cityofrosedalems.com/
h5.hbifeng.com/index.php?c=scene&a=link&id=14240604&url=https://cityofrosedalems.com/
www.bkdc.ru/bitrix/redirect.php?event1=news_out&event2=32reg.roszdravnadzor.ru/&event3=A0A0B5A09180D0%A09582A0BBA1A085%D0E2A084D0D1C2D0%A085+A0A0B5A182B0A0%C2D0D0D096+A1A0BBA0B180D0%A09795+A0A0B0A09582A1%D1D0D0D0A182B5+A0A091A08695A0%D1D0A6A185A0A085%D0D1D0D082A1A085%D0D0D1D0A095B1A0%C2D0D0D091&goto=https://cityofrosedalems.com/
record.affiliatelounge.com/WS-jvV39_rv4IdwksK4s0mNd7ZgqdRLk/7/?deeplink=https://cityofrosedalems.com/
d-click.artenaescola.org.br/u/3806/290/32826/1416_0/53052/?url=https://cityofrosedalems.com/
www.morroccoaffiliate.com/aff.php?id=883&url=https://cityofrosedalems.com/
www.omegon.eu/de/?r=https://cityofrosedalems.com/
www.flooble.com/cgi-bin/clicker.pl?id=grabbadl&url=https://cityofrosedalems.com/
www.sportsbook.ag/ctr/acctmgt/pl/openLink.ctr?ctrPage=https://cityofrosedalems.com/
www.ra2d.com/directory/redirect.asp?id=596&url=https://cityofrosedalems.com/
www.anorexicnudes.net/cgi-bin/atc/out.cgi?u=https://cityofrosedalems.com/
www.zjjiajiao.com.cn/ad/adredir.asp?url=https://cityofrosedalems.com/
https://hakobo.com/wp/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
t.agrantsem.com/tt.aspx?cus=216&eid=1&p=216-2-71016b553a1fa2c9.3b14d1d7ea8d5f86&d=https://cityofrosedalems.com/
moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
www.cheek.co.jp/location/location.php?id=keibaseminar&url=https://cityofrosedalems.com/
www.quantixtickets3.com/php-bin-8/kill_session_and_redirect.php?redirect=https://cityofrosedalems.com/
www.photokonkurs.com/cgi-bin/out.cgi?url=https://cityofrosedalems.com/
https://www.nbda.org/?URL=https://cityofrosedalems.com/
https://www.thislife.net/cgi-bin/webcams/out.cgi?id=playgirl&url=https://cityofrosedalems.com/
https://www.dans-web.nu/klick.php?url=https://cityofrosedalems.com/
https://voobrajulya.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.resnichka.ru/partner/go.php?https://cityofrosedalems.com/
www.nafta-him.com/bitrix/redirect.php?goto=https://cityofrosedalems.com/
www.kyoto-osaka.com/search/rank.cgi?mode=link&id=9143&url=https://cityofrosedalems.com/
www.findingfarm.com/redir?url=https://cityofrosedalems.com/
www.fairpoint.net/~jensen1242/gbook/go.php?url=https://cityofrosedalems.com/
www.cnainterpreta.it/redirect.asp?url=https://cityofrosedalems.com/
red.ribbon.to/~zkcsearch/zkc-search/rank.cgi?mode=link&id=156&url=https://cityofrosedalems.com/
iphoneapp.impact.co.th/i/r.php?u=https://cityofrosedalems.com/
akademik.tkyd.org/Home/SetCulture?culture=en-US&returnUrl=https://cityofrosedalems.com/
www.rencai8.com/web/jump_to_ad_url.php?id=642&url=https://cityofrosedalems.com/
http://crackstv.com/redirect.php?bnn=CabeceraHyundai&url=https://cityofrosedalems.com/
https://www.baby22.com.tw/Web/turn.php?ad_id=160&link=https://cityofrosedalems.com/
https://www.kichink.com/home/issafari?uri=https://cityofrosedalems.com/
https://blogranking.fc2.com/out.php?id=1032500&url=https://cityofrosedalems.com/
5cfxm.hxrs6.servertrust.com/v/affiliate/setCookie.asp?catId=1180&return=https://cityofrosedalems.com/
http://3dcreature.com/cgi-bin/at3/out.cgi?id=187&trade=https://cityofrosedalems.com/
https://nowlifestyle.com/redir.php?k=9a4e080456dabe5eebc8863cde7b1b48&url=https://cityofrosedalems.com/
http://slipknot1.info/go.php?url=https://cityofrosedalems.com/
www.acutenet.co.jp/cgi-bin/lcount/lcounter.cgi?link=https://cityofrosedalems.com/
http://news-matome.com/method.php?method=1&url=https://cityofrosedalems.com/
https://lifecollection.top/site/gourl?url=https://cityofrosedalems.com/
https://www.toscanapiu.com/web/lang.php?lang=DEU&oldlang=ENG&url=https://cityofrosedalems.com/
speakrus.ru/links.php?go=https://cityofrosedalems.com/
https://edcommunity.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=https://cityofrosedalems.com/
https://www.sainttropeztourisme.com/en/bannieres/redirection/index.html?id=649&lien=https://cityofrosedalems.com/
www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
www.interracialhall.com/cgi-bin/atx/out.cgi?trade=https://cityofrosedalems.com/
https://www.tumimusic.com/link.php?url=https://cityofrosedalems.com/
www.glorioustronics.com/redirect.php?link=https://cityofrosedalems.com/
mail.resen.gov.mk/redir.hsp?url=https://cityofrosedalems.com/
https://www.pompengids.net/followlink.php?id=495&link=https://cityofrosedalems.com/
https://www.hirforras.net/scripts/redir.php?url=https://cityofrosedalems.com/
https://lens-club.ru/link?go=https://cityofrosedalems.com/
https://t.raptorsmartadvisor.com/.lty?url=https://cityofrosedalems.com/&loyalty_id=14481&member_id=b01bbee6-4592-4345-a0ee-5d71ed6f1929
https://evoautoshop.com/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://beautycottageshop.com/change.php?lang=cn&url=https://cityofrosedalems.com/
http://1000love.net/lovelove/link.php?url=https://cityofrosedalems.com/
https://www.ffw-ellar.de/ref.php?url=https://cityofrosedalems.com/
https://www.contactlenshouse.com/currency.asp?c=CAD&r=https://cityofrosedalems.com/
https://www.bartaz.lt/wp-content/plugins/clikstats/ck.php?Ck_id=70&Ck_lnk=https://cityofrosedalems.com/
https://sogo.i2i.jp/link_go.php?url=https://cityofrosedalems.com/
https://ceb.bg/catalog/statistic/?id=61&location=https://cityofrosedalems.com/
https://malehealth.ie/redirect/?age=40&part=waist&illness=obesity&refer=https://cityofrosedalems.com/
https://magicode.me/affiliate/go?url=https://cityofrosedalems.com/
forum.marillion.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
https://jobregistry.net/jobclick/?RedirectURL=https://cityofrosedalems.com/&Domain=jobregistry.net&rgp_m=title13&et=4495
https://www.goatzz.com/adredirect.aspx?adType=SiteAd&ItemID=9595&ReturnURL=https://cityofrosedalems.com/
https://experts.richdadworld.com/assets/shared/php/noabp.php?oaparams=2bannerid=664zoneid=5cb=0902f987cboadest=https://cityofrosedalems.com/
https://miyagi.lawyer-search.tv/details/linkchk.aspx?type=o&url=https://cityofrosedalems.com/
https://www.snwebcastcenter.com/event/page/count_download_time.php?url=https://cityofrosedalems.com/
https://go.onelink.me/v1xd?pid=Patch&c=Mobile%20Footer&af_web_dp=https://cityofrosedalems.com/
https://www.weather.net/cgi-bin/redir?https://cityofrosedalems.com/
https://gaggedtop.com/cgi-bin/top/out.cgi?ses=sBZFnVYGjF&id=206&url=https://cityofrosedalems.com/
https://e-bike-test.net/wp-content/plugins/AND-AntiBounce/redirector.php?url=https://cityofrosedalems.com/
https://www.morhipo.com/shared/partnercookie?k=gort&url=https://cityofrosedalems.com/
https://www.luckyplants.com/cgi-bin/toplist/out.cgi?id=rmontero&url=https://cityofrosedalems.com/
https://webankety.cz/dalsi.aspx?site=https://cityofrosedalems.com/
https://runkeeper.com/apps/authorize?redirect_uri=https://cityofrosedalems.com/
https://www.emiratesvoice.com/footer/comment_like_dislike_ajax/?code=like&commentid=127&redirect=https://cityofrosedalems.com/
https://envios.uces.edu.ar/control/click.mod.php?id_envio=8147&email=gramariani@gmail.com&url=https://cityofrosedalems.com/
https://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=https://cityofrosedalems.com/
https://ulfishing.ru/forum/go.php?https://cityofrosedalems.com/
https://www.ab-search.com/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
https://www.studyrama.be/tracking.php?origine=ficheform5683&lien=http://https://cityofrosedalems.com/
https://shop.merchtable.com/users/authorize?return_url=https://cityofrosedalems.com/
https://nudewwedivas.forumcommunity.net/m/ext.php?url=https://cityofrosedalems.com/
https://cztt.ru/redir.php?url=https://cityofrosedalems.com/
https://ageoutloud.gms.sg/visit.php?item=54&uri=https://cityofrosedalems.com/
https://multimedia.inrap.fr/redirect.php?li=287&R=https://cityofrosedalems.com/
http://mientaynet.com/advclick.php?o=textlink&u=15&l=https://cityofrosedalems.com/
https://atlanticleague.com/tracker/index.html?t=ad&pool_id=11&ad_id=5&url=https://cityofrosedalems.com/
https://www.castellodivezio.it/lingua.php?lingua=EN&url=https://cityofrosedalems.com/
https://www.podcastone.com/site/rd?satype=40&said=4&aaid=email&camid=-4999600036534929178&url=https://cityofrosedalems.com/
https://www.rechnungswesen-portal.de/bitrix/redirect.php?event1=KD37107&event2=https2F/www.universal-music.de2880%25-100%25)(m/w/d)&goto=https://cityofrosedalems.com/
https://monarchbeachmembers.play18.com/ViewSwitcher/SwitchView?mobile=False&returnUrl=https://cityofrosedalems.com/
https://wayi.com.tw/wayi_center.aspx?flag=banner&url=https://cityofrosedalems.com/&idno=443
https://d.agkn.com/pixel/2389/?che=2979434297&col=22204979,1565515,238211572,435508400,111277757&l1=https://cityofrosedalems.com/
https://mobilize.org.br/handlers/anuncioshandler.aspx?anuncio=55&canal=2&redirect=https://cityofrosedalems.com/
https://bemidji.bigdealsmedia.net/include/sort.php?return_url=https://cityofrosedalems.com/&sort=a:3:{s:9:%E2%80%9Ddirection%E2%80%9D;s:3:%E2%80%9DASC%E2%80%9D;s:5:%E2%80%9Dfield%E2%80%9D;s:15:%E2%80%9DItems.PriceList%E2%80%9D;s:5:%E2%80%9Dlabel%E2%80%9D;s:9:%E2%80%9Dvalue_asc%E2%80%9D;}
https://hslda.org/content/a/LinkTracker.aspx?id=4015475&appeal=385&package=36&uri=https://cityofrosedalems.com/
https://www.sign-in-china.com/newsletter/statistics.php?type=mail2url&bs=88&i=114854&url=https://cityofrosedalems.com/
https://panarmenian.net/eng/tofv?tourl=https://cityofrosedalems.com/
https://www.kvinfo.dk/visit.php?linkType=2&linkValue=https://cityofrosedalems.com/
https://lavoro.provincia.como.it/portale/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=935
https://www.dodeley.com/?action=show_ad&url=https://cityofrosedalems.com/
https://www.ignicaodigital.com.br/affiliate/?idev_id=270&u=https://cityofrosedalems.com/
https://www.cheerunion.org/tracker/index.html?t=ad&pool_id=2&ad_id=5&url=https://cityofrosedalems.com/
https://m.wedkuje.pl/mobile/redirect.php?redir=https://cityofrosedalems.com/
https://area51.to/go/out.php?s=100&l=site&u=https://cityofrosedalems.com/
https://games4ever.3dn.ru/go?https://cityofrosedalems.com/
https://de.reasonable.shop/SetCurrency.aspx?currency=CNY&returnurl=https://cityofrosedalems.com/
https://www.pcbheaven.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
https://mobo.osport.ee/Home/SetLang?lang=cs&returnUrl=https://cityofrosedalems.com/
https://www.shop-bell.com/out.php?id=kibocase&category=ladies&url=https://cityofrosedalems.com/
https://m.addthis.com/live/redirect/?url=https://cityofrosedalems.com/
https://www.pcreducator.com/Common/SSO.aspx?returnUrl=https://cityofrosedalems.com/
https://horsesmouth.com/LinkTrack.aspx?u=https://cityofrosedalems.com/
https://soom.cz/projects/get2mail/redir.php?id=c2e52da9ad&url=https://cityofrosedalems.com/
https://www.iaai.com/VehicleInspection/InspectionProvidersUrl?name=AA%20Transit%20Pros%20Inspection%20Service&url=https://cityofrosedalems.com/
https://cingjing.fun-taiwan.com/AdRedirector.aspx?padid=303&target=https://cityofrosedalems.com/
https://rtb-asiamax.tenmax.io/bid/click/1462922913409/e95f2c30-1706-11e6-a9b4-a9f6fe33c6df/3456/5332/?rUrl=https://cityofrosedalems.com/
https://campaign.unitwise.com/click?emid=31452&emsid=ee720b9f-a315-47ce-9552-fd5ee4c1c5fa&url=https://cityofrosedalems.com/
https://www.swipeclock.com/sc/cookie.asp?sitealias=79419397&redirect=https://cityofrosedalems.com/
https://www.gutscheinaffe.de/wp-content/plugins/AND-AntiBounce/redirector.php?url=https://cityofrosedalems.com/
https://home.uceusa.com/Redirect.aspx?r=https://cityofrosedalems.com/
https://www.seankenney.com/include/jump.php?num=https://cityofrosedalems.com/
https://runningcheese.com/go?url=https://cityofrosedalems.com/
http://chtbl.com/track/118167/https://cityofrosedalems.com/
https://m.17ll.com/apply/tourl/?url=https://cityofrosedalems.com/
https://crewroom.alpa.org/SAFETY/LinkClick.aspx?link=https://cityofrosedalems.com/&mid=12872
https://rs.businesscommunity.it/snap.php?u=https://cityofrosedalems.com/
http://dstats.net/redir.php?url=https://cityofrosedalems.com/
https://www.aiac.world/pdf/October-December2015Issue?pdf_url=https://cityofrosedalems.com/
https://union.591.com.tw/stats/event/redirect?e=eyJpdiI6IjdUd1B5Z2FPTmNWQzBmZk1LblR2R0E9PSIsInZhbHVlIjoiQTI4TnVKMzdjMkxrUjcrSWlkcXdzbjRQeGRtZ0ZGbXdNSWxkSkVieENwNjQ1cHF5aDZmWmFobU92ZGVyUk5jRTlxVnI2TG5pb0dJVHZSUUlHcXFTbGo3UDliYWU5UE5MSjlMY0xOQnFmbVRQSFNoZDRGd2dqVDZXZEU4WFoyajJ0S0JITlQ2XC9SXC9jRklPekdmcnFGb09vRllqNHVtTHlYT284ZmN3d0ozOHFkclRYYnU5UlY2NTFXSGRheW5SbGxJb3BmYjQ2Mm9TWUFCTEJuXC9iT25nYkg4QXpOd2pHVlBWTWxWXC91aWRQMVhKQmVJXC9qMW9IdlZaVVlBdWlCYW4rS0JualhSMElFeVZYN3NnUW1qcUdxcWUrSlFROFhKbWttdkdvMUJ3aWVRa2I3MVV5TXpER3doa2ZuekFWNWd3OGpuQ1VSczFDREVKaklaUks0TTRIY2pUeXYrQmdZYUFTK1F4RWpTY0RRaW5Nc0krdVJ2N2VUT1wvSUxVVWVKN3hnQU92QmlCbjQyMUpRdTZKVWJcL0RCSVFOcWl0azl4V2pBazBHWmVhdWptZGREVXh0VkRNWWxkQmFSYXhBRmZtMHA5dTlxMzIzQzBVaWRKMEFqSG0wbGkxME01RDBaaElTaU5QKzIxbSswaUltS0FYSzViZlFmZjZ1XC9Yclg0U2VKdHFCc0pTNndcL09FWklUdjlQM2dcL2RuN0szQ3plWmcyYWdpQzJDQ2NIcWROczVua3dIM1Q3OXpJY3Z0XC93MVk3SHUyODZHU3Z5aHFVbWEwRFU1ZFdyMGt0YWpsb3BkQitsZER5aWk4YWMrZWYzSFNHNERhOGdDeUJWeEtoSm9wQ0hQb2EzOHZ3eHFGVTQ2Mk1QSEZERzlXZWxRRTJldjJkdnZUM0ZwaW1KcEVVc3ZXWjRHaTZWRDJOK0YxR3d4bXhMR3BhWmZBNkJ6eUYxQjR4ODVxc0d0YkFpYU8yZ2tuWGdzelBpU3dFUjJVYUVtYUlpZllSUTVpaHZMbjhySFp4VEpQR3EyYnRLTmdcLzRvKzQwRmtGNUdWWnQ0VjFpcTNPc0JubEdWenFiajRLRFg5a2dRZFJOZ1wvaUEwVHR3ZnYzakpYVmVtT294aFk1TXBUZ3FmVnF2dnNSVWJ5VEE0WGZpV3o3Y0k2SjJhM2RDK2hoQ0FvV2YrSW9QWnhuZG5QN1hlOEFaTVZrcFZ3c0pXVHhtNkRTUkpmenpibG8zdTM0cGF6Q3oxTEJsdDdiOUgwWXFOUkNHWjlEbTBFYzdIRUcyalYrcW4wYklFbnlGYlZJUG00R1VDQTZLZEVJRklIbFVNZFdpS3RkeCt5bVpTNUkrOXE3dDlxWmZ2bitjSGlSeE9wZTg5Yk9wS0V6N1wvd1EzUTNVenNtbjlvQUJhdGsxMzNkZTdjTU1LNkd4THJMYTBGUEJ4elEycFNTNGZabEJnalhJc0pYZit1c1wvWDBzSm1JMzRad3F3PT0iLCJtYWMiOiI4MjNhNDJlYTMwOTlmY2VlYzgxNmU1N2JiM2QzODk5YjI5MDFhYThhMDBkYzNhODljOTRmMTMzMzk0YTM5OGIzIn0=&source=BANNER.2913&url=https://cityofrosedalems.com/
https://fdeam.finanzen-partnerprogramm.de/tracking/?as_id=9257&c_id=595&url=https://cityofrosedalems.com/
https://pdcn.co/e/https://cityofrosedalems.com/
https://api.webconnex.com/v1/postmaster/track/click/4f8036d14ee545798599c8921fbfcd22/db005310dba511e89fb606f49a4ee876?url=https://cityofrosedalems.com/
https://www.lecake.com/stat/goto.php?url=https://cityofrosedalems.com/
https://www.im-harz.com/counter/counter.php?url=https://cityofrosedalems.com/
https://pzz.to/click?uid=8571&target_url=https://cityofrosedalems.com/
https://www.ricacorp.com/Ricapih09/redirect.aspx?link=https://cityofrosedalems.com/
https://www.tremblant.ca/Shared/LanguageSwitcher/ChangeCulture?culture=en&url=https://cityofrosedalems.com/
https://www.winxuan.com/page/cps/eqifacookieinterface.jsp?from=yiqifa&wid=8&url=https://cityofrosedalems.com/
https://moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://igert2011.videohall.com/to_client?target=https://cityofrosedalems.com/
https://sete.gr/app_plugins/newsletterstudio/pages/tracking/trackclick.aspx?nid=218221206039243162226109144018149003034132019130&e=000220142174231130224127060133189018075115154134&url=https://cityofrosedalems.com/
https://ovatu.com/e/c?url=https://cityofrosedalems.com/
https://primepartners.globalprime.com/afs/wcome.php?c=427|0|1&e=GP204519&url=https://cityofrosedalems.com/
https://ltp.org/home/setlocale?locale=en&returnUrl=https://cityofrosedalems.com/
https://www.morhipo.com/shared/partnercookie?k=gort&url=https://cityofrosedalems.com/
https://www.google.co.jp/url?q=https://cityofrosedalems.com/
https://images.google.de/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?q=https://cityofrosedalems.com/
https://za.zalo.me/v3/verifyv2/pc?token=OcNsmjfpL0XY2F3BtHzNRs4A-hhQ5q5sPXtbk3O&continue=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://cse.google.cz/url?q=https://cityofrosedalems.com/
https://scanmail.trustwave.com/?c=8510&d=4qa02KqxZJadHuhFUvy7ZCUfI_2L10yeH0EeBz7FGQ&u=https://cityofrosedalems.com/
https://images.google.co.kr/url?sa=t&url=https://cityofrosedalems.com/
https://animal.doctorsfile.jp/redirect/?ct=doctor&id=178583&url=https://cityofrosedalems.com/
https://hr.pecom.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://cse.google.ru/url?q=https://cityofrosedalems.com/
https://cse.google.com/url?q=https://cityofrosedalems.com/
https://jump2.bdimg.com/mo/q/checkurl?url=https://cityofrosedalems.com/
https://severeweather.wmo.int/cgi-bin/goto?where=https://cityofrosedalems.com/
https://beam.jpn.org/rank.cgi?mode=link&url=https://cityofrosedalems.com/
https://cse.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://blogranking.fc2.com/out.php?id=414788&url=https://cityofrosedalems.com/
https://wtk.db.com/777554543598768/optout?redirect=https://cityofrosedalems.com/
https://community.rsa.com/t5/custom/page/page-id/ExternalRedirect?url=https://cityofrosedalems.com/
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=https://cityofrosedalems.com/
https://images.google.sk/url?q=https://cityofrosedalems.com/
https://images.google.gr/url?q=https://cityofrosedalems.com/
https://click.alamode.com/?adcode=CPEMAQM0913_1&url=https://cityofrosedalems.com/
https://images.google.lv/url?sa=t&url=https://cityofrosedalems.com/
https://m.caijing.com.cn/member/logout?referer=https://cityofrosedalems.com/
https://jamesattorney.agilecrm.com/click?u=https://cityofrosedalems.com/
https://wikimapia.org/external_link?url=https://cityofrosedalems.com/
https://rsv.nta.co.jp/affiliate/set/af100101.aspx?site_id=66108024&redi_url=https://cityofrosedalems.com/
https://adengine.old.rt.ru/go.jsp?to=https://cityofrosedalems.com/
https://thediplomat.com/ads/books/ad.php?i=4&r=https://cityofrosedalems.com/
https://mitsui-shopping-park.com/lalaport/iwata/redirect.html?url=https://cityofrosedalems.com/
https://toolbarqueries.google.lt/url?q=https://cityofrosedalems.com/
https://clients1.google.de/url?q=https://cityofrosedalems.com/
https://clients1.google.es/url?q=https://cityofrosedalems.com/
https://clients1.google.co.uk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.jp/url?q=https://cityofrosedalems.com/
https://clients1.google.fr/url?q=https://cityofrosedalems.com/
https://clients1.google.it/url?q=https://cityofrosedalems.com/
https://clients1.google.com.br/url?q=https://cityofrosedalems.com/
https://clients1.google.co.in/url?q=https://cityofrosedalems.com/
https://clients1.google.ca/url?q=https://cityofrosedalems.com/
https://clients1.google.ru/url?q=https://cityofrosedalems.com/
https://clients1.google.com.hk/url?q=https://cityofrosedalems.com/
https://clients1.google.com.au/url?q=https://cityofrosedalems.com/
https://clients1.google.co.id/url?q=https://cityofrosedalems.com/
https://clients1.google.nl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tw/url?q=https://cityofrosedalems.com/
https://clients1.google.pl/url?q=https://cityofrosedalems.com/
https://clients1.google.be/url?q=https://cityofrosedalems.com/
https://clients1.google.co.th/url?q=https://cityofrosedalems.com/
https://clients1.google.at/url?q=https://cityofrosedalems.com/
https://clients1.google.cz/url?q=https://cityofrosedalems.com/
https://clients1.google.se/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mx/url?q=https://cityofrosedalems.com/
https://clients1.google.ch/url?q=https://cityofrosedalems.com/
https://clients1.google.com.vn/url?q=https://cityofrosedalems.com/
https://clients1.google.pt/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ua/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tr/url?q=https://cityofrosedalems.com/
https://clients1.google.ro/url?q=https://cityofrosedalems.com/
https://clients1.google.com.my/url?q=https://cityofrosedalems.com/
https://clients1.google.gr/url?q=https://cityofrosedalems.com/
https://clients1.google.dk/url?q=https://cityofrosedalems.com/
https://clients1.google.hu/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ar/url?q=https://cityofrosedalems.com/
https://clients1.google.fi/url?q=https://cityofrosedalems.com/
https://clients1.google.co.il/url?q=https://cityofrosedalems.com/
https://clients1.google.co.nz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.za/url?q=https://cityofrosedalems.com/
https://clients1.google.cl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.co/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sg/url?q=https://cityofrosedalems.com/
https://clients1.google.ie/url?q=https://cityofrosedalems.com/
https://clients1.google.sk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.kr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ph/url?q=https://cityofrosedalems.com/
https://clients1.google.no/url?q=https://cityofrosedalems.com/
https://clients1.google.lt/url?q=https://cityofrosedalems.com/
https://clients1.google.bg/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sa/url?q=https://cityofrosedalems.com/
https://clients1.google.hr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pe/url?q=https://cityofrosedalems.com/
https://clients1.google.ae/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ve/url?q=https://cityofrosedalems.com/
https://clients1.google.ee/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pk/url?q=https://cityofrosedalems.com/
https://clients1.google.rs/url?q=https://cityofrosedalems.com/
https://clients1.google.com.eg/url?q=https://cityofrosedalems.com/
https://clients1.google.si/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ec/url?q=https://cityofrosedalems.com/
https://clients1.google.com.qa/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pr/url?q=https://cityofrosedalems.com/
https://clients1.google.mu/url?q=https://cityofrosedalems.com/
https://clients1.google.li/url?q=https://cityofrosedalems.com/
https://clients1.google.lv/url?q=https://cityofrosedalems.com/
https://clients1.google.mn/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gt/url?q=https://cityofrosedalems.com/
https://clients1.google.co.cr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.uy/url?q=https://cityofrosedalems.com/
https://clients1.google.lu/url?q=https://cityofrosedalems.com/
https://clients1.google.ba/url?q=https://cityofrosedalems.com/
https://clients1.google.is/url?q=https://cityofrosedalems.com/
https://clients1.google.dz/url?q=https://cityofrosedalems.com/
https://clients1.google.kg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ke/url?q=https://cityofrosedalems.com/
https://clients1.google.az/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ng/url?q=https://cityofrosedalems.com/
https://clients1.google.com.np/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mt/url?q=https://cityofrosedalems.com/
https://clients1.google.bi/url?q=https://cityofrosedalems.com/
https://clients1.google.by/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bd/url?q=https://cityofrosedalems.com/
https://clients1.google.as/url?q=https://cityofrosedalems.com/
https://clients1.google.com.do/url?q=https://cityofrosedalems.com/
https://clients1.google.kz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ma/url?q=https://cityofrosedalems.com/
https://clients1.google.jo/url?q=https://cityofrosedalems.com/
https://clients1.google.lk/url?q=https://cityofrosedalems.com/
https://clients1.google.com.cu/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ai/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gi/url?q=https://cityofrosedalems.com/
https://clients1.google.cf/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ni/url?q=https://cityofrosedalems.com/
https://clients1.google.md/url?q=https://cityofrosedalems.com/
https://clients1.google.mg/url?q=https://cityofrosedalems.com/
https://clients1.google.la/url?q=https://cityofrosedalems.com/
https://clients1.google.com.jm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.vc/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tj/url?q=https://cityofrosedalems.com/
https://clients1.google.com.cy/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sv/url?q=https://cityofrosedalems.com/
https://clients1.google.rw/url?q=https://cityofrosedalems.com/
https://clients1.google.com.om/url?q=https://cityofrosedalems.com/
https://clients1.google.ps/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bo/url?q=https://cityofrosedalems.com/
https://clients1.google.tk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.mz/url?q=https://cityofrosedalems.com/
https://clients1.google.bs/url?q=https://cityofrosedalems.com/
https://clients1.google.mk/url?q=https://cityofrosedalems.com/
https://clients1.google.co.bw/url?q=https://cityofrosedalems.com/
https://clients1.google.al/url?q=https://cityofrosedalems.com/
https://clients1.google.sm/url?q=https://cityofrosedalems.com/
https://clients1.google.co.zw/url?q=https://cityofrosedalems.com/
https://clients1.google.tm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bh/url?q=https://cityofrosedalems.com/
https://clients1.google.com.af/url?q=https://cityofrosedalems.com/
https://clients1.google.com.fj/url?q=https://cityofrosedalems.com/
https://clients1.google.com.kh/url?q=https://cityofrosedalems.com/
https://clients1.google.cg/url?q=https://cityofrosedalems.com/
https://clients1.google.ki/url?q=https://cityofrosedalems.com/
https://clients1.google.mw/url?q=https://cityofrosedalems.com/
https://clients1.google.com.kw/url?q=https://cityofrosedalems.com/
https://clients1.google.bf/url?q=https://cityofrosedalems.com/
https://clients1.google.com.lb/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ls/url?q=https://cityofrosedalems.com/
https://clients1.google.ms/url?q=https://cityofrosedalems.com/
https://clients1.google.ci/url?q=https://cityofrosedalems.com/
https://clients1.google.dm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sb/url?q=https://cityofrosedalems.com/
https://clients1.google.co.vi/url?q=https://cityofrosedalems.com/
https://clients1.google.so/url?q=https://cityofrosedalems.com/
https://clients1.google.nu/url?q=https://cityofrosedalems.com/
https://clients1.google.dj/url?q=https://cityofrosedalems.com/
https://clients1.google.hn/url?q=https://cityofrosedalems.com/
https://clients1.google.nr/url?q=https://cityofrosedalems.com/
https://clients1.google.co.tz/url?q=https://cityofrosedalems.com/
https://clients1.google.mv/url?q=https://cityofrosedalems.com/
https://clients1.google.tn/url?q=https://cityofrosedalems.com/
https://clients1.google.sc/url?q=https://cityofrosedalems.com/
https://clients1.google.com.py/url?q=https://cityofrosedalems.com/
https://clients1.google.sn/url?q=https://cityofrosedalems.com/
https://clients1.google.am/url?q=https://cityofrosedalems.com/
https://clients1.google.ad/url?q=https://cityofrosedalems.com/
https://clients1.google.com.gh/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bz/url?q=https://cityofrosedalems.com/
https://clients1.google.iq/url?q=https://cityofrosedalems.com/
https://clients1.google.to/url?q=https://cityofrosedalems.com/
https://clients1.google.com.bn/url?q=https://cityofrosedalems.com/
https://clients1.google.cat/url?q=https://cityofrosedalems.com/
https://clients1.google.sh/url?q=https://cityofrosedalems.com/
https://clients1.google.cm/url?q=https://cityofrosedalems.com/
https://clients1.google.gg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ug/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ly/url?q=https://cityofrosedalems.com/
https://clients1.google.co.uz/url?q=https://cityofrosedalems.com/
https://clients1.google.co.zm/url?q=https://cityofrosedalems.com/
https://clients1.google.com.na/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ag/url?q=https://cityofrosedalems.com/
https://clients1.google.me/url?q=https://cityofrosedalems.com/
https://clients1.google.cd/url?q=https://cityofrosedalems.com/
https://clients1.google.fm/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ck/url?q=https://cityofrosedalems.com/
https://clients1.google.com.et/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pa/url?q=https://cityofrosedalems.com/
https://clients1.google.je/url?q=https://cityofrosedalems.com/
https://clients1.google.gl/url?q=https://cityofrosedalems.com/
https://clients1.google.ge/url?q=https://cityofrosedalems.com/
https://clients1.google.ht/url?q=https://cityofrosedalems.com/
https://clients1.google.im/url?q=https://cityofrosedalems.com/
https://clients1.google.gy/url?q=https://cityofrosedalems.com/
https://clients1.google.com.sl/url?q=https://cityofrosedalems.com/
https://clients1.google.bj/url?q=https://cityofrosedalems.com/
https://clients1.google.ml/url?q=https://cityofrosedalems.com/
https://clients1.google.cv/url?q=https://cityofrosedalems.com/
https://clients1.google.tl/url?q=https://cityofrosedalems.com/
https://clients1.google.com.mm/url?q=https://cityofrosedalems.com/
https://clients1.google.ga/url?q=https://cityofrosedalems.com/
https://clients1.google.bt/url?q=https://cityofrosedalems.com/
https://clients1.google.ac/url?q=https://cityofrosedalems.com/
https://clients1.google.st/url?q=https://cityofrosedalems.com/
https://clients1.google.td/url?q=https://cityofrosedalems.com/
https://clients1.google.com.pg/url?q=https://cityofrosedalems.com/
https://clients1.google.co.ao/url?q=https://cityofrosedalems.com/
https://clients1.google.gp/url?q=https://cityofrosedalems.com/
https://clients1.google.com.nf/url?q=https://cityofrosedalems.com/
https://clients1.google.ne/url?q=https://cityofrosedalems.com/
https://clients1.google.pn/url?q=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://ipv4.google.com/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://cse.google.com/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com/url?q=https://cityofrosedalems.com/
https://cse.google.com/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.com.om/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.com.om/url?q=https://cityofrosedalems.com/
https://images.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.mn/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.mn/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.mn/url?q=https://cityofrosedalems.com/
https://cse.google.mn/url?sa=i&url=https://cityofrosedalems.com/
https://www.google.mn/url?q=https://cityofrosedalems.com/
https://maps.google.dz/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.kg/url?q=https://cityofrosedalems.com/
https://www.google.kg/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://maps.google.iq/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.iq/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.co.uz/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://images.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.dz/url?q=https://cityofrosedalems.com/
https://images.google.dz/url?q=https://cityofrosedalems.com/
https://images.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.ga/url?q=https://cityofrosedalems.com/
https://images.google.nu/url?q=https://cityofrosedalems.com/
https://images.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.nu/url?q=https://cityofrosedalems.com/
https://www.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.kg/url?q=https://cityofrosedalems.com/
https://maps.google.sc/url?q=https://cityofrosedalems.com/
https://maps.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https://cityofrosedalems.com/
https://images.google.sc/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.sc/url?q=https://cityofrosedalems.com/
https://maps.google.iq/url?q=https://cityofrosedalems.com/
https://images.google.ki/url?q=https://cityofrosedalems.com/
https://images.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.ga/url?q=https://cityofrosedalems.com/
https://maps.google.nu/url?q=https://cityofrosedalems.com/
https://maps.google.bt/url?q=https://cityofrosedalems.com/
https://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https%3A%2F%2Fwww.https://cityofrosedalems.com/
https://www.google.bt/url?q=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://asia.google.com/url?q=https://cityofrosedalems.com/
http://www.macro.ua/out.php?link=https://cityofrosedalems.com/
http://www.aurki.com/jarioa/redirect?id_feed=510&url=https://cityofrosedalems.com/
http://www.cnainterpreta.it/redirect.asp?url=https://cityofrosedalems.com/
http://www.site-navi.net/sponavi/rank.cgi?mode=link&id=890&url=https://cityofrosedalems.com/
https://www.mattias.nu/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
http://teenstgp.us/cgi-bin/out.cgi?u=https://cityofrosedalems.com/
http://congovibes.com/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.humaniplex.com/jscs.html?hj=y&ru=https://cityofrosedalems.com/
http://www.qlt-online.de/cgi-bin/click/clicknlog.pl?link=https://cityofrosedalems.com/
http://ww.sdam-snimu.ru/redirect.php?url=https://cityofrosedalems.com/
http://www.uktrademarkregistration.co.uk/JumpTo.aspx?url=https://cityofrosedalems.com/
http://fosteringsuccessmichigan.com/?URL=https://cityofrosedalems.com/
http://fotos24.org/url?q=https://cityofrosedalems.com/
http://fouillez-tout.com/cgi-bin/redirurl.cgi?https://cityofrosedalems.com/
http://frag-den-doc.de/index.php?s=verlassen&url=https://cityofrosedalems.com/
http://freethailand.com/goto.php?url=https://cityofrosedalems.com/
http://freshcannedfrozen.ca/?URL=https://cityofrosedalems.com/
http://funkhouse.de/url?q=https://cityofrosedalems.com/
http://ga.naaar.nl/link/?url=https://cityofrosedalems.com/
http://gb.poetzelsberger.org/show.php?c453c4=https://cityofrosedalems.com/
http://getmethecd.com/?URL=https://cityofrosedalems.com/
http://goldankauf-oberberg.de/out.php?link=https://cityofrosedalems.com/
http://distributors.hrsprings.com/?URL=https://cityofrosedalems.com/
http://dorf-v8.de/url?q=https://cityofrosedalems.com/
http://doverwx.com/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://dr-guitar.de/quit.php?url=https://cityofrosedalems.com/
http://drdrum.biz/quit.php?url=https://cityofrosedalems.com/
http://ds-media.info/url?q=https://cityofrosedalems.com/
http://excitingperformances.com/?URL=https://cityofrosedalems.com/
http://familie-huettler.de/link.php?link=https://cityofrosedalems.com/
http://forum.vizslancs.hu/lnks.php?uid=net&url=https://cityofrosedalems.com/
http://forum.wonaruto.com/redirection.php?redirection=https://cityofrosedalems.com/
https://www.fairsandfestivals.net/?URL=https://cityofrosedalems.com/
http://chatx2.whocares.jp/redir.jsp?u=https://cityofrosedalems.com/
http://chuanroi.com/Ajax/dl.aspx?u=https://cityofrosedalems.com/
http://club.dcrjs.com/link.php?url=https://cityofrosedalems.com/
http://conny-grote.de/url?q=https://cityofrosedalems.com/
http://crazyfrag91.free.fr/?URL=https://cityofrosedalems.com/
http://crewe.de/url?q=https://cityofrosedalems.com/
http://cwa4100.org/uebimiau/redir.php?https://cityofrosedalems.com/
http://d-quintet.com/i/index.cgi?id=1&mode=redirect&no=494&ref_eid=33&url=https://cityofrosedalems.com/
http://data.allie.dbcls.jp/fct/rdfdesc/usage.vsp?g=https://cityofrosedalems.com/
http://data.linkedevents.org/describe/?url=https://cityofrosedalems.com/
http://dayviews.com/externalLinkRedirect.php?url=https://cityofrosedalems.com/
http://db.cbservices.org/cbs.nsf/forward?openform&https://cityofrosedalems.com/
http://simvol-veri.ru/xp/?goto=https://cityofrosedalems.com/
http://www.skladcom.ru/(S(qdiwhk55jkcyok45u4ti0a55))/banners.aspx?url=https://cityofrosedalems.com/
https://stroim100.ru/redirect?url=https://cityofrosedalems.com/
https://kakaku-navi.net/items/detail.php?url=https://cityofrosedalems.com/
http://www.paladiny.ru/go.php?url=https://cityofrosedalems.com/
http://tido.al/vazhdo.php?url=https://cityofrosedalems.com/
http://smile.wjp.am/link-free/link3.cgi?mode=cnt&no=8&hpurl=https://cityofrosedalems.com/
https://www.usjournal.com/go.php?campusID=190&url=https://cityofrosedalems.com/
https://temptationsaga.com/buy.php?url=https://cityofrosedalems.com/
https://meguro.keizai.biz/banner.php?type=image_banner&position=right&id=13&uri=https://cityofrosedalems.com/
http://ads.cars.cz/adclick.php?bannerid=333&zoneid=237&source=&dest=https://cityofrosedalems.com/
https://spb-medcom.ru/redirect.php?https://cityofrosedalems.com/
https://www.info-realty.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.sermemole.com/public/serbook/redirect.php?url=https://cityofrosedalems.com/
https://www.hradycz.cz/redir.php?b=445&t=https://cityofrosedalems.com/
https://www.moneydj.com/ads/adredir.aspx?bannerid=39863&url=https://cityofrosedalems.com/
https://uk.kindofbook.com/redirect.php/?red=https://cityofrosedalems.com/
http://m.17ll.com/apply/tourl/?url=https://cityofrosedalems.com/
http://auyttrv.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bartos.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://cllfather.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://littlejohnny.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://semesmemos.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://faultypirations.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mmurugesamnfo.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://katihfmaxtron.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ikkemandar.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://googlejfgdlenewstoday.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bhrecadominicana.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://anupam-bestprice89.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bhrepublica.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://allinspirations.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://weallfreieds.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://shanmegurad.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://manualmfuctional.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://verybeayurifull.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://prasat.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://meri.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://prabu.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://easehipranaam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://nidatyi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://krodyit.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://naine.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://breajwasi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://beithe.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://allthingnbeyondblog.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://usafunworldt.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://financialallorner.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mjghouthernmatron.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://chandancomputers.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bingshopping.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ptritam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tech-universes.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://littleboy.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://correctinspiration.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://esujkamien.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ujkaeltnx.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ausnangck2809.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://supermu.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://buyfcyclingteam.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tumari.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://hariomm.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://lejano.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://jotumko.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bestandroid.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://discoverable.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://puriagatratt.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://azizlemon.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ptrfsiitan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://boardgame-breking.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://flowwergulab.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bingcreater-uk.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://skinnyskin.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://amil.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mohan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://dilip.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://avinasg.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://virat.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://hsadyttk.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://ashu-quality.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://tinko.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://konkaruna.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://udavhav.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://matura.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://patana.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://bopal.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://gwl.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://delhi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://gopi.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://kripa.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://charanraj.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://pream.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://sang.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://pasas.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://kanchan.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
http://assadaaka.nl/?URL=https://cityofrosedalems.com/
http://atchs.jp/jump?u=https://cityofrosedalems.com/
http://away3d.com/?URL=https://cityofrosedalems.com/
http://blackberryvietnam.net/proxy.php?link=https://cityofrosedalems.com/
http://boogiewoogie.com/?URL=https://cityofrosedalems.com/
http://bsumzug.de/url?q=https://cityofrosedalems.com/
http://business.eatonton.com/list?globaltemplate=https://cityofrosedalems.com/
http://archives.midweek.com/?URL=https://cityofrosedalems.com/
http://armdrag.com/mb/get.php?url=https://cityofrosedalems.com/
http://asai-kota.com/acc/acc.cgi?REDIRECT=https://cityofrosedalems.com/
http://ass-media.de/wbb2/redir.php?url=https://cityofrosedalems.com/
http://cdiabetes.com/redirects/offer.php?URL=https://cityofrosedalems.com/
https://padletcdn.com/cgi/fetch?disposition=attachment&url=https://cityofrosedalems.com/
http://cdn.iframe.ly/api/iframe?url=https://cityofrosedalems.com/
http://centuryofaction.org/?URL=https://cityofrosedalems.com/
http://Somewh.a.T.dfqw@www.newsdiffs.org/article-history/www.findabeautyschool.com/map.aspx?url=https://cityofrosedalems.com/
http://actontv.org/?URL=https://cityofrosedalems.com/
http://bigbarganz.com/g/?https://cityofrosedalems.com/
http://andreasgraef.de/url?q=https://cityofrosedalems.com/
http://app.espace.cool/ClientApi/SubscribeToCalendar/1039?url=https://cityofrosedalems.com/
http://archive.paulrucker.com/?URL=https://cityofrosedalems.com/
http://baseballpodcasts.net/Feed2JS/feed2js.php?src=https://cityofrosedalems.com/
http://bbs.mottoki.com/index?bbs=hpsenden&act=link&page=94&linkk=https://cityofrosedalems.com/
http://bernhardbabel.com/url?q=https://cityofrosedalems.com/
http://albertaaerialapplicators.com/?URL=https://cityofrosedalems.com/
http://alexanderroth.de/url?q=https://cityofrosedalems.com/
http://alt.baunetzwissen.de/rd/nl-track/?obj=bnw&cg1=redirect&cg2=wissen-newsletter:beton&cg3=partner&datum=2017-01&titel=partnerklick-beton&firma=informationszentrumbeton&link=https://cityofrosedalems.com/
https://duckduckgo.com/?q=https%3A%2F%2Fspo1top.com&t=h_&ia=web
https://www.google.com/search?q=https%3A%2F%2Fspo1top.com&sca_esv=567933587&sxsrf=AM9HkKkLNk4hFHvgRG-dTHcW476TZakh3Q%3A1695527512326&ei=WLIPZZPIE66t4-EPwYOS4AE&ved=0ahUKEwiT1NCYrMKBAxWu1jgGHcGBBBwQ4dUDCBA&uact=5&oq=https%3A%2F%2Fspo1top.com&gs_lp=Egxnd3Mtd2l6LXNlcnAiE2h0dHBzOi8vc3BvMXRvcC5jb20yBBAjGCdI2x9QkAtY2xpwAXgBkAEAmAGHAaABygeqAQMwLji4AQPIAQD4AQHCAgoQABhHGNYEGLAD4gMEGAAgQYgGAZAGBg&sclient=gws-wiz-serp
http://client.paltalk.com/client/webapp/client/External.wmt?url=https://cityofrosedalems.com/
http://community.nxp.com/t5/custom/page/page-id/ExternalRedirect?url=https://cityofrosedalems.com/
http://foro.infojardin.com/proxy.php?link=https://cityofrosedalems.com/
http://ceskapozice.lidovky.cz/redir.aspx?url=https://cityofrosedalems.com/
http://mitsui-shopping-park.com/lalaport/ebina/redirect.html?url=https://cityofrosedalems.com/
http://www.interempresas.net/estadisticas/r.asp?idsector=129&e=221083&c=195&d=https://cityofrosedalems.com/
http://fishbiz.seagrant.uaf.edu/click-thru.html?url=https://cityofrosedalems.com/
http://kupiauto.zr.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://ipx.bcove.me/?url=https://cityofrosedalems.com/
https://toolbarqueries.google.td/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
https://home.guanzhuang.org/link.php?url=https://cityofrosedalems.com/
http://dvd24online.de/url?q=https://cityofrosedalems.com/
http://twindish-electronics.de/url?q=https://cityofrosedalems.com/
http://www.beigebraunapartment.de/url?q=https://cityofrosedalems.com/
http://www.mix-choice.com/yomi/rank.cgi?mode=link&id=391&url=https://cityofrosedalems.com/
https://cse.google.fm/url?q=https://cityofrosedalems.com/
http://www.1soft-tennis.com/search/rank.cgi?mode=link&id=17&url=https://cityofrosedalems.com/
http://big-data-fr.com/linkedin.php?lien=https://cityofrosedalems.com/
http://reg.kost.ru/cgi-bin/go?https://cityofrosedalems.com/
http://www.kirstenulrich.de/url?q=https://cityofrosedalems.com/
http://www.inkwell.ru/redirect/?url=https://cityofrosedalems.com/
https://cse.google.co.je/url?q=https://cityofrosedalems.com/
http://click-navi.jp/cgi/service-search/rank.cgi?mode=link&id=121&url=https://cityofrosedalems.com/
https://n1653.funny.ge/redirect.php?url=https://cityofrosedalems.com/
https://www.livecmc.com/?lang=fr&id=Ld9efT&url=https://cityofrosedalems.com/
http://maps.google.com/url?q=https://cityofrosedalems.com/
http://maps.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://plus.google.com/url?q=https://cityofrosedalems.com/
http://cse.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://clients1.google.de/url?sa=t&url=https://cityofrosedalems.com/
http://es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
http://forum.solidworks.com/external-link.jspa?url=https://cityofrosedalems.com/
http://www.guru-pon.jp/search/rank.cgi?mode=link&id=107&url=https://cityofrosedalems.com/
https://www.google.to/url?q=https://cityofrosedalems.com/
https://maps.google.bi/url?q=https://cityofrosedalems.com/
http://www.nickl-architects.com/url?q=https://cityofrosedalems.com/
https://www.ocbin.com/out.php?url=https://cityofrosedalems.com/
http://www.lobenhausen.de/url?q=https://cityofrosedalems.com/
https://image.google.bs/url?q=https://cityofrosedalems.com/
http://redirect.me/?https://cityofrosedalems.com/
http://kank.o.oo7.jp/cgi-bin/ys4/rank.cgi?mode=link&id=569&url=https://cityofrosedalems.com/
http://ruslog.com/forum/noreg.php?https://cityofrosedalems.com/
http://forum.ahigh.ru/away.htm?link=https://cityofrosedalems.com/
http://www.wildner-medien.de/url?q=https://cityofrosedalems.com/
http://www.tifosy.de/url?q=https://cityofrosedalems.com/
https://image.google.co.ck/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
http://ads.icorp.ro/others/STS/?t=CeNortjKxUjK0NDFVsgZcMBADAm4-&g=https://cityofrosedalems.com/
http://deai-ranking.org/search/rank.cgi?mode=link&id=28&url=https://cityofrosedalems.com/
https://izispicy.com/go.php?url=https://cityofrosedalems.com/
http://demo.vieclamcantho.vn/baohiemthatnghiep/Redirect.aspx?sms=90bb20bb20tbb20thc3%B4ng&link=https://cityofrosedalems.com/
http://www.city-fs.de/url?q=https://cityofrosedalems.com/
http://p.profmagic.com/urllink.php?url=https://cityofrosedalems.com/
https://www.google.md/url?q=https://cityofrosedalems.com/
https://maps.google.com.pa/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?sa=j&source=web&rct=j&url=https://cityofrosedalems.com/
http://www.arndt-am-abend.de/url?q=https://cityofrosedalems.com/
https://maps.google.com.vc/url?q=https://cityofrosedalems.com/
http://maxnetworks.org/searchlink/rank.cgi?mode=link&id=321&url=https://cityofrosedalems.com/
https://image.google.com.ng/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://www.kenzai-navi.com/location/location_topbanner.php?bs=238&m=899&href=https://cityofrosedalems.com/
https://www.anybeats.jp/jump/?https://cityofrosedalems.com/
http://url-collector.appspot.com/positiveVotes?topic=Hate%20speech&url=https://cityofrosedalems.com/
https://cse.google.com.cy/url?q=https://cityofrosedalems.com/
https://maps.google.be/url?sa=j&url=https://cityofrosedalems.com/
https://top.hange.jp/linkdispatch/dispatch?targetUrl=https://cityofrosedalems.com/
http://www.link.gokinjyo-eikaiwa.com/rank.cgi?mode=link&id=5&url=https://cityofrosedalems.com/
http://www.air-dive.com/au/mt4i.cgi?mode=redirect&ref_eid=697&url=https://cityofrosedalems.com/
http://okozukai.j-web.jp/j-web/okozukai/ys4/rank.cgi?mode=link&id=6817&url=https://cityofrosedalems.com/
https://sfmission.com/rss/feed2js.php?src=https://cityofrosedalems.com/
https://www.watersportstaff.co.uk/extern.aspx?src=https://cityofrosedalems.com/&cu=60096&page=1&t=1&s=42"
http://siamcafe.net/board/go/go.php?https://cityofrosedalems.com/
http://www.whitening-navi.info/cgi/search-smartphone/rank.cgi?mode=link&id=1431&url=https://cityofrosedalems.com/
http://www.youtube.com/redirect?q=https://cityofrosedalems.com/
http://www.youtube.com/redirect?event=channeldescription&q=https://cityofrosedalems.com/
http://www.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://go.e-frontier.co.jp/rd2.php?uri=https://cityofrosedalems.com/
http://adchem.net/Click.aspx?url=https://cityofrosedalems.com/
http://www.reddotmedia.de/url?q=https://cityofrosedalems.com/
https://10ways.com/fbredir.php?orig=https://cityofrosedalems.com/
https://emailtrackerapi.leadforensics.com/api/URLOpen?EmailSentRecordID=17006&URL=https://cityofrosedalems.com/
http://search.haga-f.net/rank.cgi?mode=link&url=https://cityofrosedalems.com/
http://7ba.ru/out.php?url=https://cityofrosedalems.com/
http://www.51queqiao.net/link.php?url=https://cityofrosedalems.com/
https://cse.google.dm/url?q=https://cityofrosedalems.com/
https://rsyosetsu.bookmarks.jp/ys4/rank.cgi?mode=link&id=3519&url=https://cityofrosedalems.com/
https://ulfishing.ru/forum/go.php?https://cityofrosedalems.com/
https://underwood.ru/away.html?url=https://cityofrosedalems.com/
https://unicom.ru/links.php?go=https://cityofrosedalems.com/
http://www.unifin.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://uogorod.ru/feed/520?redirect=https://cityofrosedalems.com/
http://uvbnb.ru/go?https://cityofrosedalems.com/
https://skibaza.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://smartservices.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.topkam.ru/gtu/?url=https://cityofrosedalems.com/
https://torggrad.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://torgi-rybinsk.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://tpprt.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://clients1.google.al/url?q=https://cityofrosedalems.com/
https://cse.google.al/url?q=https://cityofrosedalems.com/
https://images.google.al/url?q=https://cityofrosedalems.com/
http://toolbarqueries.google.al/url?q=https://cityofrosedalems.com/
https://www.google.al/url?q=https://cityofrosedalems.com/
https://www.snek.ai/redirect?url=https://cityofrosedalems.com/
http://gals.graphis.ne.jp/mkr/out.cgi?id=01019&go=https://cityofrosedalems.com/
http://www.capitalbikepark.se/bok/go.php?url=https://cityofrosedalems.com/
http://cooltgp.org/tgp/click.php?id=370646&u=https://cityofrosedalems.com/
https://joomlinks.org/?url=https://cityofrosedalems.com/
https://managementportal.de/modules/mod_jw_srfr/redir.php?url=https://cityofrosedalems.com/
http://www.green-cross.pro/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://m.adlf.jp/jump.php?l=https://cityofrosedalems.com/
https://www.moonbbs.com/dm/dmlink.php?dmurl=https://cityofrosedalems.com/
http://www.mac52ipod.cn/urlredirect.php?go=https://cityofrosedalems.com/
http://chrison.net/ct.ashx?id=dad2849a-8862-4a6d-b842-ef628cbfd3c3&url=https://cityofrosedalems.com/
http://datasheetcatalog.biz/url.php?url=https://cityofrosedalems.com/
http://minlove.biz/out.html?id=nhmode&go=https://cityofrosedalems.com/
http://www.diwaxx.ru/win/redir.php?redir=https://cityofrosedalems.com/
http://request-response.com/blog/ct.ashx?url=https://cityofrosedalems.com/
https://turbazar.ru/url/index?url=https://cityofrosedalems.com/
http://members.asoa.org/sso/logout.aspx?returnurl=https://cityofrosedalems.com/
http://login.mediafort.ru/autologin/mail/?code=14844×02ef859015x290299&url=https://cityofrosedalems.com/
https://www.sports-central.org/cgi-bin/axs/ax.pl?https://cityofrosedalems.com/
http://dot.boss.sk/o/rs.php?ssid=1&szid=4&dsid=12&dzid=32&url=https://cityofrosedalems.com/
http://www.americanstylefridgefreezer.co.uk/go.php?url=https://cityofrosedalems.com/
https://www.newzealandgirls.co.nz/auckland/escorts/clicks.php?click_id=NavBar_AF_AdultDirectory&target=https://cityofrosedalems.com/
https://wdesk.ru/go?https://cityofrosedalems.com/
http://edcommunity.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://page.yicha.cn/tp/j?url=https://cityofrosedalems.com/
http://www.internettrafficreport.com/cgi-bin/cgirdir.exe?https://cityofrosedalems.com/
http://www.interfacelift.com/goto.php?url=https://cityofrosedalems.com/
https://good-surf.ru/r.php?g=https://cityofrosedalems.com/
http://mbrf.ae/knowledgeaward/language/ar/?redirect_url=https://cityofrosedalems.com/
https://gektor-nsk.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://lekoufa.ru/banner/go?banner_id=4&link=https://cityofrosedalems.com/
https://forsto.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://www.cossa.ru/bitrix/redirect.php?event1=click&event2=&event3=&goto=https://cityofrosedalems.com/
http://www.gigatran.ru/go?url=https://cityofrosedalems.com/
http://www.gigaalert.com/view.php?h=&s=https://cityofrosedalems.com/
http://www.jkes.tyc.edu.tw/dyna/webs/gotourl.php?id=357&url=https://cityofrosedalems.com/
http://www.laopinpai.com/gourl.asp?url=/gourl.asp?url=https://cityofrosedalems.com/
http://www.humanbrainmapping.org/i4a/etrack/track.cfm?rType=2&campaignID=3572&contactID=4524&origURL=https://cityofrosedalems.com/
https://gcup.ru/go?https://cityofrosedalems.com/
http://www.gearguide.ru/phpbb/go.php?https://cityofrosedalems.com/
https://es.catholic.net/ligas/ligasframe.phtml?liga=https://cityofrosedalems.com/
https://passport.sfacg.com/LoginOut.aspx?Returnurl=https://cityofrosedalems.com/
https://offers.sidex.ru/stat_ym_new.php?redir=https://cityofrosedalems.com/&hash=1577762
https://globalmedia51.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://ramset.com.au/document/url/?url=https://cityofrosedalems.com/
https://www.vicsport.com.au/analytics/outbound?url=https://cityofrosedalems.com/
http://www.cyprus-net.com/banner_click.php?banid=4&link=https://cityofrosedalems.com/
http://a.gongkong.com/db/adredir.asp?id=16757&url=https://cityofrosedalems.com/
http://gfaq.ru/go?https://cityofrosedalems.com/
http://gbi-12.ru/links.php?go=https://cityofrosedalems.com/
http://www.global-flat.com/mobile/mobile_switch.php?dir=tofull&url=https://cityofrosedalems.com/
https://golden-resort.ru/out.php?out=https://cityofrosedalems.com/
http://avalon.gondor.ru/away.php?link=https://cityofrosedalems.com/
http://www.laosubenben.com/home/link.php?url=https://cityofrosedalems.com/
http://www.lifeshow.com.tw/show.php?ty5_id=1599&url=https://cityofrosedalems.com/
http://games.cheapdealuk.co.uk/go.php?url=https://cityofrosedalems.com/
https://www.netwalkerstore.com/redirect.asp?accid=18244&adcampaignid=2991&adcampaigntype=2&affduration=30&url=https://cityofrosedalems.com/
http://tsugarubrand.jp/blog/?wptouch_switch=mobile&redirect=https://cityofrosedalems.com/
http://blackhistorydaily.com/black_historylinks/link.asp?linkid=5&URL=https://cityofrosedalems.com/
http://www.kitchencabinetsdirectory.com/redirect.asp?url=https://cityofrosedalems.com/
http://cityprague.ru/go.php?go=https://cityofrosedalems.com/
https://www.hachimantaishi.com/click3/click3.cgi?cnt=c5&url=https://cityofrosedalems.com/
http://earnupdates.com/goto.php?url=https://cityofrosedalems.com/
http://www.careerbuilder.com.cn/cn/tallyredirector.aspx?task=Ushi&objName=SnsShare&redirectUrl=https://cityofrosedalems.com/&methodName=SetSnsShareLink
http://banners.knollenstein.com/adnetwork/servlet/advertBeans.TrackingServlet?seid=27709655&t=100&redirectTo=https://cityofrosedalems.com/&cid=DECONL&vid=986809&externalsource=jobbsquareppc
https://meyeucon.org/ext-click.php?url=https://cityofrosedalems.com/
http://www.cnmhe.fr/spip.php?action=cookie&url=https://cityofrosedalems.com/
https://perezvoni.com/blog/away?url=https://cityofrosedalems.com/
https://www.ciymca.org/af/register-redirect/71104?url=https://cityofrosedalems.com/
https://whizpr.nl/tracker.php?u=https://cityofrosedalems.com/
http://bw.irr.by/knock.php?bid=252583&link=https://cityofrosedalems.com/
http://www.beeicons.com/redirect.php?site=https://cityofrosedalems.com/
http://www.diwaxx.ru/hak/redir.php?redir=https://cityofrosedalems.com/
http://www.actuaries.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.isadatalab.com/redirect?clientId=ee5a64e1-3743-9b4c-d923-6e6d092ae409&appId=69&value=[EMVFIELD]EMAIL[EMV/FIELD]&cat=Techniquesculturales&url=https://cityofrosedalems.com/
https://www.jukujo.gs/bin/out.cgi?id=kimiomof&url=https://cityofrosedalems.com/
http://hansolav.net/blog/ct.ashx?id=0af6301b-e71f-44be-838f-905709eee792&url=https://cityofrosedalems.com/
http://bitrix.adlr.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://m.snek.ai/redirect?url=https://cityofrosedalems.com/
http://www.officeservice.com.br/trackMKT/trackerFolha.aspx?DESCRIPTION=HotSite_EcrmContabil&FROM=Workshop&DESTINATION=https://cityofrosedalems.com/
https://www.arbsport.ru/gotourl.php?url=https://cityofrosedalems.com/
http://www.strana.co.il/finance/redir.aspx?site=https://cityofrosedalems.com/
https://www.tourplanisrael.com/redir/?url=https://cityofrosedalems.com/
http://www.mutualgravity.com/archangel/webcontact/d_signinsimple.php?action=signup&CID=240&EID=&S=default.css&return=https://cityofrosedalems.com/
http://buildingreputation.com/lib/exe/fetch.php?media=https://cityofrosedalems.com/
http://gals.graphis.ne.jp/mkr/out.cgi?id=04489&go=https://cityofrosedalems.com/
http://dir.tetsumania.net/search/rank.cgi?mode=link&id=3267&url=https://cityofrosedalems.com/
http://www.project24.info/mmview.php?dest=https://cityofrosedalems.com/
http://tfads.testfunda.com/TFServeAds.aspx?strTFAdVars=4a086196-2c64-4dd1-bff7-aa0c7823a393,TFvar,00319d4f-d81c-4818-81b1-a8413dc614e6,TFvar,GYDH-Y363-YCFJ-DFGH-5R6H,TFvar,https://cityofrosedalems.com/
http://www.request-response.com/blog/ct.ashx?id=d827b163-39dd-48f3-b767-002147c94e05&url=https://cityofrosedalems.com/
http://www.cooltgp.org/tgp/click.php?id=370646&u=https://cityofrosedalems.com/
http://sdchamber.biz/admin/mod_newsletter/redirect.aspx?message_id=785&redirect=https://cityofrosedalems.com/
http://www.kyoto-osaka.com/search/rank.cgi?mode=link&url=https://cityofrosedalems.com/
http://metalist.co.il/redirect.asp?url=https://cityofrosedalems.com/
http://i-marine.eu/pages/goto.aspx?link=https://cityofrosedalems.com/
http://www.teenlove.biz/cgibin/atc/out.cgi?id=24&u=https://cityofrosedalems.com/
http://www.altoona.com/newsletter/click.php?volume=52&url=https://cityofrosedalems.com/
http://www.katjushik.ru/link.php?to=https://cityofrosedalems.com/
http://gondor.ru/go.php?url=https://cityofrosedalems.com/
http://proekt-gaz.ru/go?https://cityofrosedalems.com/
https://www.tricitiesapartmentguide.com/MobileDefault.aspx?reff=https://cityofrosedalems.com/
http://www.vietnamsingle.com/vn/banners/redirect.asp?url=https://cityofrosedalems.com/
https://www.voxlocalis.net/enlazar/?url=https://cityofrosedalems.com/
http://no-smok.net/nsmk/InterWiki?action=goto&oe=EUC-KR&url=https://cityofrosedalems.com/
http://www.stalker-modi.ru/go?https://cityofrosedalems.com/
http://www.p1-uranai.com/rank.cgi?mode=link&id=538&url=https://cityofrosedalems.com/
http://earthsciencescanada.com/modules/babel/redirect.php?newlang=en_us&newurl=https://cityofrosedalems.com/
http://psykodynamiskt.nu/?wptouch_switch=desktop&redirect=https://cityofrosedalems.com/
http://www.pcbheaven.com/forum/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.castellodivezio.it/lingua.php?lingua=EN&url=https://cityofrosedalems.com/
http://moscowdesignmuseum.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.fairpoint.net/~jensen1242/gbook/go.php?url=https://cityofrosedalems.com/
https://lirasport.com.ar/bloques/bannerclick.php?id=7&url=https://cityofrosedalems.com/
http://www.knabstrupper.se/guestbook/go.php?url=https://cityofrosedalems.com/
https://www.pba.ph/redirect?url=https://cityofrosedalems.com/&id=3&type=tab
https://bondage-guru.net/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://www.westbloomfieldlibrary.org/includes/statistics.php?StatType=Link&StatID=Facebook&weblink=https://cityofrosedalems.com/
https://tenisnews.com.br/clickTracker/clickTracker.php?u=https://cityofrosedalems.com/
http://www.stevestechspot.com/ct.ashx?id=9fdcf4a4-6e09-4807-bc31-ac1adf836f6c&url=https://cityofrosedalems.com/
http://rcoi71.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://www.laselection.net/redir.php3?cat=int&url=https://cityofrosedalems.com/
http://www.masai-mara.com/cgi-bin/link2.pl?grp=mm&link=https://cityofrosedalems.com/
http://evenemangskalender.se/redirect/?id=15723&lank=https://cityofrosedalems.com/
http://anifre.com/out.html?go=https://cityofrosedalems.com/
http://www.restavracije-gostilne.si/banner.php?id=44&url=https://cityofrosedalems.com/
http://hobbyplastic.co.uk/trigger.php?r_link=https://cityofrosedalems.com/
http://ads.pukpik.com/myads/click.php?banner_id=316&banner_url=https://cityofrosedalems.com/
https://www.adminer.org/redirect/?sa=t&url=https://cityofrosedalems.com/
http://www.bigblackmamas.com/cgi-bin/sites/out.cgi?url=https://cityofrosedalems.com/
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=https://cityofrosedalems.com/
https://primorye.ru/go.php?id=19&url=https://cityofrosedalems.com/
https://www.123gomme.it/it/ViewSwitcher/SwitchView?mobile=True&returnUrl=https://cityofrosedalems.com/
http://youmydaddy.com/cgi-bin/at3/out.cgi?id=88&trade=https://cityofrosedalems.com/
https://naviking.localking.com.tw/about/redirect.aspx?mid=7&url=https://cityofrosedalems.com/
http://sms-muzeji.si/Home/ChangeCulture?lang=sl&returnUrl=https://cityofrosedalems.com/
http://r.emeraldexpoinfo.com/s.ashx?ms=EXI2:125462_115115&e=krubin723@aol.com&eId=440186886&c=h&url=https://cityofrosedalems.com/
https://apps.firstrain.com/service/openurl.jsp?action=titleclick&src=rss&u=https://cityofrosedalems.com/
http://cdp.thegoldwater.com/click.php?id=101&url=https://cityofrosedalems.com/
http://www.appenninobianco.it/ads/adclick.php?bannerid=159&zoneid=8&source=&dest=https://cityofrosedalems.com/
https://www.eduplus.hk/special/emailalert/goURL.jsp?clickURL=https://cityofrosedalems.com/
http://www.japan.road.jp/navi/navi.cgi?jump=226&url=https://cityofrosedalems.com/
http://www.arch.iped.pl/artykuly.php?id=1&cookie=1&url=https://cityofrosedalems.com/
http://hirlevelcenter.eu/click.php?hirlevel_id=13145441085508&url=https://cityofrosedalems.com/
https://birds.cz/avif/redirect.php?from=avif.obs.php&url=https://cityofrosedalems.com/
http://www.bigblackbootywatchers.com/cgi-bin/sites/out.cgi?url=https://cityofrosedalems.com/
http://barykin.com/go.php?https://cityofrosedalems.com/
https://forum.everleap.com/proxy.php?link=https://cityofrosedalems.com/
http://www.mosig-online.de/url?q=https://cityofrosedalems.com/
http://www.hccincorporated.com/?URL=https://cityofrosedalems.com/
http://fatnews.com/?URL=https://cityofrosedalems.com/
https://csirealty.com/?URL=https://cityofrosedalems.com/
http://asadi.de/url?q=https://cityofrosedalems.com/
http://treblin.de/url?q=https://cityofrosedalems.com/
https://kentbroom.com/?URL=https://cityofrosedalems.com/
http://0845.boo.jp/cgi/mt3/mt4i.cgi?id=24&mode=redirect&no=15&ref_eid=3387&url=https://cityofrosedalems.com/
http://110.164.66.211/ULIB6//dublin.linkout.php?url=https://cityofrosedalems.com/
http://110.164.92.12/ULIB//dublin.linkout.php?url=https://cityofrosedalems.com/
http://198.54.125.86.myopenlink.net/describe/?url=https://cityofrosedalems.com/
http://202.144.225.38/jmp?url=https://cityofrosedalems.com/
http://2cool2.be/url?q=https://cityofrosedalems.com/
http://4coma.net/cgi/mt4/mt4i.cgi?cat=12&mode=redirect&ref_eid=3231&url=https://cityofrosedalems.com/
http://4travel.jp/dynamic/redirect.php?mode=dm_tour&url=https://cityofrosedalems.com/
http://imaginingourselves.globalfundforwomen.org/pb/External.aspx?url=https://cityofrosedalems.com/
https://clients1.google.cd/url?q=https://cityofrosedalems.com/
https://clients1.google.co.id/url?q=https://cityofrosedalems.com/
https://clients1.google.co.in/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ag/url?q=https://cityofrosedalems.com/
https://clients1.google.com.et/url?q=https://cityofrosedalems.com/
https://clients1.google.com.tr/url?q=https://cityofrosedalems.com/
https://clients1.google.com.ua/url?q=https://cityofrosedalems.com/
https://clients1.google.fm/url?q=https://cityofrosedalems.com/
https://clients1.google.hu/url?q=https://cityofrosedalems.com/
https://clients1.google.md/url?q=https://cityofrosedalems.com/
https://clients1.google.mw/url?q=https://cityofrosedalems.com/
https://clients1.google.nu/url?sa=j&url=https://cityofrosedalems.com/
https://clients1.google.rw/url?q=https://cityofrosedalems.com/
http://search.pointcom.com/k.php?ai=&url=https://cityofrosedalems.com/
http://www.cercasostituto.it/index.php?name=GestBanner&file=counter&idbanner=40&dir_link=https://cityofrosedalems.com/
http://vsvejr.dk/mt/plugins/stationExtremes/redirect.php?url=https://cityofrosedalems.com/
http://www.meteo-leran.fr/meteotemplate/template/plugins/deviations/redirect.php?url=https://cityofrosedalems.com/
https://www.eurobichons.com/fda%20alerts.php?url=https://cityofrosedalems.com/
https://mydojo.at/de_AT/karate/weiterleitung?redirect=https://cityofrosedalems.com/
http://click.phosphodiesterase4.com/k.php?ai=&url=https://cityofrosedalems.com/
http://www.mariahownersclub.com/forum/redirect-to/?redirect=https://cityofrosedalems.com/
http://www.wildromance.com/buy.php?url=https://cityofrosedalems.com/&store=iBooks&book=omk-ibooks-us
http://mapleriverweather.com/mobile/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://www.archijob.co.il/index/comp_website.asp?companyId=1469&website=https://cityofrosedalems.com/
https://student-helpr.rminds.dev/redirect?redirectTo=https://cityofrosedalems.com/
http://www.kalinna.de/url?q=https://cityofrosedalems.com/
http://www.hartmanngmbh.de/url?q=https://cityofrosedalems.com/
https://www.the-mainboard.com/proxy.php?link=https://cityofrosedalems.com/
https://www.betamachinery.com/?URL=https://cityofrosedalems.com/
http://nishiyama-takeshi.com/mobile2/mt4i.cgi?id=3&mode=redirect&no=67&ref_eid=671&url=https://cityofrosedalems.com/
http://www.sprang.net/url?q=https://cityofrosedalems.com/
https://img.2chan.net/bin/jump.php?https://cityofrosedalems.com/
http://www.is.kyusan-u.ac.jp/htmllint/htmllint.cgi?ViewSource=on;URL=https://cityofrosedalems.com/
http://local.rongbachkim.com/rdr.php?url=https://cityofrosedalems.com/
http://bachecauniversitaria.it/link/frm_top.php?url=https://cityofrosedalems.com/
https://www.stcwdirect.com/redirect.php?url=https://cityofrosedalems.com/
http://forum.vcoderz.com/externalredirect.php?url=https://cityofrosedalems.com/
https://www.momentumstudio.com/?URL=https://cityofrosedalems.com/
https://s-p.me/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://ww.thesteelbrothers.com/buy.php?store=iBooks&url=https://cityofrosedalems.com/
http://thdt.vn/convert/convert.php?link=https://cityofrosedalems.com/
http://www.doitweb365.de/scripts/doitweb.exe/rasklickzaehler2?https://cityofrosedalems.com/
https://www.guadamur.eu/template/pages/station/redirect.php?url=https://cityofrosedalems.com/
https://weather.cube.com.gr/pages/station/redirect.php?url=https://cityofrosedalems.com/
http://www.fimmgviterbo.org/mobfimmgviterbo/index.php?nametm=counter&idbanner=4&dir_link=https://cityofrosedalems.com/
http://www.mckinneyfarm.com/template/plugins/stationExtremes/redirect.php?url=https://cityofrosedalems.com/
https://utmagazine.ru/r?url=https://cityofrosedalems.com/
http://www.evrika41.ru/redirect?url=https://cityofrosedalems.com/
http://expomodel.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://facto.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://fallout3.ru/utils/ref.php?url=https://cityofrosedalems.com/
https://fc-zenit.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://forum-region.ru/forum/away.php?s=https://cityofrosedalems.com/
http://forumdate.ru/redirect-to/?redirect=https://cityofrosedalems.com/
http://shckp.ru/ext_link?url=https://cityofrosedalems.com/
https://shinglas.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://sibran.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://sintez-oka.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://app.espace.cool/clientapi/subscribetocalendar/974?url=https://cityofrosedalems.com/
http://111056.net/yomisearch/rank.cgi?mode=link&id=6205&url=https://cityofrosedalems.com/
http://stanko.tw1.ru/redirect.php?url=https://cityofrosedalems.com/
http://www.sozialemoderne.de/url?q=https://cityofrosedalems.com/
http://www.showb.com/search/ranking.cgi?mode=link&id=7083&url=https://cityofrosedalems.com/
http://imagelibrary.asprey.com/?URL=www.https://cityofrosedalems.com/
http://ime.nu/https://cityofrosedalems.com/
http://interflex.biz/url?q=https://cityofrosedalems.com/
http://ivvb.de/url?q=https://cityofrosedalems.com/
http://j.lix7.net/?https://cityofrosedalems.com/
http://jacobberger.com/?URL=www.https://cityofrosedalems.com/
http://jahn.eu/url?q=https://cityofrosedalems.com/
http://jamesvelvet.com/?URL=www.https://cityofrosedalems.com/
http://jla.drmuller.net/r.php?url=https://cityofrosedalems.com/
http://jump.pagecs.net/https://cityofrosedalems.com/
http://kagarin.net/cgi/mt/mt4i.cgi?id=2&mode=redirect&no=330&ref_eid=103&url=https://cityofrosedalems.com/
http://karkom.de/url?q=https://cityofrosedalems.com/
http://kens.de/url?q=https://cityofrosedalems.com/
http://kinderundjugendpsychotherapie.de/url?q=https://cityofrosedalems.com/
http://kinhtexaydung.net/redirect/?url=https://cityofrosedalems.com/
http://neoromance.info/link/rank.cgi?mode=link&id=26&url=https://cityofrosedalems.com/
http://www.hainberg-gymnasium.com/url?q=https://cityofrosedalems.com/
https://befonts.com/checkout/redirect?url=https://cityofrosedalems.com/
https://www.usap.gov/externalsite.cfm?https://cityofrosedalems.com/
https://maps.google.com.ua/url?rct=j&sa=t&url=https://cityofrosedalems.com/
http://images.google.hu/url?q=https://cityofrosedalems.com/
http://images.google.com.mx/url?q=https://cityofrosedalems.com/
http://www.google.com.mx/url?q=https://cityofrosedalems.com/
http://images.google.com.hk/url?q=https://cityofrosedalems.com/
http://images.google.fi/url?q=https://cityofrosedalems.com/
http://maps.google.fi/url?q=https://cityofrosedalems.com/
http://www.shinobi.jp/etc/goto.html?https://cityofrosedalems.com/
http://images.google.co.id/url?q=https://cityofrosedalems.com/
http://maps.google.co.id/url?q=https://cityofrosedalems.com/
http://images.google.no/url?q=https://cityofrosedalems.com/
http://maps.google.no/url?q=https://cityofrosedalems.com/
http://images.google.co.th/url?q=https://cityofrosedalems.com/
http://maps.google.co.th/url?q=https://cityofrosedalems.com/
http://www.google.co.th/url?q=https://cityofrosedalems.com/
http://maps.google.co.za/url?q=https://cityofrosedalems.com/
http://images.google.ro/url?q=https://cityofrosedalems.com/
http://maps.google.ro/url?q=https://cityofrosedalems.com/
http://cse.google.dk/url?q=https://cityofrosedalems.com/
http://cse.google.com.tr/url?q=https://cityofrosedalems.com/
http://cse.google.hu/url?q=https://cityofrosedalems.com/
http://cse.google.com.hk/url?q=https://cityofrosedalems.com/
http://cse.google.fi/url?q=https://cityofrosedalems.com/
http://images.google.com.sg/url?q=https://cityofrosedalems.com/
http://cse.google.pt/url?q=https://cityofrosedalems.com/
http://cse.google.co.nz/url?q=https://cityofrosedalems.com/
http://images.google.com.ar/url?q=https://cityofrosedalems.com/
http://cse.google.co.id/url?q=https://cityofrosedalems.com/
http://images.google.com.ua/url?q=https://cityofrosedalems.com/
http://cse.google.no/url?q=https://cityofrosedalems.com/
http://cse.google.co.th/url?q=https://cityofrosedalems.com/
http://cse.google.ro/url?q=https://cityofrosedalems.com/
http://images.google.com.tr/url?q=https://cityofrosedalems.com/
http://maps.google.dk/url?q=https://cityofrosedalems.com/
http://www.google.fi/url?q=https://cityofrosedalems.com/
https://supplier-portal-uat.daimler.com/external-link.jspa?url=https://cityofrosedalems.com/
https://strelmag.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://spb90.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://spartak.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://sovaisova.ru/bitrix/redirect.php?event1=2009_mainpage&event2=go_www&event3=&goto=https://cityofrosedalems.com/
https://slashwrestling.com/cgi-bin/redirect.cgi?https://cityofrosedalems.com/
https://seoandme.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://s-online.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://rssfeeds.wtsp.com/~/t/0/0/wtsp/home/~https://cityofrosedalems.com/
https://rostovmama.ru/redirect?url=https://cityofrosedalems.com/
https://rev1.reversion.jp/redirect?url=https://cityofrosedalems.com/
https://www.star174.ru/redir.php?url=https://cityofrosedalems.com/
https://staten.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://stopcran.ru/go?https://cityofrosedalems.com/
http://studioad.ru/go?https://cityofrosedalems.com/
http://swepub.kb.se/setattribute?language=en&redirect=https://cityofrosedalems.com/
https://www.licnioglasi.org/index.php?thememode=full;redirect=https://cityofrosedalems.com/
http://www.rexart.com/cgi-rexart/al/affiliates.cgi?aid=872&redirect=https://cityofrosedalems.com/
http://www2.smartmail.com.ar/tl.php?p=hqf/f94/rs/1fp/4c0/rs//https://cityofrosedalems.com/
http://old.roofnet.org/external.php?link=https://cityofrosedalems.com/
http://www.bucatareasa.ro/link.php?url=https://cityofrosedalems.com/
http://mosprogulka.ru/go?https://cityofrosedalems.com/
https://uniline.co.nz/Document/Url/?url=https://cityofrosedalems.com/
https://tracker.onrecruit.net/api/v1/redirect/?redirect_to=https://cityofrosedalems.com/
http://slipknot1.info/go.php?url=https://cityofrosedalems.com/
https://www.samovar-forum.ru/go?https://cityofrosedalems.com/
http://staldver.ru/go.php?go=https://cityofrosedalems.com/
https://posts.google.com/url?q=https://cityofrosedalems.com/
https://plus.google.com/url?q=https://cityofrosedalems.com/
https://naruto.su/link.ext.php?url=https://cityofrosedalems.com/
https://justpaste.it/redirect/172fy/https://cityofrosedalems.com/
https://jt-pr-dot-yamm-track.appspot.com/Redirect?ukey=1iXi3dF8AuzUIsgifbcVnqqx-anF4B8R-9PC3UGhHO3E-1131306248&key=YAMMID-79725512&link=https://cityofrosedalems.com/
https://ipv4.google.com/url?q=https://cityofrosedalems.com/
https://forum.solidworks.com/external-link.jspa?url=https://cityofrosedalems.com/
https://foro.infojardin.com/proxy.php?link=https://cityofrosedalems.com/
https://ditu.google.com/url?q=https://cityofrosedalems.com/
https://de.flavii.de/index.php?flavii=linker&link=https://cityofrosedalems.com/
https://dakke.co/redirect/?url=https://cityofrosedalems.com/
https://creativecommons.org/choose/results-one?q_1=2&q_1=1&field_attribute_to_url=https://cityofrosedalems.com/
https://contacts.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://community.rsa.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.nxp.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.esri.com/external-link.jspa?url=https://cityofrosedalems.com/
https://community.cypress.com/external-link.jspa?url=https://cityofrosedalems.com/
https://club.panasonic.jp/member/terms/?siteId=B1&returnURL=https://cityofrosedalems.com/
https://cdn.iframe.ly/api/iframe?url=https://cityofrosedalems.com/
https://bukkit.org/proxy.php?link=https://cityofrosedalems.com/
https://branch.app.link/?$deeplink_path=article%2Fjan%2F123&$fallback_url=https://cityofrosedalems.com/&channel=facebook&feature=affiliate
https://boowiki.info/go.php?go=https://cityofrosedalems.com/
https://blaze.su/bitrix/redirect.php?event1=&event2=&event3=&goto=https://cityofrosedalems.com/
https://bbs.pku.edu.cn/v2/jump-to.php?url=https://cityofrosedalems.com/
https://bares.blog.idnes.cz/redir.aspx?url=https://cityofrosedalems.com/
https://www.flyingsamaritans.net/Startup/SetupSite.asp?RestartPage=https://cityofrosedalems.com/
https://www.ewind.cz/index.php?page=home/redirect&url=https://cityofrosedalems.com/
https://www.eas-racing.se/gbook/go.php?url=https://cityofrosedalems.com/
https://www.direkt-einkauf.de/includes/refer.php?id=170&url=https://cityofrosedalems.com/
https://www.dialogportal.com/Services/Forward.aspx?link=https://cityofrosedalems.com/
https://www.curseforge.com/linkout?remoteUrl=https://cityofrosedalems.com/
https://www.counterwelt.com/charts/click.php?user=14137&link=https://cityofrosedalems.com/
https://www.bing.com/news/apiclick.aspx?ref=FexRss&aid=&tid=9BB77FDA801248A5AD23FDBDD5922800&url=https://cityofrosedalems.com/
https://www.autopartskart.com/buyfromamzon.php?url=https://cityofrosedalems.com/
https://www.autoandrv.com/linkout.aspx?websiteurl=https://cityofrosedalems.com/
https://www.art-prizes.com/AdRedirector.aspx?ad=MelbPrizeSculpture_2017&target=https://cityofrosedalems.com/
https://wirelessestimator.com/advertise/newsletter_redirect.php?url=https://cityofrosedalems.com/
https://wasitviewed.com/index.php?href=https://cityofrosedalems.com/
https://tvtropes.org/pmwiki/no_outbounds.php?o=https://cityofrosedalems.com/
https://trello.com/add-card?source=mode=popup&name=click+here&desc=https://cityofrosedalems.com/
https://sutd.ru/links.php?go=https://cityofrosedalems.com/
https://abiznes.com.ua/bitrix/redirect.php?event1=&event2=&event3=&goto=https://cityofrosedalems.com/
http://www.sv-mama.ru/shared/go.php?url=https://cityofrosedalems.com/
http://www.sofion.ru/banner.php?r1=41&r2=2234&goto=https://cityofrosedalems.com/
http://www.rss.geodles.com/fwd.php?url=https://cityofrosedalems.com/
http://www.nuttenzone.at/jump.php?url=https://cityofrosedalems.com/
http://www.myhottiewife.com/cgi-bin/arpro/out.cgi?id=Jojo&url=https://cityofrosedalems.com/
http://www.mistress-and-slave.com/cgi-bin/out.cgi?id=123crush&url=https://cityofrosedalems.com/
http://www.johnvorhees.com/gbook/go.php?url=https://cityofrosedalems.com/
http://www.imsnet.at/LangChange.aspx?uri=https://cityofrosedalems.com/
http://www.hon-cafe.net/cgi-bin/re.cgi?lid=hmw&url=https://cityofrosedalems.com/
http://www.glorioustronics.com/redirect.php?link=https://cityofrosedalems.com/
http://www.etis.ford.com/externalURL.do?url=https://cityofrosedalems.com/
http://www.chungshingelectronic.com/redirect.asp?url=https://cityofrosedalems.com/
http://www.bdsmandfetish.com/cgi-bin/sites/out.cgi?id=mandymon&url=https://cityofrosedalems.com/
http://visits.seogaa.ru/redirect/?g=https://cityofrosedalems.com/
http://tharp.me/?url_to_shorten=https://cityofrosedalems.com/
http://speakrus.ru/links.php?go=https://cityofrosedalems.com/
http://spbstroy.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://solo-center.ru/links.php?go=https://cityofrosedalems.com/
http://sennheiserstore.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://school364.spb.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://sc.sie.gov.hk/TuniS/https://cityofrosedalems.com/
http://rzngmu.ru/go?https://cityofrosedalems.com/
http://rostovklad.ru/go.php?https://cityofrosedalems.com/
http://portalnp.snauka.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
http://markiza.me/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://jump.5ch.net/?https://cityofrosedalems.com/
http://imperialoptical.com/news-redirect.aspx?url=https://cityofrosedalems.com/
http://hellothai.com/wwwlink/wwwredirect.asp?hp_id=1242&url=https://cityofrosedalems.com/
http://guru.sanook.com/?URL=https://cityofrosedalems.com/
http://fr.knubic.com/redirect_to?url=https://cityofrosedalems.com/
http://branch.app.link/?$deeplink_path=article/jan/123&$fallback_url=https://cityofrosedalems.com/
http://biz-tech.org/bitrix/rk.php?goto=https://cityofrosedalems.com/
http://2010.russianinternetweek.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://xat.com/web_gear/chat/linkvalidator.php?link=https://cityofrosedalems.com/
https://www.yeaah.com/disco/DiscoGo.asp?ID=3435&Site=https://cityofrosedalems.com/
https://www.woodlist.us/delete-company?nid=13964&element=https://cityofrosedalems.com/
https://www.viecngay.vn/go?to=https://cityofrosedalems.com/
https://www.uts.edu.co/portal/externo.php?id=https://cityofrosedalems.com/
https://www.talgov.com/Main/exit.aspx?url=https://cityofrosedalems.com/
https://www.serie-a.ru/bitrix/redirect.php?goto=https://cityofrosedalems.com/
https://www.russianrobotics.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.ruchnoi.ru/ext_link?url=https://cityofrosedalems.com/
https://www.rprofi.ru/bitrix/rk.php?goto=https://cityofrosedalems.com/
https://www.roccotube.com/cgi-bin/at3/out.cgi?id=27&tag=toplist&trade=https://cityofrosedalems.com/
https://www.nyl0ns.com/cgi-bin/a2/out.cgi?id=43&l=btop&u=https://cityofrosedalems.com/
https://www.meetme.com/apps/redirect/?url=https://cityofrosedalems.com/
https://www.kath-kirche-kaernten.at/pfarren/pfarre/C3014?URL=https://cityofrosedalems.com/
https://www.interpals.net/url_redirect.php?href=https://cityofrosedalems.com/
https://www.interecm.com/interecm/tracker?op=click&id=5204.db2&url=https://cityofrosedalems.com/
https://www.hottystop.com/cgi-bin/at3/out.cgi?id=12&trade=https://cityofrosedalems.com/
https://www.hobowars.com/game/linker.php?url=https://cityofrosedalems.com/
https://www.hentainiches.com/index.php?id=derris&tour=https://cityofrosedalems.com/
https://www.gudarjavalambre.com/sections/miscelany/link.php?url=https://cityofrosedalems.com/
https://www.greencom.ru/catalog/irrigation_systems.html?jumpsite=2008&url=https://cityofrosedalems.com/
https://www.girlznation.com/cgi-bin/atc/out.cgi?id=50&l=side&u=https://cityofrosedalems.com/
https://www.funeralunion.org/delete-company?nid=39&element=https://cityofrosedalems.com/
https://www.fudbal91.com/tz.php?zone=America/Iqaluit&r=https://cityofrosedalems.com/
https://www.frodida.org/BannerClick.php?BannerID=29&LocationURL=https://cityofrosedalems.com/
https://kryvbas.at.ua/go?https://cityofrosedalems.com/
https://google.cat/url?q=https://cityofrosedalems.com/
https://joomluck.com/go/?https://cityofrosedalems.com/
https://www.anonymz.com/?https://cityofrosedalems.com/
https://weburg.net/redirect?url=https://cityofrosedalems.com/
https://tw6.jp/jump/?url=https://cityofrosedalems.com/
https://www.spainexpat.com/?URL=https://cityofrosedalems.com/
https://www.fotka.pl/link.php?u=https://cityofrosedalems.com/
https://www.lolinez.com/?https://cityofrosedalems.com/
https://nanos.jp/jmp?url=https://cityofrosedalems.com/
https://www.fca.gov/?URL=https://cityofrosedalems.com/
https://savvylion.com/?bmDomain=https://cityofrosedalems.com/
https://www.soyyooestacaido.com/https://cityofrosedalems.com/
https://www.gta.ru/redirect/www.https://cityofrosedalems.com/
https://directx10.org/go?https://cityofrosedalems.com/
https://mejeriet.dk/link.php?id=https://cityofrosedalems.com/
https://ezdihan.do.am/go?https://cityofrosedalems.com/
https://fishki.net/click?https://cityofrosedalems.com/
https://hiddenrefer.com/?https://cityofrosedalems.com/
https://romhacking.ru/go?https://cityofrosedalems.com/
https://turion.my1.ru/go?https://cityofrosedalems.com/
https://kassirs.ru/sweb.asp?url=https://cityofrosedalems.com/
https://www.allods.net/redirect/https://cityofrosedalems.com/
https://icook.ucoz.ru/go?https://cityofrosedalems.com/
https://www.pasco.k12.fl.us/?URL=https://cityofrosedalems.com/
https://anolink.com/?link=https://cityofrosedalems.com/
https://www.questsociety.ca/?URL=https://cityofrosedalems.com/
https://www.disl.edu/?URL=https://cityofrosedalems.com/
https://holidaykitchens.com/?URL=https://cityofrosedalems.com/
https://www.mbcarolinas.org/?URL=https://cityofrosedalems.com/
https://www.anibox.org/go?https://cityofrosedalems.com/
https://google.info/url?q=https://cityofrosedalems.com/
https://atlantis-tv.ru/go?https://cityofrosedalems.com/
https://otziv.ucoz.com/go?https://cityofrosedalems.com/
https://www.sgvavia.ru/go?https://cityofrosedalems.com/
https://element.lv/go?url=https://cityofrosedalems.com/
https://karanova.ru/?goto=https://cityofrosedalems.com/
https://789.ru/go.php?url=https://cityofrosedalems.com/
https://krasnoeselo.su/go?https://cityofrosedalems.com/
https://game-era.do.am/go?https://cityofrosedalems.com/
https://kudago.com/go/?to=https://cityofrosedalems.com/
https://after.ucoz.net/go?https://cityofrosedalems.com/
https://kinteatr.at.ua/go?https://cityofrosedalems.com/
https://nervetumours.org.uk/?URL=https://cityofrosedalems.com/
https://kopyten.clan.su/go?https://cityofrosedalems.com/
https://www.taker.im/go/?u=https://cityofrosedalems.com/
https://usehelp.clan.su/go?https://cityofrosedalems.com/
https://sepoa.fr/wp/go.php?https://cityofrosedalems.com/
https://world-source.ru/go?https://cityofrosedalems.com/
https://mail2.mclink.it/SRedirect/https://cityofrosedalems.com/
https://www.swleague.ru/go?https://cityofrosedalems.com/
https://nazgull.ucoz.ru/go?https://cityofrosedalems.com/
https://www.rosbooks.ru/go?https://cityofrosedalems.com/
https://beskuda.ucoz.ru/go?https://cityofrosedalems.com/
https://richmonkey.biz/go/?https://cityofrosedalems.com/
https://vlpacific.ru/?goto=https://cityofrosedalems.com/
https://google.co.ck/url?q=https://cityofrosedalems.com/
https://google.co.uz/url?q=https://cityofrosedalems.com/
https://google.co.ls/url?q=https://cityofrosedalems.com/
https://google.co.zm/url?q=https://cityofrosedalems.com/
https://google.co.ve/url?q=https://cityofrosedalems.com/
https://google.co.zw/url?q=https://cityofrosedalems.com/
https://google.co.uk/url?q=https://cityofrosedalems.com/
https://google.co.ao/url?q=https://cityofrosedalems.com/
https://google.co.cr/url?q=https://cityofrosedalems.com/
https://google.co.nz/url?q=https://cityofrosedalems.com/
https://google.co.th/url?q=https://cityofrosedalems.com/
https://google.co.ug/url?q=https://cityofrosedalems.com/
https://google.co.ma/url?q=https://cityofrosedalems.com/
https://google.co.za/url?q=https://cityofrosedalems.com/
https://google.co.kr/url?q=https://cityofrosedalems.com/
https://google.co.mz/url?q=https://cityofrosedalems.com/
https://google.co.vi/url?q=https://cityofrosedalems.com/
https://google.co.ke/url?q=https://cityofrosedalems.com/
https://google.co.hu/url?q=https://cityofrosedalems.com/
https://google.co.tz/url?q=https://cityofrosedalems.com/
https://google.co.jp/url?q=https://cityofrosedalems.com/
https://eric.ed.gov/?redir=https://cityofrosedalems.com/
https://www.usich.gov/?URL=https://cityofrosedalems.com/
https://sec.pn.to/jump.php?https://cityofrosedalems.com/
https://www.earth-policy.org/?URL=https://cityofrosedalems.com/
https://www.silverdart.co.uk/?URL=https://cityofrosedalems.com/
https://pr-cy.ru/jump/?url=https://cityofrosedalems.com/
https://google.co.bw/url?q=https://cityofrosedalems.com/
https://google.co.id/url?q=https://cityofrosedalems.com/
https://google.co.in/url?q=https://cityofrosedalems.com/
https://google.co.il/url?q=https://cityofrosedalems.com/
https://pikmlm.ru/out.php?p=https://cityofrosedalems.com/
https://regnopol.clan.su/go?https://cityofrosedalems.com/
https://mss.in.ua/go.php?to=https://cityofrosedalems.com/
https://owohho.com/away?url=https://cityofrosedalems.com/
https://www.youa.eu/r.php?u=https://cityofrosedalems.com/
https://cool4you.ucoz.ru/go?https://cityofrosedalems.com/
https://rg4u.clan.su/go?https://cityofrosedalems.com/
https://dawnofwar.org.ru/go?https://cityofrosedalems.com/
https://www.de-online.ru/go?https://cityofrosedalems.com/
https://www.allpn.ru/redirect/?url=https://cityofrosedalems.com/
https://click.start.me/?url=https://cityofrosedalems.com/
https://prizraks.clan.su/go?https://cityofrosedalems.com/
https://flyd.ru/away.php?to=https://cityofrosedalems.com/
https://risunok.ucoz.com/go?https://cityofrosedalems.com/
https://www.google.ca/url?q=https://cityofrosedalems.com/
https://www.google.fr/url?q=https://cityofrosedalems.com/
https://cse.google.mk/url?q=https://cityofrosedalems.com/
https://cse.google.ki/url?q=https://cityofrosedalems.com/
https://www.google.sn/url?q=https://cityofrosedalems.com/
https://cse.google.sr/url?q=https://cityofrosedalems.com/
https://www.google.so/url?q=https://cityofrosedalems.com/
https://www.google.cl/url?q=https://cityofrosedalems.com/
https://www.google.sc/url?q=https://cityofrosedalems.com/
https://www.google.iq/url?q=https://cityofrosedalems.com/
https://www.semanticjuice.com/site/https://cityofrosedalems.com/
https://cse.google.kz/url?q=https://cityofrosedalems.com/
https://www.google.gy/url?q=https://cityofrosedalems.com/
https://s79457.gridserver.com/?URL=https://cityofrosedalems.com/
https://cdl.su/redirect?url=https://cityofrosedalems.com/
https://www.fondbtvrtkovic.hr/?URL=https://cityofrosedalems.com/
https://lostnationarchery.com/?URL=https://cityofrosedalems.com/
https://www.booktrix.com/live/?URL=https://cityofrosedalems.com/
https://www.google.ro/url?q=https://cityofrosedalems.com/
https://www.google.tm/url?q=https://cityofrosedalems.com/
https://www.marcellusmatters.psu.edu/?URL=https://cityofrosedalems.com/
https://cse.google.vu/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.vg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.tg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.td/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.so/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.sn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.se/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ne/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.mu/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ml/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.kz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.hn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gy/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gp/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.gl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ge/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.dj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cv/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.vc/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.tj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.sl/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.sb/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.py/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ph/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.pg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.np/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.nf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.mt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ly/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.lb/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.kw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.kh/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.jm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.gi/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.gh/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.fj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.et/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.do/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.cy/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bo/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.bn/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ai/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.ag/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.com.af/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.zw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.zm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.vi/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.uz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.tz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.mz/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ma/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ls/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ke/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.ck/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.co.bw/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cm/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ci/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cg/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cd/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.cat/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bj/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.bf/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.am/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.al/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ad/url?sa=i&url=https://cityofrosedalems.com/
https://cse.google.ac/url?sa=i&url=https://cityofrosedalems.com/
https://maps.google.ws/url?q=https://cityofrosedalems.com/
https://maps.google.tn/url?q=https://cityofrosedalems.com/
https://maps.google.tl/url?q=https://cityofrosedalems.com/
https://maps.google.tk/url?q=https://cityofrosedalems.com/
https://maps.google.td/url?q=https://cityofrosedalems.com/
https://maps.google.st/url?q=https://cityofrosedalems.com/
https://maps.google.sn/url?q=https://cityofrosedalems.com/
https://maps.google.sm/url?q=https://cityofrosedalems.com/
https://maps.google.si/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.sh/url?q=https://cityofrosedalems.com/
https://maps.google.se/url?q=https://cityofrosedalems.com/
https://maps.google.rw/url?q=https://cityofrosedalems.com/
https://maps.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ru/url?q=https://cityofrosedalems.com/
https://maps.google.rs/url?q=https://cityofrosedalems.com/
https://maps.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.pt/url?q=https://cityofrosedalems.com/
https://maps.google.pn/url?q=https://cityofrosedalems.com/
https://maps.google.pl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.pl/url?q=https://cityofrosedalems.com/
https://maps.google.nr/url?q=https://cityofrosedalems.com/
https://maps.google.no/url?q=https://cityofrosedalems.com/
https://maps.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ne/url?q=https://cityofrosedalems.com/
https://maps.google.mw/url?q=https://cityofrosedalems.com/
https://maps.google.mu/url?q=https://cityofrosedalems.com/
https://maps.google.ms/url?q=https://cityofrosedalems.com/
https://maps.google.mn/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ml/url?q=https://cityofrosedalems.com/
https://maps.google.mk/url?q=https://cityofrosedalems.com/
https://maps.google.mg/url?q=https://cityofrosedalems.com/
https://maps.google.lv/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.lt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.lt/url?q=https://cityofrosedalems.com/
https://maps.google.lk/url?q=https://cityofrosedalems.com/
https://maps.google.li/url?q=https://cityofrosedalems.com/
https://maps.google.la/url?q=https://cityofrosedalems.com/
https://maps.google.kz/url?q=https://cityofrosedalems.com/
https://maps.google.ki/url?q=https://cityofrosedalems.com/
https://maps.google.jo/url?q=https://cityofrosedalems.com/
https://maps.google.je/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?q=https://cityofrosedalems.com/
https://maps.google.gg/url?q=https://cityofrosedalems.com/
https://maps.google.ge/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ge/url?q=https://cityofrosedalems.com/
https://maps.google.fr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fr/url?q=https://cityofrosedalems.com/
https://maps.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ee/url?q=https://cityofrosedalems.com/
https://maps.google.dm/url?q=https://cityofrosedalems.com/
https://maps.google.dk/url?q=https://cityofrosedalems.com/
https://maps.google.de/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cz/url?q=https://cityofrosedalems.com/
https://maps.google.cv/url?q=https://cityofrosedalems.com/
https://maps.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com/url?q=https://cityofrosedalems.com/
https://maps.google.com.uy/url?q=https://cityofrosedalems.com/
https://maps.google.com.ua/url?q=https://cityofrosedalems.com/
https://maps.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tw/url?q=https://cityofrosedalems.com/
https://maps.google.com.sg/url?q=https://cityofrosedalems.com/
https://maps.google.com.sb/url?q=https://cityofrosedalems.com/
https://maps.google.com.qa/url?q=https://cityofrosedalems.com/
https://maps.google.com.py/url?q=https://cityofrosedalems.com/
https://maps.google.com.ph/url?q=https://cityofrosedalems.com/
https://maps.google.com.om/url?q=https://cityofrosedalems.com/
https://maps.google.com.ni/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ni/url?q=https://cityofrosedalems.com/
https://maps.google.com.na/url?q=https://cityofrosedalems.com/
https://maps.google.com.mx/url?q=https://cityofrosedalems.com/
https://maps.google.com.mt/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ly/url?q=https://cityofrosedalems.com/
https://maps.google.com.lb/url?q=https://cityofrosedalems.com/
https://maps.google.com.kw/url?q=https://cityofrosedalems.com/
https://maps.google.com.kh/url?q=https://cityofrosedalems.com/
https://maps.google.com.jm/url?q=https://cityofrosedalems.com/
https://maps.google.com.gt/url?q=https://cityofrosedalems.com/
https://maps.google.com.gh/url?q=https://cityofrosedalems.com/
https://maps.google.com.fj/url?q=https://cityofrosedalems.com/
https://maps.google.com.et/url?q=https://cityofrosedalems.com/
https://maps.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bz/url?q=https://cityofrosedalems.com/
https://maps.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?q=https://cityofrosedalems.com/
https://maps.google.com.bn/url?q=https://cityofrosedalems.com/
https://maps.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.au/url?q=https://cityofrosedalems.com/
https://maps.google.com.ar/url?q=https://cityofrosedalems.com/
https://maps.google.com.ai/url?q=https://cityofrosedalems.com/
https://maps.google.com.ag/url?q=https://cityofrosedalems.com/
https://maps.google.co.zm/url?q=https://cityofrosedalems.com/
https://maps.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.vi/url?q=https://cityofrosedalems.com/
https://maps.google.co.ug/url?q=https://cityofrosedalems.com/
https://maps.google.co.tz/url?q=https://cityofrosedalems.com/
https://maps.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.nz/url?q=https://cityofrosedalems.com/
https://maps.google.co.ls/url?q=https://cityofrosedalems.com/
https://maps.google.co.kr/url?q=https://cityofrosedalems.com/
https://maps.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.il/url?q=https://cityofrosedalems.com/
https://maps.google.co.id/url?q=https://cityofrosedalems.com/
https://maps.google.co.cr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.ck/url?q=https://cityofrosedalems.com/
https://maps.google.co.bw/url?q=https://cityofrosedalems.com/
https://maps.google.co.ao/url?q=https://cityofrosedalems.com/
https://maps.google.cm/url?q=https://cityofrosedalems.com/
https://maps.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ci/url?q=https://cityofrosedalems.com/
https://maps.google.ch/url?q=https://cityofrosedalems.com/
https://maps.google.cg/url?q=https://cityofrosedalems.com/
https://maps.google.cf/url?q=https://cityofrosedalems.com/
https://maps.google.cd/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.cd/url?q=https://cityofrosedalems.com/
https://maps.google.bs/url?q=https://cityofrosedalems.com/
https://maps.google.bj/url?q=https://cityofrosedalems.com/
https://maps.google.bi/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?q=https://cityofrosedalems.com/
https://maps.google.bf/url?q=https://cityofrosedalems.com/
https://maps.google.be/url?q=https://cityofrosedalems.com/
https://maps.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.at/url?q=https://cityofrosedalems.com/
https://maps.google.ad/url?q=https://cityofrosedalems.com/
https://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.vg/url?q=https://cityofrosedalems.com/
https://images.google.tt/url?q=https://cityofrosedalems.com/
https://images.google.tm/url?q=https://cityofrosedalems.com/
https://images.google.tk/url?q=https://cityofrosedalems.com/
https://images.google.tg/url?q=https://cityofrosedalems.com/
https://images.google.sk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.si/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.sh/url?q=https://cityofrosedalems.com/
https://images.google.se/url?q=https://cityofrosedalems.com/
https://images.google.pt/url?q=https://cityofrosedalems.com/
https://images.google.ps/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pn/url?q=https://cityofrosedalems.com/
https://images.google.pl/url?q=https://cityofrosedalems.com/
https://images.google.nr/url?q=https://cityofrosedalems.com/
https://images.google.no/url?q=https://cityofrosedalems.com/
https://images.google.mw/url?q=https://cityofrosedalems.com/
https://images.google.mv/url?q=https://cityofrosedalems.com/
https://images.google.ml/url?q=https://cityofrosedalems.com/
https://images.google.mg/url?q=https://cityofrosedalems.com/
https://images.google.me/url?q=https://cityofrosedalems.com/
https://images.google.lk/url?q=https://cityofrosedalems.com/
https://images.google.li/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.la/url?q=https://cityofrosedalems.com/
https://images.google.kz/url?q=https://cityofrosedalems.com/
https://images.google.kg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.kg/url?q=https://cityofrosedalems.com/
https://images.google.je/url?q=https://cityofrosedalems.com/
https://images.google.it/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.it/url?q=https://cityofrosedalems.com/
https://images.google.im/url?q=https://cityofrosedalems.com/
https://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hu/url?q=https://cityofrosedalems.com/
https://images.google.ht/url?q=https://cityofrosedalems.com/
https://images.google.hn/url?q=https://cityofrosedalems.com/
https://images.google.gy/url?q=https://cityofrosedalems.com/
https://images.google.gp/url?q=https://cityofrosedalems.com/
https://images.google.gm/url?q=https://cityofrosedalems.com/
https://images.google.gg/url?q=https://cityofrosedalems.com/
https://images.google.ge/url?q=https://cityofrosedalems.com/
https://images.google.fr/url?q=https://cityofrosedalems.com/
https://images.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.fi/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dm/url?q=https://cityofrosedalems.com/
https://images.google.de/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?q=https://cityofrosedalems.com/
https://images.google.com/url?q=https://cityofrosedalems.com/
https://images.google.com.vn/url?q=https://cityofrosedalems.com/
https://images.google.com.vc/url?q=https://cityofrosedalems.com/
https://images.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tj/url?q=https://cityofrosedalems.com/
https://images.google.com.sl/url?q=https://cityofrosedalems.com/
https://images.google.com.sb/url?q=https://cityofrosedalems.com/
https://images.google.com.qa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.py/url?q=https://cityofrosedalems.com/
https://images.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ph/url?q=https://cityofrosedalems.com/
https://images.google.com.pa/url?q=https://cityofrosedalems.com/
https://images.google.com.ni/url?q=https://cityofrosedalems.com/
https://images.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.na/url?q=https://cityofrosedalems.com/
https://images.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.mm/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ly/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ly/url?q=https://cityofrosedalems.com/
https://images.google.com.lb/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kw/url?q=https://cityofrosedalems.com/
https://images.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.kh/url?q=https://cityofrosedalems.com/
https://images.google.com.jm/url?q=https://cityofrosedalems.com/
https://images.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.gt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.gi/url?q=https://cityofrosedalems.com/
https://images.google.com.gh/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.fj/url?q=https://cityofrosedalems.com/
https://images.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.eg/url?q=https://cityofrosedalems.com/
https://images.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cy/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cy/url?q=https://cityofrosedalems.com/
https://images.google.com.bz/url?q=https://cityofrosedalems.com/
https://images.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bd/url?q=https://cityofrosedalems.com/
https://images.google.com.au/url?q=https://cityofrosedalems.com/
https://images.google.com.ag/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.ag/url?q=https://cityofrosedalems.com/
https://images.google.co.zw/url?q=https://cityofrosedalems.com/
https://images.google.co.zm/url?q=https://cityofrosedalems.com/
https://images.google.co.za/url?q=https://cityofrosedalems.com/
https://images.google.co.vi/url?q=https://cityofrosedalems.com/
https://images.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.ve/url?q=https://cityofrosedalems.com/
https://images.google.co.uz/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?q=https://cityofrosedalems.com/
https://images.google.co.ug/url?q=https://cityofrosedalems.com/
https://images.google.co.tz/url?q=https://cityofrosedalems.com/
https://images.google.co.nz/url?q=https://cityofrosedalems.com/
https://images.google.co.mz/url?q=https://cityofrosedalems.com/
https://images.google.co.ma/url?q=https://cityofrosedalems.com/
https://images.google.co.jp/url?q=https://cityofrosedalems.com/
https://images.google.co.id/url?q=https://cityofrosedalems.com/
https://images.google.co.cr/url?q=https://cityofrosedalems.com/
https://images.google.co.ck/url?q=https://cityofrosedalems.com/
https://images.google.co.bw/url?q=https://cityofrosedalems.com/
https://images.google.cm/url?q=https://cityofrosedalems.com/
https://images.google.ci/url?q=https://cityofrosedalems.com/
https://images.google.ch/url?q=https://cityofrosedalems.com/
https://images.google.cg/url?q=https://cityofrosedalems.com/
https://images.google.cf/url?q=https://cityofrosedalems.com/
https://images.google.cat/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ca/url?q=https://cityofrosedalems.com/
https://images.google.by/url?q=https://cityofrosedalems.com/
https://images.google.bt/url?q=https://cityofrosedalems.com/
https://images.google.bs/url?q=https://cityofrosedalems.com/
https://images.google.bj/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.bf/url?q=https://cityofrosedalems.com/
https://images.google.be/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ba/url?q=https://cityofrosedalems.com/
https://images.google.at/url?q=https://cityofrosedalems.com/
https://images.google.am/url?q=https://cityofrosedalems.com/
https://images.google.ad/url?q=https://cityofrosedalems.com/
https://images.google.ac/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.iq/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hu/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ht/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.hn/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gy/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gp/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gl/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.gg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ge/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ga/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fr/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.fi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.es/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ee/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dk/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.dj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.de/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cv/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.kh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.hk/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gt/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.gh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.fj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.et/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.eg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ec/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.do/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.cy/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.cu/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.co/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bz/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.br/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bo/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bn/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bh/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.bd/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.au/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ar/url?sa=i&url=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ar/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ai/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.ag/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.com.af/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.il/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.id/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.ck/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.ao/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cm/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cl/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ci/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ch/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cf/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cd/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cc/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.cat/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ca/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.by/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bt/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bs/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bj/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bi/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.bf/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.be/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ba/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.az/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.at/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.as/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.am/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.al/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ae/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ad/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ac/url?q=https://cityofrosedalems.com/
http://maps.google.vu/url?q=https://cityofrosedalems.com/
http://maps.google.vg/url?q=https://cityofrosedalems.com/
http://maps.google.tt/url?q=https://cityofrosedalems.com/
http://maps.google.sk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.si/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.sc/url?q=https://cityofrosedalems.com/
http://maps.google.ru/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ro/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.pt/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.pl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.nl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.mv/url?q=https://cityofrosedalems.com/
http://maps.google.mn/url?q=https://cityofrosedalems.com/
http://maps.google.ml/url?q=https://cityofrosedalems.com/
http://maps.google.lv/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.lt/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.je/url?q=https://cityofrosedalems.com/
http://maps.google.it/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.im/url?q=https://cityofrosedalems.com/
http://maps.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ie/url?q=https://cityofrosedalems.com/
http://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ht/url?q=https://cityofrosedalems.com/
http://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.gm/url?q=https://cityofrosedalems.com/
http://maps.google.fr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.fm/url?q=https://cityofrosedalems.com/
http://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.es/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ee/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.dk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.dj/url?q=https://cityofrosedalems.com/
http://maps.google.cz/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.om/url?q=https://cityofrosedalems.com/
http://maps.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.mm/url?q=https://cityofrosedalems.com/
http://maps.google.com.ly/url?q=https://cityofrosedalems.com/
http://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.gi/url?q=https://cityofrosedalems.com/
http://maps.google.com.fj/url?q=https://cityofrosedalems.com/
http://maps.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.cu/url?q=https://cityofrosedalems.com/
http://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.com.bh/url?q=https://cityofrosedalems.com/
http://maps.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.zw/url?q=https://cityofrosedalems.com/
http://maps.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.mz/url?q=https://cityofrosedalems.com/
http://maps.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.cl/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ch/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.cg/url?q=https://cityofrosedalems.com/
http://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.bt/url?q=https://cityofrosedalems.com/
http://maps.google.bi/url?q=https://cityofrosedalems.com/
http://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.be/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.ba/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.at/url?sa=t&url=https://cityofrosedalems.com/
http://maps.google.as/url?q=https://cityofrosedalems.com/
http://maps.google.ae/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.vu/url?q=https://cityofrosedalems.com/
http://images.google.vg/url?q=https://cityofrosedalems.com/
http://images.google.sr/url?q=https://cityofrosedalems.com/
http://images.google.sn/url?q=https://cityofrosedalems.com/
http://images.google.sm/url?q=https://cityofrosedalems.com/
http://images.google.sk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.si/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.sh/url?q=https://cityofrosedalems.com/
http://images.google.sc/url?q=https://cityofrosedalems.com/
http://images.google.rw/url?q=https://cityofrosedalems.com/
http://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ro/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ps/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.nr/url?q=https://cityofrosedalems.com/
http://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.mw/url?q=https://cityofrosedalems.com/
http://images.google.mv/url?q=https://cityofrosedalems.com/
http://images.google.ms/url?q=https://cityofrosedalems.com/
http://images.google.mn/url?q=https://cityofrosedalems.com/
http://images.google.ml/url?q=https://cityofrosedalems.com/
http://images.google.mg/url?q=https://cityofrosedalems.com/
http://images.google.md/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.lk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.li/url?q=https://cityofrosedalems.com/
http://images.google.la/url?q=https://cityofrosedalems.com/
http://images.google.kg/url?q=https://cityofrosedalems.com/
http://images.google.je/url?q=https://cityofrosedalems.com/
http://images.google.it/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.iq/url?q=https://cityofrosedalems.com/
http://images.google.im/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?q=https://cityofrosedalems.com/
http://images.google.hu/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ht/url?q=https://cityofrosedalems.com/
http://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.gm/url?q=https://cityofrosedalems.com/
http://images.google.gg/url?q=https://cityofrosedalems.com/
http://images.google.fr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.fm/url?q=https://cityofrosedalems.com/
http://images.google.fi/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ee/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.dm/url?q=https://cityofrosedalems.com/
http://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.dj/url?q=https://cityofrosedalems.com/
http://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.uy/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.tj/url?q=https://cityofrosedalems.com/
http://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.na/url?q=https://cityofrosedalems.com/
http://images.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.mm/url?q=https://cityofrosedalems.com/
http://images.google.com.jm/url?q=https://cityofrosedalems.com/
http://images.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.gi/url?q=https://cityofrosedalems.com/
http://images.google.com.fj/url?q=https://cityofrosedalems.com/
http://images.google.com.et/url?q=https://cityofrosedalems.com/
http://images.google.com.eg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ec/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.do/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.cy/url?q=https://cityofrosedalems.com/
http://images.google.com.cu/url?q=https://cityofrosedalems.com/
http://images.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.bz/url?q=https://cityofrosedalems.com/
http://images.google.com.br/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.bn/url?q=https://cityofrosedalems.com/
http://images.google.com.bh/url?q=https://cityofrosedalems.com/
http://images.google.com.bd/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.ag/url?q=https://cityofrosedalems.com/
http://images.google.com.af/url?q=https://cityofrosedalems.com/
http://images.google.co.zw/url?q=https://cityofrosedalems.com/
http://images.google.co.zm/url?q=https://cityofrosedalems.com/
http://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.vi/url?q=https://cityofrosedalems.com/
http://images.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.tz/url?q=https://cityofrosedalems.com/
http://images.google.co.th/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.nz/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.mz/url?q=https://cityofrosedalems.com/
http://images.google.co.ma/url?q=https://cityofrosedalems.com/
http://images.google.co.ls/url?q=https://cityofrosedalems.com/
http://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.co.ck/url?q=https://cityofrosedalems.com/
http://images.google.co.bw/url?q=https://cityofrosedalems.com/
http://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ci/url?q=https://cityofrosedalems.com/
http://images.google.ch/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.cg/url?q=https://cityofrosedalems.com/
http://images.google.cd/url?q=https://cityofrosedalems.com/
http://images.google.ca/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.bt/url?q=https://cityofrosedalems.com/
http://images.google.bs/url?q=https://cityofrosedalems.com/
http://images.google.bj/url?q=https://cityofrosedalems.com/
http://images.google.bi/url?q=https://cityofrosedalems.com/
http://images.google.bg/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.be/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.az/url?q=https://cityofrosedalems.com/
http://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.as/url?q=https://cityofrosedalems.com/
http://images.google.al/url?q=https://cityofrosedalems.com/
http://images.google.ae/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.ad/url?q=https://cityofrosedalems.com/
http://cse.google.com.ly/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.lb/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.kw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.kh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.jm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.hk/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gt/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.gh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.fj/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.et/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.eg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ec/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.do/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.cy/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.co/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.br/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bo/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bn/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bh/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.bd/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.au/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ai/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.ag/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.com.af/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.zw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.zm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.za/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.vi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ve/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.uz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.uk/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ug/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.tz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.th/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.nz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.mz/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ma/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ls/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.kr/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ke/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.jp/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.in/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.il/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.id/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.cr/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ck/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.bw/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.co.ao/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cm/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cl/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ci/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ch/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cf/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cd/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.cat/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ca/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.by/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bt/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bs/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bj/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bi/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bg/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.bf/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.be/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ba/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.az/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.at/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.as/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.am/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.al/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ae/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ad/url?sa=i&url=https://cityofrosedalems.com/
http://cse.google.ac/url?sa=i&url=https://cityofrosedalems.com/
https://google.ws/url?q=https://cityofrosedalems.com/
https://google.vu/url?q=https://cityofrosedalems.com/
https://google.vg/url?q=https://cityofrosedalems.com/
https://google.tt/url?q=https://cityofrosedalems.com/
https://google.to/url?q=https://cityofrosedalems.com/
https://google.tm/url?q=https://cityofrosedalems.com/
https://google.tl/url?q=https://cityofrosedalems.com/
https://google.tk/url?q=https://cityofrosedalems.com/
https://google.tg/url?q=https://cityofrosedalems.com/
https://google.st/url?q=https://cityofrosedalems.com/
https://google.sr/url?q=https://cityofrosedalems.com/
https://google.so/url?q=https://cityofrosedalems.com/
https://google.sm/url?q=https://cityofrosedalems.com/
https://google.sh/url?q=https://cityofrosedalems.com/
https://google.sc/url?q=https://cityofrosedalems.com/
https://google.rw/url?q=https://cityofrosedalems.com/
https://google.ps/url?q=https://cityofrosedalems.com/
https://google.pn/url?q=https://cityofrosedalems.com/
https://google.nu/url?q=https://cityofrosedalems.com/
https://google.nr/url?q=https://cityofrosedalems.com/
https://google.ne/url?q=https://cityofrosedalems.com/
https://google.mw/url?q=https://cityofrosedalems.com/
https://google.mv/url?q=https://cityofrosedalems.com/
https://google.ms/url?q=https://cityofrosedalems.com/
https://google.ml/url?q=https://cityofrosedalems.com/
https://google.mg/url?q=https://cityofrosedalems.com/
https://google.md/url?q=https://cityofrosedalems.com/
https://google.lk/url?q=https://cityofrosedalems.com/
https://google.la/url?q=https://cityofrosedalems.com/
https://google.kz/url?q=https://cityofrosedalems.com/
https://google.ki/url?q=https://cityofrosedalems.com/
https://google.kg/url?q=https://cityofrosedalems.com/
https://google.iq/url?q=https://cityofrosedalems.com/
https://google.im/url?q=https://cityofrosedalems.com/
https://google.ht/url?q=https://cityofrosedalems.com/
https://google.hn/url?sa=t&url=https://cityofrosedalems.com/
https://google.gm/url?q=https://cityofrosedalems.com/
https://google.gl/url?q=https://cityofrosedalems.com/
https://google.gg/url?q=https://cityofrosedalems.com/
https://google.ge/url?q=https://cityofrosedalems.com/
https://google.ga/url?q=https://cityofrosedalems.com/
https://google.dz/url?q=https://cityofrosedalems.com/
https://google.dm/url?q=https://cityofrosedalems.com/
https://google.dj/url?q=https://cityofrosedalems.com/
https://google.cv/url?q=https://cityofrosedalems.com/
https://google.com.vc/url?q=https://cityofrosedalems.com/
https://google.com.tj/url?q=https://cityofrosedalems.com/
https://google.com.sv/url?sa=t&url=https://cityofrosedalems.com/
https://google.com.sb/url?q=https://cityofrosedalems.com/
https://google.com.pa/url?q=https://cityofrosedalems.com/
https://google.com.om/url?q=https://cityofrosedalems.com/
https://google.com.ni/url?q=https://cityofrosedalems.com/
https://google.com.na/url?q=https://cityofrosedalems.com/
https://google.com.kw/url?q=https://cityofrosedalems.com/
https://google.com.kh/url?q=https://cityofrosedalems.com/
https://google.com.jm/url?q=https://cityofrosedalems.com/
https://google.com.gi/url?q=https://cityofrosedalems.com/
https://google.com.gh/url?q=https://cityofrosedalems.com/
https://google.com.fj/url?q=https://cityofrosedalems.com/
https://google.com.et/url?q=https://cityofrosedalems.com/
https://google.com.do/url?q=https://cityofrosedalems.com/
https://google.com.bz/url?q=https://cityofrosedalems.com/
https://google.com.ai/url?q=https://cityofrosedalems.com/
https://google.com.ag/url?q=https://cityofrosedalems.com/
https://google.com.af/url?q=https://cityofrosedalems.com/
https://google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://google.cm/url?q=https://cityofrosedalems.com/
https://google.ci/url?sa=t&url=https://cityofrosedalems.com/
https://google.cg/url?q=https://cityofrosedalems.com/
https://google.cf/url?q=https://cityofrosedalems.com/
https://google.cd/url?q=https://cityofrosedalems.com/
https://google.bt/url?q=https://cityofrosedalems.com/
https://google.bj/url?q=https://cityofrosedalems.com/
https://google.bf/url?q=https://cityofrosedalems.com/
https://google.am/url?q=https://cityofrosedalems.com/
https://google.al/url?q=https://cityofrosedalems.com/
https://google.ad/url?q=https://cityofrosedalems.com/
https://google.ac/url?q=https://cityofrosedalems.com/
https://www.google.vg/url?q=https://cityofrosedalems.com/
https://www.google.tt/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.tl/url?q=https://cityofrosedalems.com/
https://www.google.st/url?q=https://cityofrosedalems.com/
https://www.google.nu/url?q=https://cityofrosedalems.com/
https://www.google.ms/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.it/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.it/url?q=https://cityofrosedalems.com/
https://www.google.is/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.gl/url?q=https://cityofrosedalems.com/
https://www.google.fm/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.es/url?q=https://cityofrosedalems.com/
https://www.google.dm/url?q=https://cityofrosedalems.com/
https://www.google.cz/url?q=https://cityofrosedalems.com/
https://www.google.com/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.uy/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.ua/url?q=https://cityofrosedalems.com/
https://www.google.com.sl/url?q=https://cityofrosedalems.com/
https://www.google.com.sg/url?q=https://cityofrosedalems.com/
https://www.google.com.pr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.pk/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.om/url?q=https://cityofrosedalems.com/
https://www.google.com.ng/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.ec/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.au/url?q=https://cityofrosedalems.com/
https://www.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ma/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ck/url?q=https://cityofrosedalems.com/
https://www.google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.ch/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.cd/url?q=https://cityofrosedalems.com/
https://www.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.by/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.bt/url?q=https://cityofrosedalems.com/
https://www.google.be/url?q=https://cityofrosedalems.com/
https://www.google.as/url?sa=t&url=https://cityofrosedalems.com/
http://www.google.sk/url?q=https://cityofrosedalems.com/
http://www.google.ro/url?q=https://cityofrosedalems.com/
http://www.google.pt/url?q=https://cityofrosedalems.com/
http://www.google.no/url?q=https://cityofrosedalems.com/
http://www.google.lt/url?q=https://cityofrosedalems.com/
http://www.google.ie/url?q=https://cityofrosedalems.com/
http://www.google.com.vn/url?q=https://cityofrosedalems.com/
http://www.google.com.ua/url?q=https://cityofrosedalems.com/
http://www.google.com.tr/url?q=https://cityofrosedalems.com/
http://www.google.com.ph/url?q=https://cityofrosedalems.com/
http://www.google.com.ar/url?q=https://cityofrosedalems.com/
http://www.google.co.za/url?q=https://cityofrosedalems.com/
http://www.google.co.nz/url?q=https://cityofrosedalems.com/
http://www.google.co.kr/url?q=https://cityofrosedalems.com/
http://www.google.co.il/url?q=https://cityofrosedalems.com/
http://www.google.co.id/url?q=https://cityofrosedalems.com/
https://www.google.ac/url?q=https://cityofrosedalems.com/
https://www.google.ad/url?q=https://cityofrosedalems.com/
https://www.google.ae/url?q=https://cityofrosedalems.com/
https://www.google.am/url?q=https://cityofrosedalems.com/
https://www.google.as/url?q=https://cityofrosedalems.com/
https://www.google.at/url?q=https://cityofrosedalems.com/
https://www.google.az/url?q=https://cityofrosedalems.com/
https://www.google.bg/url?q=https://cityofrosedalems.com/
https://www.google.bi/url?q=https://cityofrosedalems.com/
https://www.google.bj/url?q=https://cityofrosedalems.com/
https://www.google.bs/url?q=https://cityofrosedalems.com/
https://www.google.by/url?q=https://cityofrosedalems.com/
https://www.google.cf/url?q=https://cityofrosedalems.com/
https://www.google.cg/url?q=https://cityofrosedalems.com/
https://www.google.ch/url?q=https://cityofrosedalems.com/
https://www.google.ci/url?q=https://cityofrosedalems.com/
https://www.google.cm/url?q=https://cityofrosedalems.com/
https://www.google.co.ao/url?q=https://cityofrosedalems.com/
https://www.google.co.bw/url?q=https://cityofrosedalems.com/
https://www.google.co.cr/url?q=https://cityofrosedalems.com/
https://www.google.co.id/url?q=https://cityofrosedalems.com/
https://www.google.co.in/url?q=https://cityofrosedalems.com/
https://www.google.co.kr/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ma/url?q=https://cityofrosedalems.com/
https://www.google.co.mz/url?q=https://cityofrosedalems.com/
https://www.google.co.nz/url?q=https://cityofrosedalems.com/
https://www.google.co.th/url?q=https://cityofrosedalems.com/
https://www.google.co.tz/url?q=https://cityofrosedalems.com/
https://www.google.co.ug/url?q=https://cityofrosedalems.com/
https://www.google.co.uk/url?q=https://cityofrosedalems.com/
https://www.google.co.uz/url?q=https://cityofrosedalems.com/
https://www.google.co.uz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.co.ve/url?q=https://cityofrosedalems.com/
https://www.google.co.vi/url?q=https://cityofrosedalems.com/
https://www.google.co.za/url?q=https://cityofrosedalems.com/
https://www.google.co.zm/url?q=https://cityofrosedalems.com/
https://www.google.co.zw/url?q=https://cityofrosedalems.com/
https://www.google.com.ai/url?q=https://cityofrosedalems.com/
https://www.google.com.ar/url?q=https://cityofrosedalems.com/
https://www.google.com.bd/url?q=https://cityofrosedalems.com/
https://www.google.com.bh/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.bo/url?q=https://cityofrosedalems.com/
https://www.google.com.br/url?q=https://cityofrosedalems.com/
https://www.google.com.bz/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://www.google.com.cu/url?q=https://cityofrosedalems.com/
https://www.google.com.cy/url?q=https://cityofrosedalems.com/
https://www.google.com.ec/url?q=https://cityofrosedalems.com/
https://www.google.com.fj/url?q=https://cityofrosedalems.com/
https://www.google.com.gh/url?q=https://cityofrosedalems.com/
https://www.google.com.hk/url?q=https://cityofrosedalems.com/
https://www.google.com.jm/url?q=https://cityofrosedalems.com/
https://www.google.com.kh/url?q=https://cityofrosedalems.com/
https://www.google.com.kw/url?q=https://cityofrosedalems.com/
https://www.google.com.lb/url?q=https://cityofrosedalems.com/
https://www.google.com.ly/url?q=https://cityofrosedalems.com/
https://www.google.com.mm/url?q=https://cityofrosedalems.com/
https://www.google.com.mt/url?q=https://cityofrosedalems.com/
https://www.google.com.mx/url?q=https://cityofrosedalems.com/
https://www.google.com.my/url?q=https://cityofrosedalems.com/
https://www.google.com.nf/url?q=https://cityofrosedalems.com/
https://www.google.com.ng/url?q=https://cityofrosedalems.com/
https://www.google.com.ni/url?q=https://cityofrosedalems.com/
https://www.google.com.pa/url?q=https://cityofrosedalems.com/
https://www.google.com.pe/url?q=https://cityofrosedalems.com/
https://www.google.com.pg/url?q=https://cityofrosedalems.com/
https://www.google.com.ph/url?q=https://cityofrosedalems.com/
https://www.google.com.pk/url?q=https://cityofrosedalems.com/
https://www.google.com.pr/url?q=https://cityofrosedalems.com/
https://www.google.com.py/url?q=https://cityofrosedalems.com/
https://www.google.com.qa/url?q=https://cityofrosedalems.com/
https://www.google.com.sa/url?q=https://cityofrosedalems.com/
https://www.google.com.sb/url?q=https://cityofrosedalems.com/
https://www.google.com.sv/url?q=https://cityofrosedalems.com/
https://www.google.com.tj/url?sa=i&url=https://cityofrosedalems.com/
https://www.google.com.tr/url?q=https://cityofrosedalems.com/
https://www.google.com.tw/url?q=https://cityofrosedalems.com/
https://www.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.com.uy/url?q=https://cityofrosedalems.com/
https://www.google.com.vn/url?q=https://cityofrosedalems.com/
https://www.google.com/url?q=https://cityofrosedalems.com/
https://www.google.com/url?sa=i&rct=j&url=https://cityofrosedalems.com/
https://www.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.de/url?q=https://cityofrosedalems.com/
https://www.google.dj/url?q=https://cityofrosedalems.com/
https://www.google.dk/url?q=https://cityofrosedalems.com/
https://www.google.dz/url?q=https://cityofrosedalems.com/
https://www.google.ee/url?q=https://cityofrosedalems.com/
https://www.google.fi/url?q=https://cityofrosedalems.com/
https://www.google.fm/url?q=https://cityofrosedalems.com/
https://www.google.ga/url?q=https://cityofrosedalems.com/
https://www.google.ge/url?q=https://cityofrosedalems.com/
https://www.google.gg/url?q=https://cityofrosedalems.com/
https://www.google.gm/url?q=https://cityofrosedalems.com/
https://www.google.gp/url?q=https://cityofrosedalems.com/
https://www.google.gr/url?q=https://cityofrosedalems.com/
https://www.google.hn/url?q=https://cityofrosedalems.com/
https://www.google.hr/url?q=https://cityofrosedalems.com/
https://www.google.ht/url?q=https://cityofrosedalems.com/
https://www.google.hu/url?q=https://cityofrosedalems.com/
https://www.google.ie/url?q=https://cityofrosedalems.com/
https://www.google.jo/url?q=https://cityofrosedalems.com/
https://www.google.ki/url?q=https://cityofrosedalems.com/
https://www.google.la/url?q=https://cityofrosedalems.com/
https://www.google.lk/url?q=https://cityofrosedalems.com/
https://www.google.lt/url?q=https://cityofrosedalems.com/
https://www.google.lu/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.lv/url?q=https://cityofrosedalems.com/
https://www.google.mg/url?sa=t&url=https://cityofrosedalems.com/
https://www.google.mk/url?q=https://cityofrosedalems.com/
https://www.google.ml/url?q=https://cityofrosedalems.com/
https://www.google.mn/url?q=https://cityofrosedalems.com/
https://www.google.ms/url?q=https://cityofrosedalems.com/
https://www.google.mu/url?q=https://cityofrosedalems.com/
https://www.google.mv/url?q=https://cityofrosedalems.com/
https://www.google.mw/url?q=https://cityofrosedalems.com/
https://www.google.ne/url?q=https://cityofrosedalems.com/
https://www.google.nl/url?q=https://cityofrosedalems.com/
https://www.google.no/url?q=https://cityofrosedalems.com/
https://www.google.nr/url?q=https://cityofrosedalems.com/
https://www.google.pl/url?q=https://cityofrosedalems.com/
https://www.google.pt/url?q=https://cityofrosedalems.com/
https://www.google.rs/url?q=https://cityofrosedalems.com/
https://www.google.ru/url?q=https://cityofrosedalems.com/
https://www.google.se/url?q=https://cityofrosedalems.com/
https://www.google.sh/url?q=https://cityofrosedalems.com/
https://www.google.si/url?q=https://cityofrosedalems.com/
https://www.google.sk/url?q=https://cityofrosedalems.com/
https://www.google.sm/url?q=https://cityofrosedalems.com/
https://www.google.sr/url?q=https://cityofrosedalems.com/
https://www.google.tg/url?q=https://cityofrosedalems.com/
https://www.google.tk/url?q=https://cityofrosedalems.com/
https://www.google.tn/url?q=https://cityofrosedalems.com/
https://www.google.tt/url?q=https://cityofrosedalems.com/
https://www.google.vu/url?q=https://cityofrosedalems.com/
https://www.google.ws/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.co.uk/url?sa=i&url=https://cityofrosedalems.com/
https://toolbarqueries.google.je/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.ms/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.vg/url?q=https://cityofrosedalems.com/
https://toolbarqueries.google.vu/url?q=https://cityofrosedalems.com/
http://toolbarqueries.google.com.tj/url?sa=t&url=https://cityofrosedalems.com/
https://cse.google.ba/url?q=https://cityofrosedalems.com/
https://cse.google.bj/url?q=https://cityofrosedalems.com/
https://cse.google.cat/url?q=https://cityofrosedalems.com/
https://cse.google.co.bw/url?q=https://cityofrosedalems.com/
https://cse.google.co.kr/url?q=https://cityofrosedalems.com/
https://cse.google.co.nz/url?q=https://cityofrosedalems.com/
https://cse.google.co.zw/url?q=https://cityofrosedalems.com/
https://cse.google.com.ai/url?q=https://cityofrosedalems.com/
https://cse.google.com.ly/url?q=https://cityofrosedalems.com/
https://cse.google.com.sb/url?q=https://cityofrosedalems.com/
https://cse.google.com.sv/url?q=https://cityofrosedalems.com/
https://cse.google.com.vc/url?q=https://cityofrosedalems.com/
https://cse.google.ge/url?q=https://cityofrosedalems.com/
https://cse.google.gy/url?q=https://cityofrosedalems.com/
https://cse.google.hn/url?q=https://cityofrosedalems.com/
https://cse.google.ht/url?q=https://cityofrosedalems.com/
https://cse.google.iq/url?q=https://cityofrosedalems.com/
https://cse.google.lk/url?q=https://cityofrosedalems.com/
https://cse.google.no/url?q=https://cityofrosedalems.com/
https://cse.google.se/url?q=https://cityofrosedalems.com/
https://cse.google.sn/url?q=https://cityofrosedalems.com/
https://cse.google.st/url?q=https://cityofrosedalems.com/
https://cse.google.td/url?q=https://cityofrosedalems.com/
https://cse.google.ws/url?q=https://cityofrosedalems.com/
http://maps.google.ad/url?q=https://cityofrosedalems.com/
http://maps.google.at/url?q=https://cityofrosedalems.com/
http://maps.google.ba/url?q=https://cityofrosedalems.com/
http://maps.google.be/url?q=https://cityofrosedalems.com/
http://maps.google.bf/url?q=https://cityofrosedalems.com/
http://maps.google.bg/url?q=https://cityofrosedalems.com/
http://maps.google.bj/url?q=https://cityofrosedalems.com/
http://maps.google.bs/url?q=https://cityofrosedalems.com/
http://maps.google.by/url?q=https://cityofrosedalems.com/
http://maps.google.cd/url?q=https://cityofrosedalems.com/
http://maps.google.cf/url?q=https://cityofrosedalems.com/
http://maps.google.ch/url?q=https://cityofrosedalems.com/
http://maps.google.ci/url?q=https://cityofrosedalems.com/
http://maps.google.cl/url?q=https://cityofrosedalems.com/
http://maps.google.cm/url?q=https://cityofrosedalems.com/
http://maps.google.co.ao/url?q=https://cityofrosedalems.com/
http://maps.google.co.bw/url?q=https://cityofrosedalems.com/
http://maps.google.co.ck/url?q=https://cityofrosedalems.com/
http://maps.google.co.cr/url?q=https://cityofrosedalems.com/
http://maps.google.co.il/url?q=https://cityofrosedalems.com/
http://maps.google.co.kr/url?q=https://cityofrosedalems.com/
http://maps.google.co.ls/url?q=https://cityofrosedalems.com/
http://maps.google.co.nz/url?q=https://cityofrosedalems.com/
http://maps.google.co.tz/url?q=https://cityofrosedalems.com/
http://maps.google.co.ug/url?q=https://cityofrosedalems.com/
http://maps.google.co.ve/url?q=https://cityofrosedalems.com/
http://maps.google.co.vi/url?q=https://cityofrosedalems.com/
http://maps.google.co.zm/url?q=https://cityofrosedalems.com/
http://maps.google.com.ag/url?q=https://cityofrosedalems.com/
http://maps.google.com.ai/url?q=https://cityofrosedalems.com/
http://maps.google.com.ar/url?q=https://cityofrosedalems.com/
http://maps.google.com.au/url?q=https://cityofrosedalems.com/
http://maps.google.com.bn/url?q=https://cityofrosedalems.com/
http://maps.google.com.bz/url?q=https://cityofrosedalems.com/
http://maps.google.com.ec/url?q=https://cityofrosedalems.com/
http://maps.google.com.eg/url?q=https://cityofrosedalems.com/
http://maps.google.com.et/url?q=https://cityofrosedalems.com/
http://maps.google.com.gh/url?q=https://cityofrosedalems.com/
http://maps.google.com.gt/url?q=https://cityofrosedalems.com/
http://maps.google.com.jm/url?q=https://cityofrosedalems.com/
http://maps.google.com.mt/url?q=https://cityofrosedalems.com/
http://maps.google.com.mx/url?q=https://cityofrosedalems.com/
http://maps.google.com.na/url?q=https://cityofrosedalems.com/
http://maps.google.com.ng/url?q=https://cityofrosedalems.com/
http://maps.google.com.pe/url?q=https://cityofrosedalems.com/
http://maps.google.com.ph/url?q=https://cityofrosedalems.com/
http://maps.google.com.pr/url?q=https://cityofrosedalems.com/
http://maps.google.com.py/url?q=https://cityofrosedalems.com/
http://maps.google.com.qa/url?q=https://cityofrosedalems.com/
http://maps.google.com.sb/url?q=https://cityofrosedalems.com/
http://maps.google.com.sg/url?q=https://cityofrosedalems.com/
http://maps.google.com.tw/url?q=https://cityofrosedalems.com/
http://maps.google.com.uy/url?q=https://cityofrosedalems.com/
http://maps.google.com.vc/url?q=https://cityofrosedalems.com/
http://maps.google.cv/url?q=https://cityofrosedalems.com/
http://maps.google.cz/url?q=https://cityofrosedalems.com/
http://maps.google.dm/url?q=https://cityofrosedalems.com/
http://maps.google.dz/url?q=https://cityofrosedalems.com/
http://maps.google.ee/url?q=https://cityofrosedalems.com/
http://maps.google.fr/url?q=https://cityofrosedalems.com/
http://maps.google.ga/url?q=https://cityofrosedalems.com/
http://maps.google.ge/url?q=https://cityofrosedalems.com/
http://maps.google.gg/url?q=https://cityofrosedalems.com/
http://maps.google.gp/url?q=https://cityofrosedalems.com/
http://maps.google.hr/url?q=https://cityofrosedalems.com/
http://maps.google.hu/url?q=https://cityofrosedalems.com/
http://maps.google.iq/url?q=https://cityofrosedalems.com/
http://maps.google.kg/url?q=https://cityofrosedalems.com/
http://maps.google.ki/url?q=https://cityofrosedalems.com/
http://maps.google.kz/url?q=https://cityofrosedalems.com/
http://maps.google.la/url?q=https://cityofrosedalems.com/
http://maps.google.li/url?q=https://cityofrosedalems.com/
http://maps.google.lt/url?q=https://cityofrosedalems.com/
http://maps.google.mg/url?q=https://cityofrosedalems.com/
http://maps.google.mk/url?q=https://cityofrosedalems.com/
http://maps.google.ms/url?q=https://cityofrosedalems.com/
http://maps.google.mu/url?q=https://cityofrosedalems.com/
http://maps.google.mw/url?q=https://cityofrosedalems.com/
http://maps.google.ne/url?q=https://cityofrosedalems.com/
http://maps.google.nr/url?q=https://cityofrosedalems.com/
http://maps.google.pl/url?q=https://cityofrosedalems.com/
http://maps.google.pn/url?q=https://cityofrosedalems.com/
http://maps.google.pt/url?q=https://cityofrosedalems.com/
http://maps.google.rs/url?q=https://cityofrosedalems.com/
http://maps.google.ru/url?q=https://cityofrosedalems.com/
http://maps.google.rw/url?q=https://cityofrosedalems.com/
http://maps.google.se/url?q=https://cityofrosedalems.com/
http://maps.google.sh/url?q=https://cityofrosedalems.com/
http://maps.google.si/url?q=https://cityofrosedalems.com/
http://maps.google.sm/url?q=https://cityofrosedalems.com/
http://maps.google.sn/url?q=https://cityofrosedalems.com/
http://maps.google.st/url?q=https://cityofrosedalems.com/
http://maps.google.td/url?q=https://cityofrosedalems.com/
http://maps.google.tl/url?q=https://cityofrosedalems.com/
http://maps.google.tn/url?q=https://cityofrosedalems.com/
http://maps.google.ws/url?q=https://cityofrosedalems.com/
https://maps.google.ae/url?q=https://cityofrosedalems.com/
https://maps.google.as/url?q=https://cityofrosedalems.com/
https://maps.google.cat/url?q=https://cityofrosedalems.com/
https://maps.google.co.ck/url?sa=t&source=web&rct=j&url=https://cityofrosedalems.com/
https://maps.google.co.cr/url?q=https://cityofrosedalems.com/
https://maps.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.in/url?q=https://cityofrosedalems.com/
https://maps.google.co.jp/url?q=https://cityofrosedalems.com/
https://maps.google.co.ke/url?q=https://cityofrosedalems.com/
https://maps.google.co.mz/url?q=https://cityofrosedalems.com/
https://maps.google.co.th/url?q=https://cityofrosedalems.com/
https://maps.google.co.ug/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?q=https://cityofrosedalems.com/
https://maps.google.co.za/url?q=https://cityofrosedalems.com/
https://maps.google.co.zw/url?q=https://cityofrosedalems.com/
https://maps.google.com.bd/url?q=https://cityofrosedalems.com/
https://maps.google.com.bh/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.bo/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.br/url?q=https://cityofrosedalems.com/
https://maps.google.com.co/url?q=https://cityofrosedalems.com/
https://maps.google.com.cu/url?q=https://cityofrosedalems.com/
https://maps.google.com.do/url?q=https://cityofrosedalems.com/
https://maps.google.com.fj/url?sa=t&rct=j&url=https://cityofrosedalems.com/
https://maps.google.com.gi/url?q=https://cityofrosedalems.com/
https://maps.google.com.hk/url?q=https://cityofrosedalems.com/
https://maps.google.com.kh/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ly/url?sa=i&rct=j&url=https://cityofrosedalems.com/
https://maps.google.com.mm/url?q=https://cityofrosedalems.com/
https://maps.google.com.my/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.np/url?q=https://cityofrosedalems.com/
https://maps.google.com.om/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.pa/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.pg/url?q=https://cityofrosedalems.com/
https://maps.google.com.sa/url?q=https://cityofrosedalems.com/
https://maps.google.com.sl/url?q=https://cityofrosedalems.com/
https://maps.google.com.sv/url?q=https://cityofrosedalems.com/
https://maps.google.com.tr/url?q=https://cityofrosedalems.com/
https://maps.google.de/url?q=https://cityofrosedalems.com/
https://maps.google.dj/url?q=https://cityofrosedalems.com/
https://maps.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.es/url?q=https://cityofrosedalems.com/
https://maps.google.fi/url?q=https://cityofrosedalems.com/
https://maps.google.fm/url?q=https://cityofrosedalems.com/
https://maps.google.gl/url?q=https://cityofrosedalems.com/
https://maps.google.gm/url?q=https://cityofrosedalems.com/
https://maps.google.gr/url?q=https://cityofrosedalems.com/
https://maps.google.gy/url?q=https://cityofrosedalems.com/
https://maps.google.hn/url?q=https://cityofrosedalems.com/
https://maps.google.ht/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?q=https://cityofrosedalems.com/
https://maps.google.ie/url?sa=j&rct=j&url=https://cityofrosedalems.com/
https://maps.google.im/url?q=https://cityofrosedalems.com/
https://maps.google.is/url?q=https://cityofrosedalems.com/
https://maps.google.it/url?q=https://cityofrosedalems.com/
https://maps.google.lk/url?rct=j&sa=t&url=https://cityofrosedalems.com/
https://maps.google.lv/url?q=https://cityofrosedalems.com/
https://maps.google.ml/url?sa=i&url=https://cityofrosedalems.com/
https://maps.google.mn/url?q=https://cityofrosedalems.com/
https://maps.google.mv/url?q=https://cityofrosedalems.com/
https://maps.google.nl/url?q=https://cityofrosedalems.com/
https://maps.google.no/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ro/url?q=https://cityofrosedalems.com/
https://maps.google.sk/url?q=https://cityofrosedalems.com/
https://maps.google.so/url?q=https://cityofrosedalems.com/
https://maps.google.tg/url?q=https://cityofrosedalems.com/
https://maps.google.to/url?q=https://cityofrosedalems.com/
https://maps.google.tt/url?q=https://cityofrosedalems.com/
https://maps.google.vg/url?q=https://cityofrosedalems.com/
https://maps.google.vu/url?q=https://cityofrosedalems.com/
http://www.google.be/url?q=https://cityofrosedalems.com/
http://www.google.bf/url?q=https://cityofrosedalems.com/
http://www.google.bt/url?q=https://cityofrosedalems.com/
http://www.google.ca/url?q=https://cityofrosedalems.com/
http://www.google.cd/url?q=https://cityofrosedalems.com/
http://www.google.cl/url?q=https://cityofrosedalems.com/
http://www.google.co.ck/url?q=https://cityofrosedalems.com/
http://www.google.co.ls/url?q=https://cityofrosedalems.com/
http://www.google.com.af/url?q=https://cityofrosedalems.com/
http://www.google.com.au/url?q=https://cityofrosedalems.com/
http://www.google.com.bn/url?q=https://cityofrosedalems.com/
http://www.google.com.do/url?q=https://cityofrosedalems.com/
http://www.google.com.eg/url?q=https://cityofrosedalems.com/
http://www.google.com.et/url?q=https://cityofrosedalems.com/
http://www.google.com.gi/url?q=https://cityofrosedalems.com/
http://www.google.com.na/url?q=https://cityofrosedalems.com/
http://www.google.com.np/url?q=https://cityofrosedalems.com/
http://www.google.com.sg/url?q=https://cityofrosedalems.com/
http://www.google.com/url?q=https://cityofrosedalems.com/
http://www.google.cv/url?q=https://cityofrosedalems.com/
http://www.google.dm/url?q=https://cityofrosedalems.com/
http://www.google.es/url?q=https://cityofrosedalems.com/
http://www.google.iq/url?q=https://cityofrosedalems.com/
http://www.google.it/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0cdiqfjaa&url=https://cityofrosedalems.com/
http://www.google.kg/url?q=https://cityofrosedalems.com/
http://www.google.kz/url?q=https://cityofrosedalems.com/
http://www.google.li/url?q=https://cityofrosedalems.com/
http://www.google.me/url?q=https://cityofrosedalems.com/
http://www.google.pn/url?q=https://cityofrosedalems.com/
http://www.google.ps/url?q=https://cityofrosedalems.com/
http://www.google.sn/url?q=https://cityofrosedalems.com/
http://www.google.so/url?q=https://cityofrosedalems.com/
http://www.google.st/url?q=https://cityofrosedalems.com/
http://www.google.td/url?q=https://cityofrosedalems.com/
http://www.google.tm/url?q=https://cityofrosedalems.com/
https://image.google.com.kw/url?sa=t&rct=j&url=https://cityofrosedalems.com/
https://image.google.com.nf/url?sa=j&url=https://cityofrosedalems.com/
https://image.google.tn/url?q=j&sa=t&url=https://cityofrosedalems.com/
https://images.google.ad/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.as/url?q=https://cityofrosedalems.com/
https://images.google.az/url?q=https://cityofrosedalems.com/
https://images.google.be/url?q=https://cityofrosedalems.com/
https://images.google.bg/url?q=https://cityofrosedalems.com/
https://images.google.bi/url?q=https://cityofrosedalems.com/
https://images.google.bs/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cat/url?q=https://cityofrosedalems.com/
https://images.google.cd/url?q=https://cityofrosedalems.com/
https://images.google.cl/url?q=https://cityofrosedalems.com/
https://images.google.co.bw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.il/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?q=https://cityofrosedalems.com/
https://images.google.co.ke/url?q=https://cityofrosedalems.com/
https://images.google.co.kr/url?q=https://cityofrosedalems.com/
https://images.google.co.ls/url?q=https://cityofrosedalems.com/
https://images.google.co.th/url?q=https://cityofrosedalems.com/
https://images.google.co.zm/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.af/url?q=https://cityofrosedalems.com/
https://images.google.com.ai/url?q=https://cityofrosedalems.com/
https://images.google.com.ar/url?q=https://cityofrosedalems.com/
https://images.google.com.bd/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.bn/url?q=https://cityofrosedalems.com/
https://images.google.com.bo/url?q=https://cityofrosedalems.com/
https://images.google.com.br/url?q=https://cityofrosedalems.com/
https://images.google.com.bz/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.cu/url?q=https://cityofrosedalems.com/
https://images.google.com.do/url?q=https://cityofrosedalems.com/
https://images.google.com.et/url?q=https://cityofrosedalems.com/
https://images.google.com.gh/url?q=https://cityofrosedalems.com/
https://images.google.com.hk/url?q=https://cityofrosedalems.com/
https://images.google.com.lb/url?q=https://cityofrosedalems.com/
https://images.google.com.mm/url?q=https://cityofrosedalems.com/
https://images.google.com.mx/url?q=https://cityofrosedalems.com/
https://images.google.com.my/url?q=https://cityofrosedalems.com/
https://images.google.com.np/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pe/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.pg/url?q=https://cityofrosedalems.com/
https://images.google.com.pk/url?q=https://cityofrosedalems.com/
https://images.google.com.pr/url?q=https://cityofrosedalems.com/
https://images.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?q=https://cityofrosedalems.com/
https://images.google.com.sv/url?q=https://cityofrosedalems.com/
https://images.google.com.tr/url?q=https://cityofrosedalems.com/
https://images.google.com.tw/url?q=https://cityofrosedalems.com/
https://images.google.com.ua/url?q=https://cityofrosedalems.com/
https://images.google.cv/url?q=https://cityofrosedalems.com/
https://images.google.cz/url?sa=i&url=https://cityofrosedalems.com/
https://images.google.dj/url?q=https://cityofrosedalems.com/
https://images.google.dk/url?q=https://cityofrosedalems.com/
https://images.google.ee/url?q=https://cityofrosedalems.com/
https://images.google.es/url?q=https://cityofrosedalems.com/
https://images.google.fm/url?q=https://cityofrosedalems.com/
https://images.google.fr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gl/url?q=https://cityofrosedalems.com/
https://images.google.hr/url?q=https://cityofrosedalems.com/
https://images.google.iq/url?q=https://cityofrosedalems.com/
https://images.google.jo/url?q=https://cityofrosedalems.com/
https://images.google.lk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.lt/url?q=https://cityofrosedalems.com/
https://images.google.lu/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.md/url?q=https://cityofrosedalems.com/
https://images.google.mk/url?q=https://cityofrosedalems.com/
https://images.google.mn/url?q=https://cityofrosedalems.com/
https://images.google.ms/url?q=https://cityofrosedalems.com/
https://images.google.ne/url?q=https://cityofrosedalems.com/
https://images.google.ng/url?q=https://cityofrosedalems.com/
https://images.google.nl/url?q=https://cityofrosedalems.com/
https://images.google.ps/url?q=https://cityofrosedalems.com/
https://images.google.ro/url?q=https://cityofrosedalems.com/
https://images.google.ru/url?q=https://cityofrosedalems.com/
https://images.google.rw/url?q=https://cityofrosedalems.com/
https://images.google.si/url?q=https://cityofrosedalems.com/
https://images.google.sm/url?q=https://cityofrosedalems.com/
https://images.google.sn/url?q=https://cityofrosedalems.com/
https://images.google.so/url?q=https://cityofrosedalems.com/
https://images.google.sr/url?q=https://cityofrosedalems.com/
https://images.google.st/url?q=https://cityofrosedalems.com/
https://images.google.tl/url?q=https://cityofrosedalems.com/
https://images.google.tn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.to/url?q=https://cityofrosedalems.com/
https://images.google.vu/url?q=https://cityofrosedalems.com/
http://images.google.am/url?q=https://cityofrosedalems.com/
http://images.google.ba/url?q=https://cityofrosedalems.com/
http://images.google.bf/url?q=https://cityofrosedalems.com/
http://images.google.co.ao/url?q=https://cityofrosedalems.com/
http://images.google.co.jp/url?q=https://cityofrosedalems.com/
http://images.google.co.nz/url?q=https://cityofrosedalems.com/
http://images.google.co.ug/url?q=https://cityofrosedalems.com/
http://images.google.co.uk/url?q=https://cityofrosedalems.com/
http://images.google.co.uz/url?q=https://cityofrosedalems.com/
http://images.google.co.ve/url?q=https://cityofrosedalems.com/
http://images.google.com.co/url?q=https://cityofrosedalems.com/
http://images.google.com.ly/url?q=https://cityofrosedalems.com/
http://images.google.com.ng/url?q=https://cityofrosedalems.com/
http://images.google.com.om/url?q=https://cityofrosedalems.com/
http://images.google.com.qa/url?q=https://cityofrosedalems.com/
http://images.google.com.sb/url?q=https://cityofrosedalems.com/
http://images.google.com.sl/url?q=https://cityofrosedalems.com/
http://images.google.com.uy/url?q=https://cityofrosedalems.com/
http://images.google.com.vc/url?q=https://cityofrosedalems.com/
http://images.google.de/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.is/url?q=https://cityofrosedalems.com/
http://images.google.it/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?q=https://cityofrosedalems.com/
http://images.google.me/url?q=https://cityofrosedalems.com/
http://images.google.mu/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?q=https://cityofrosedalems.com/
http://images.google.rs/url?q=https://cityofrosedalems.com/
http://images.google.td/url?q=https://cityofrosedalems.com/
http://images.google.tm/url?q=https://cityofrosedalems.com/
http://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.com.om/url?q=https://cityofrosedalems.com/
http://images.google.com.qa/url?q=https://cityofrosedalems.com/
http://images.google.com.sb/url?q=https://cityofrosedalems.com/
http://images.google.com.sl/url?q=https://cityofrosedalems.com/
http://images.google.com.uy/url?q=https://cityofrosedalems.com/
http://images.google.com.vc/url?q=https://cityofrosedalems.com/
http://images.google.de/url?q=https://cityofrosedalems.com/
http://images.google.ie/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.is/url?q=https://cityofrosedalems.com/
http://images.google.it/url?q=https://cityofrosedalems.com/
http://images.google.lv/url?q=https://cityofrosedalems.com/
http://images.google.me/url?q=https://cityofrosedalems.com/
http://images.google.mu/url?q=https://cityofrosedalems.com/
http://images.google.pl/url?q=https://cityofrosedalems.com/
http://images.google.pn/url?sa=t&url=https://cityofrosedalems.com/
http://images.google.pt/url?q=https://cityofrosedalems.com/
http://images.google.rs/url?q=https://cityofrosedalems.com/
http://images.google.td/url?q=https://cityofrosedalems.com/
http://images.google.tm/url?q=https://cityofrosedalems.com/
http://images.google.ws/url?q=https://cityofrosedalems.com/
https://images.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.co.uk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.jp/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.es/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.ca/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.hk/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.nl/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.in/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.ru/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.au/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.tw/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.id/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.at/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cz/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.ua/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.tr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.mx/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.dk/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hu/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.fi/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.vn/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.pt/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.co.za/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.com.sg/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.gr/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.cl/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.bg/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.co/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.com.sa/url?sa=t&url=https://cityofrosedalems.com/
https://images.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://maps.google.hr/url?sa=t&url=https://cityofrosedalems.com/
https://medium.com/@adam.scotty.best
https://maps.google.co.ve/url?sa=t&url=https://cityofrosedalems.com/