Acetone: Present in Every Nail Salon
Acetone is a very simple and common solvent. In which it allows others substances to be dissolved in it. Most females are very familiar with acetone and its use to remove nail polish or acrylic. It is known as the simplest form of a ketone with a Carbonyl group and hydrocarbons.
Acetone is a volatile substance which means it evaporates very quickly. When one touches a nail polish remover with acetone they may notice that their skin becomes very dry. Due to the volatility of acetone the substance is evaporated quickly, therefore, leaving the skin very dry. Many dermatologists benefit from the drying effect of acetone by using it on a patient’s skin before a chemical peel. This allows the skin to be very clean and dry by removing any excess fat and oils in a process call defatting.
Acetone is not only available in nail polish removers but also skin care products, perfumes, colognes, and chemistry lab cleaning equipment. Acetone is commonly used to clean lab equipment to remove any excess water while drying quickly.
What does Acetone look like in Chemistry?
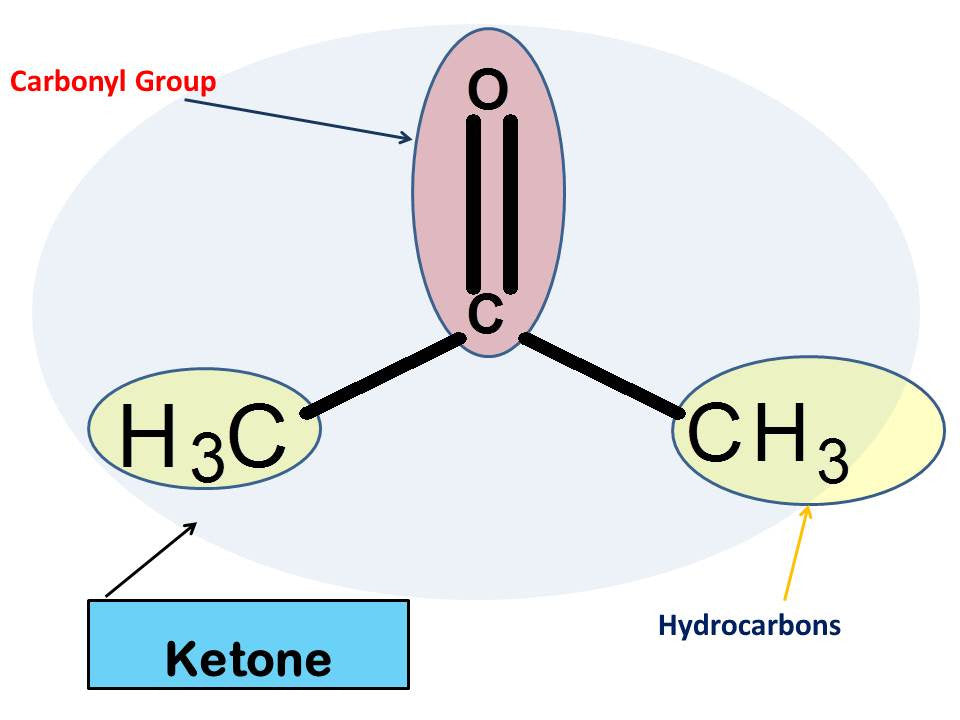
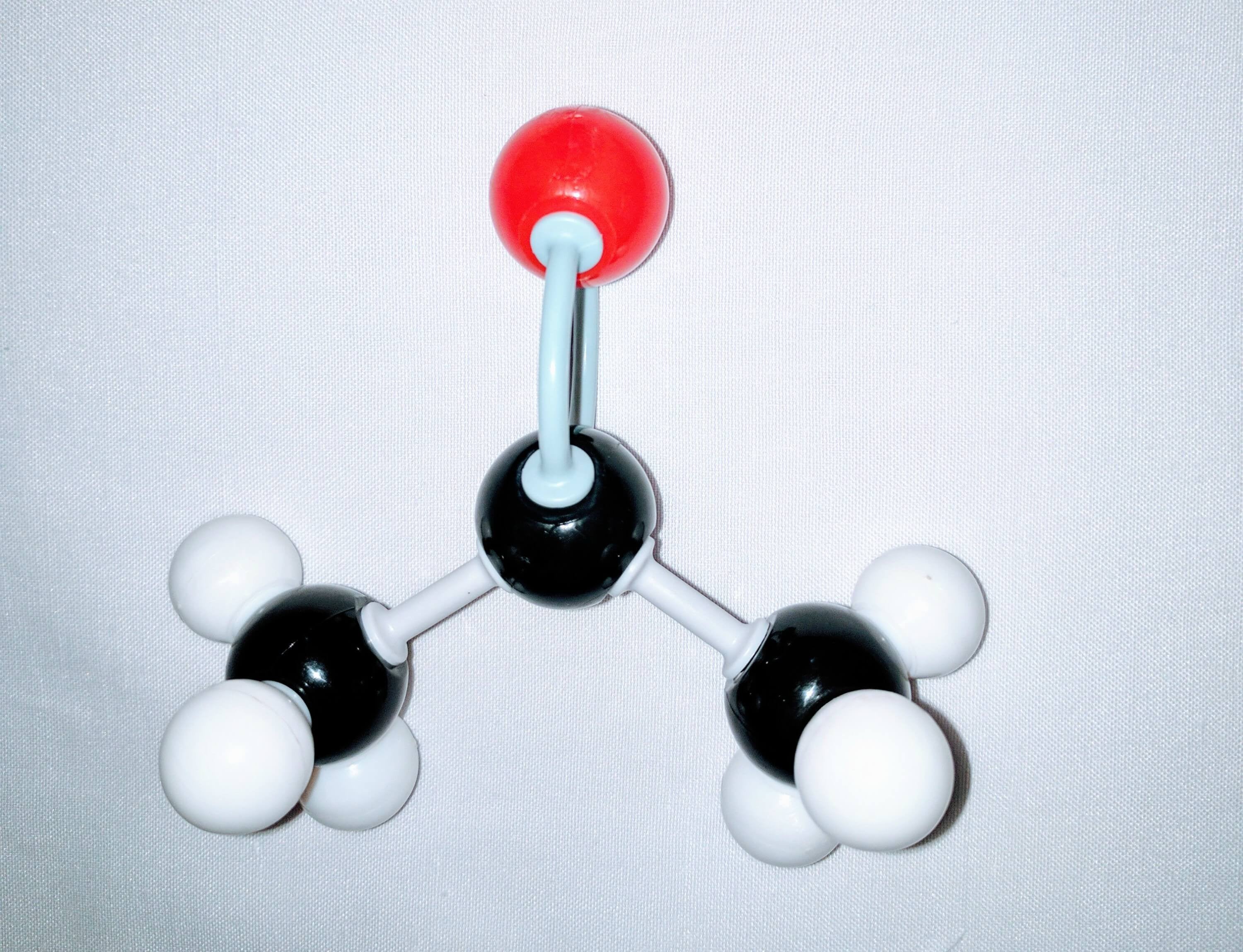
Let’s Get Building!
Using your Student Molecular Set from Duluth Labs let’s create Acetone! You’ll need:
-
3 Carbon Atoms
-
1 Oxygen atoms
-
6 Hydrogen atoms
-
6 Small connectors (compact small bonds for hydrogen)
-
2 Medium Connectors
-
2 Long connectors
-
Molecular Tool (for Disassembly)
Put aside all the atoms and connectors needed.
Let’s Start Building!
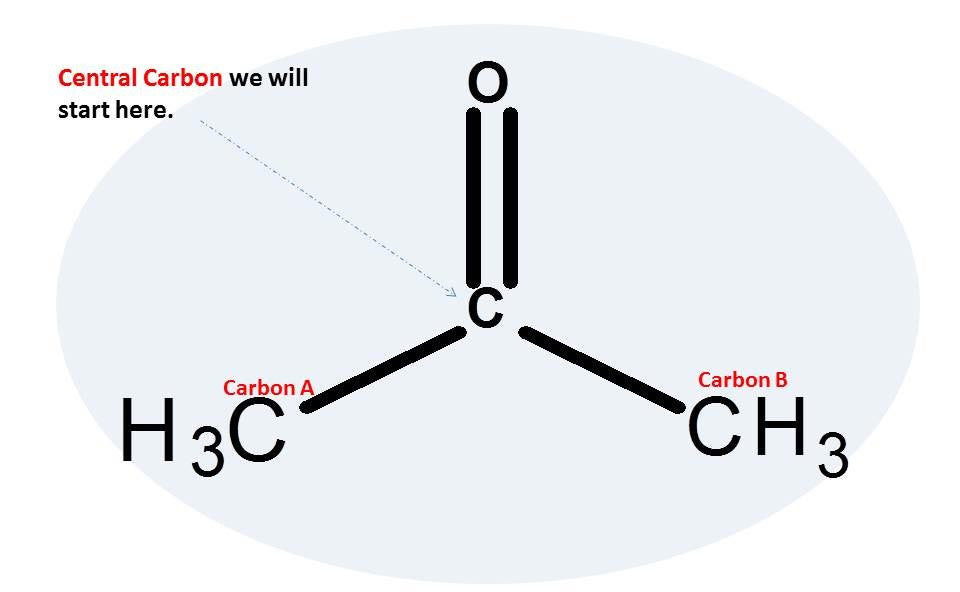
Note: Let’s begin with the central carbon atom.
Let’s start!
Steps:
-
1

1.Get one carbon atom (Central Carbon) and using 2 long connectors attach a oxygen atom to it. This will be your carbonyl group.
-
2
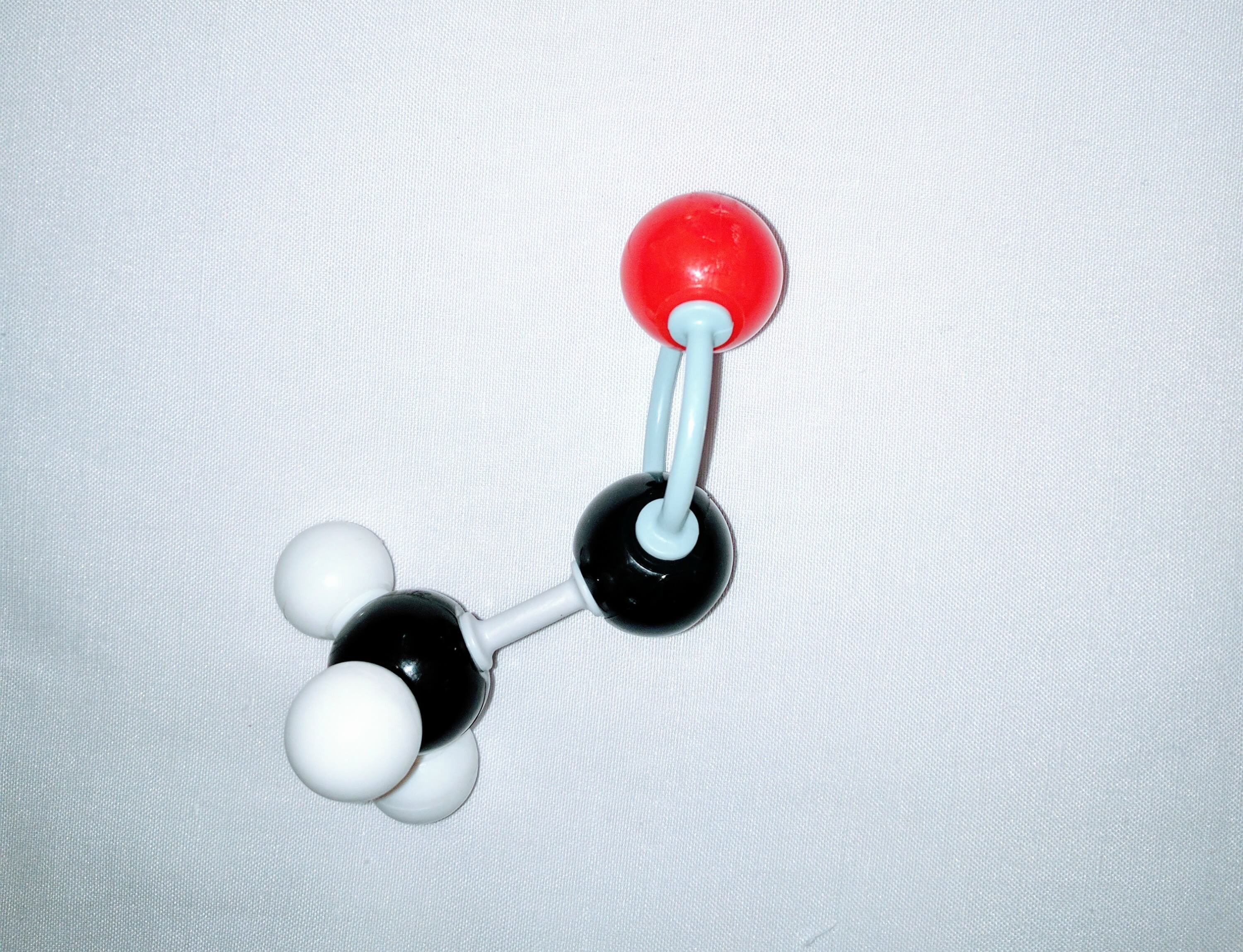
2.Using another carbon (Carbon A) atom and a medium connector attach this to the central carbon. Then using 3 small connectors attach 3 hydrogen atoms to Carbon A.
-
3
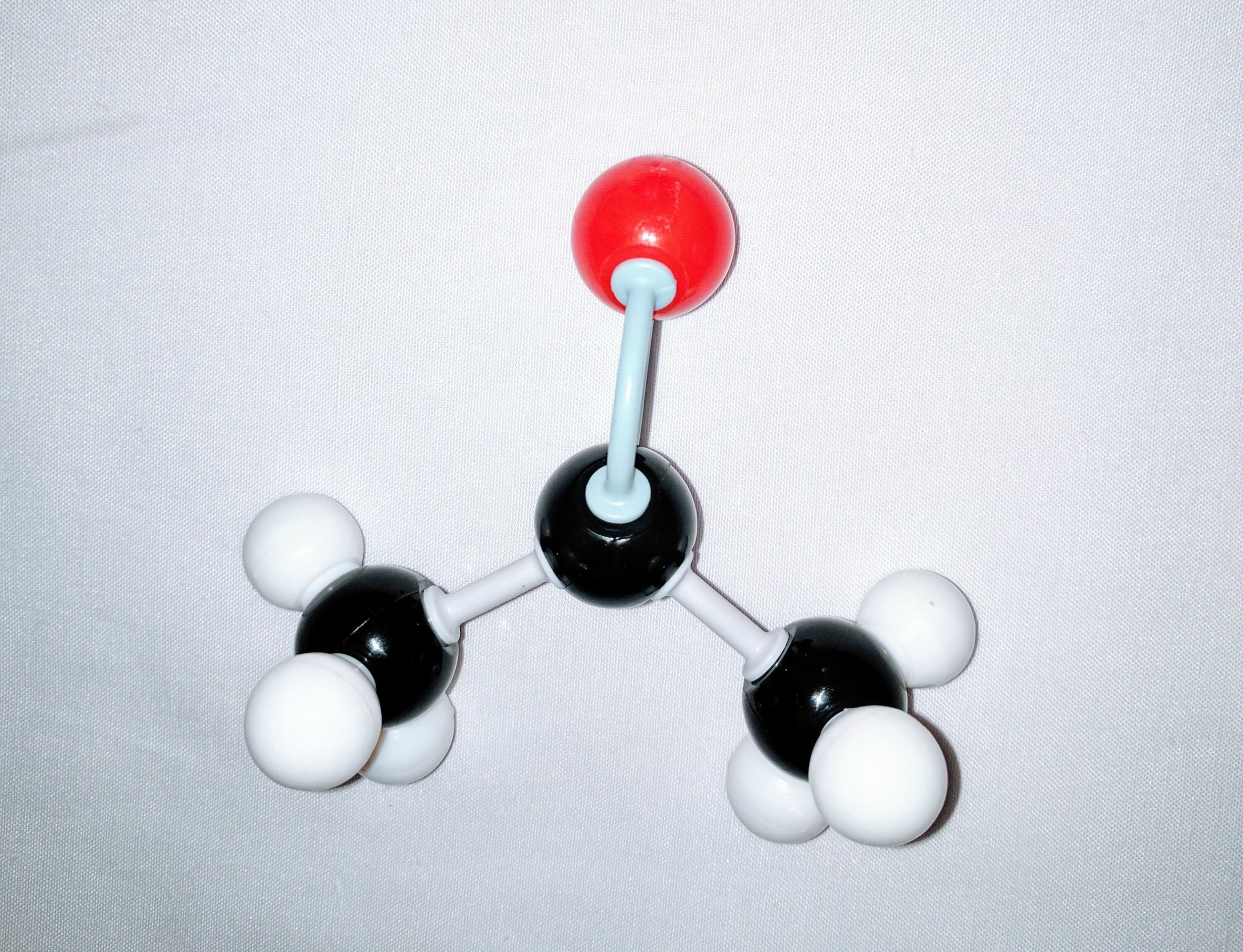
3.Using another carbon (Carbon B) and a medium connector attach this to remaining hole on the central carbon. Then using 3 small connectors attach 3 hydrogen atoms to Carbon B.
Great work! It was that easy! Now we have our newly-built Acetone Molecule!
Feel free to show us how your Acetone molecule turned out!
Comment and share pictures below!
Tune in next week for another Molecule of the week!
See you then xoxo :)









